Abstract
Mosquitoes are the most critical group of insects in the context of public health, since they transmit key parasites and pathogens, causing millions of deaths annually. Culex tritaeniorhynchus is an important vector of Japanese encephalitis (JE) across urban and semi-urban areas of Asia. In this study, we bio-fabricated silver nanoparticles (Ag NP) using the leaf extract of Bougainvillea glabra as reducing and stabilizing agent. The synthesis of Ag NP was confirmed analyzing the excitation of surface Plasmon resonance using ultraviolet–visible (UV–vis) spectrophotometry. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) showed the clustered and irregular shapes of Ag NP. The presence of silver was determined by energy dispersive X-ray (EDX) spectroscopy. Fourier transform infrared (FTIR) spectroscopy analysis investigated the identity of secondary metabolites, which may act as Ag NP capping agents. The acute toxicity of B. glabra extract, synthesized Ag NP and a combined treatment testing blends of both mosquitocidals was evaluated against larvae and pupae of Cx. tritaeniorhynchus. B. glabra showed LC50 of 198.93 (larva I), 234.50 (II), 309.18 (III), 371.69 (IV) and 466.09 (pupa) µg/ml, Ag NP LC50 ranged from 7.77 (I) to 19.44 µg/ml (pupa). Combined treatments with B. glabra leaf extract plus 5.12.5 µg/ml of Ag NP lowered the botanical LC50 to 66.09 (I), 76.48 (II), 99.02 (III), 133.43 (IV) and 179.74 µg/ml (IV), respectively. The effectiveness of green-fabricated Ag NP against the JE vector was confirmed in adulticidal tests, as well as evaluating the impact of Ag NP on fecundity and longevity of adult mosquitoes. Lastly, the larvicidal effectiveness of Ag NP was confirmed in the field, treating sewage water bodies. Overall, this study suggests that the green-synthesized Ag NP fabricated using B. glabra can be considered a potential mosquito control device against the JE vector, C. tritaeniorhynchus in Asian regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mosquitoes are the dangerous vectors of deadly pathogens and parasites, which may hit as epidemics or pandemics in the increasing world population of humans and animals [1, 2]. Culex quinquefasciatus, commonly known as the southern house mosquito, is a major vector of lymphatic filariasis, a neglected tropical disease commonly known as elephantiasis. Lymphatic filariasis is caused by Filariodea nematodes, namely Wuchereria bancrofti, which is responsible for 90 % of cases, Brugia malayi, and Brugia timori. Recently, more than 1.4 billion people in 73 countries are living in areas where lymphatic filariasis is transmitted and are at risk of being infected. Globally, an estimated 25 million men suffer with genital disease and over 15 million people are affected with lymphoedema. Eliminating lymphatic filariasis can prevent unnecessary suffering and contribute to the reduction of poverty [3]. Culex tritaeniorhynchus is a primary vector of Japanese encephalitis (JE) virus, with a distribution throughout Southeast Asia and South Asia. Keiser et al. [4] have reported that global annual incidence and mortality estimates for JE are 30,000 to 50,000 and 10,000 respectively. Cx. tritaeniorhynchus bites throughout the night with minor peaks in activity at 9 pm and 2.30 am [5]. Females have a preference for cattle and pigs, but also occasionally feed on birds and humans [6]. In India, Cx. tritaeniorhynchus predominantly feed on cattle, and to lesser extent on ducks, fowl, goats and humans [7]. In southern India this species is predominantly collected resting outdoors [8] however it has been found occurring in unusually high numbers resting indoors during the daytime [9].
The genus Bougainvillea comprises 18 species, some of them are used in traditional medicine to treat disorders such as diarrhea, stomach acidity, cough, sore throat, leucorrohea, hepatitis, and as an anti-inflammatory, antiviral and antibacterial agent [10, 11]. Leaves and inflorescences of B. glabra are also used in Mexican traditional medicine as a remedy for such respiratory illnesses as cough, cold, bronchitis and asthma [12, 13]. Its antimicrobial effect can be associated with the presence of betalains [14], as well as steroidal compounds with anti-inflammatory activity [15].
Bougainvillea glabra (Nyctaginaceae) is a common plant in Asia, used as a source of effective natural dyeing agents and botanicals used against the pests attacking stored rice. As an additional advantage, these plants can be grown easily in the farmer’s residential or cropping area. Pesticides of natural origin are economically viable and environmentally safe are easily available for the user [16, 17]. Kalirajan et al. [18] showed the flower extract of B. glabra has a promising potential as natural colouring agent and as a biopesticide. Recently, eco-friendly control tools have been implemented to enhance mosquito control. Significant efforts have been carried out to investigating the efficacy of botanical products, and many plant-borne compounds have been reported as excellent toxins against mosquitoes, acting as adulticidal, larvicidal, ovicidal, oviposition deterrent, growth and/or reproduction inhibitors and/or adult repellents [19–22]. In particularly, green-synthesized silver nanoparticles are emerging as multi-purpose materials, since their biosynthesis is easy and cheap; they are stable over time, and highly effective against mosquito vectors [23–28].
In this study, we focused on the swift fabrication of silver nanoparticles using B. glabra and on its potential against the JE vector Cx. tritaeniorhynchus. From a bio-physical point of view, the synthesized Ag NP were characterized by UV–vis spectrophotometry, Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), and X-ray diffraction (XRD). Furthermore, the toxicity of B. glabra extract, synthesized Ag NP and a combined treatment testing blends of both mosquitocidals was evaluated against larvae and pupae of Cx. tritaeniorhynchus. The effectivess of green-fabricated Ag NP against the JE vector was confirmed in adulticidal tests, as well as evaluating the impact of Ag NP on fecundity and longevity of adult mosquitoes. Lastly, larvicidal effectiveness of Ag NP was validated in the field treating sewage water bodies.
Materials and Methods
Collection of Plant Materials
The fresh leaves of B. glabra were collected in Kerala (Thenjippalam, Malappuram, India). The plants were identified at Department of Botany, Bharathiar University, Coimbatore. Voucher specimens were deposited in our laboratory and are available upon request.
Synthesis and Characterization of Silver Nanoparticles
Leaves of B. glabra were thoroughly washed with distilled water and dried for 2 days at room temperature. A plant leaf extract was prepared placing 10 g of leaves in a 300-mL Erlenmeyer flask with 100 mL of sterile distilled water. The mixture was boiled at 5 min, decanted for 2 h, stored at -4 °C, and used within 5 days. The crude extract was treated with an aqueous solution of AgNO3 (1 mM) and incubated at room temperature with dark condition. The solution turned to yellow-brownish, indicating the formation of Ag NP. The effects of reaction time on synthesis rate and particle size of the Ag NP were studied carrying out the reaction in a water bath (from 10 min to 4 h at 95 °C with reflux). AgNO3 solution was purified by repeated centrifugation at 15,000 rpm for 20 min, followed by re-dispersion of the pellet in deionizer water. The presence of synthesized Ag NP was confirmed by sampling the reaction mixture at regular intervals and absorption maxima was scanned by ultraviolet–visible (UV–vis) spectrum at the wavelength of 200–800 nm in a UV-3600 Shimadzu spectrophotometer operating at a resolution of 1 nm. Furthermore, the reaction mixture was subjected to centrifugation at 15,000 rpm for 20 min. The resulting pellet was dissolved in deionized water and filtered through a Millipore filter (0.45 μm). After freeze-drying of the purified Ag NP, the structure and composition were analyzed by 30-kV ultra-high resolution SEM (FEI QUANTA-200 SEM), TEM and EDX spectroscopy. The surface groups of the Ag NP were qualitatively confirmed using FTIR spectroscopy. FTIR spectra were recorded on a Perkin-Elmer 2000 FTIR spectrophotometer. To investigate the crystalline structure of silver nanoparticles, XRD analyses were conducted using a Cukα radiation (PAN analytical X’pert Pro MPD diffractometer [27].
Mosquito Rearing
Following the method reported by Murugan [25, 26] the eggs of Cx. tritaeniorhynchus were collected from Kallar at Mettupalayam, Tamil Nadu, India. Batches of 100–110 eggs were transferred into18 cm L × 13 cm W × 4 cm enamel trays containing 500 mL of water and they were allowed to hatch at laboratory conditions [27 ± 2 °C and 75–85 % R.H.; 14:10 (L:D) photoperiod]. Larvae were daily fed with 5 g of dog biscuits (Pedigree, USA) and brewer’s yeast (Sigma–Aldrich, Germany) in 3:1 ratio. I to IV instar larvae and recently pupated individuals (i.e., pupation occurred within 24 h) were collected and used in the experiments.
Larvicidal and Pupicidal Potential
Larvicidal activity of the aqueous extract and Ag NP from B. glabra was evaluated according to WHO protocol [29] with slight modification [25, 26]. 25 Cx. tritaeniorhynchus larvae (I, II, III, IV instar) and pupae were placed for 24 h in a 500-mL glass beaker filled with 249 mL of dechlorinated water plus 1 mL of the desired concentration of B. glabra aqueous extract or B. glabra- synthesized Ag NP. Larval food (0.5 mg) was also provided. Each concentration was replicated five times against all larval instars and pupae. In control treatments, 25 larvae or pupae were transferred in 250 mL of dechlorinated water. Percentage mortality was calculated as follows:
Mortality (%) = (Number of dead individuals/Number of treated individuals) × 100.
Impact on Longevity and Fecundity
Following the method reported by Naresh Kumar et al. [30] the longevity and fecundity experiments were conducted by taking an equal number of male and female larvae which had emerged from the control and treated sites. They were settled in wooden cages (30 × 30 cm) individually for each concentration. Three days after the blood meal, eggs were collected daily from the small plastic bowls containing water kept in the cages. The fecundity was calculated by the number of eggs laid in ovitrap divided by number of females let to mate (20 n, the death of adults in the experiments was also considered). Longevity was calculated by the number of days lived by the adult. The emergence day and mortal days of the adults were recorded and the mean longevity in days was calculated.
Field Experiments
The aqueous leaf extract of B. glabra and green-synthesized Ag NP were applied into sewage water bodies at Kalveerampalaym, Coimbatore, using a knapsack sprayer (Private Limited 2008, Ignition Products, India). Post-treatment observations were conducted after 24, 48, and 72 h using a larval dipper. Toxicity was assessed against larvae. All larvae were counted and identified to specific level Almirón and Brewer [31]. More than 91 % of all surveyed larvae belong to Cx. tritaeniorhynchus. Six trials were conducted for each site over similar weather conditions (28 ± 2 °C; 80 % RH). The required concentration of mosquitocidal was calculated on the basis of the total surface area and volume (0.25 m3 and 250 L) using 10 × LC50 values [32]. After 24 h, the percentage reduction of the larval density was calculated using the formula:
Percentage reduction = (C − T)/C × 100, where C is the total number of mosquitoes in the control and T is the total number of mosquitoes in the treatment [27].
Data Analysis
All mosquito toxicity data were subjected to ANOVA followed by Tukey’s HSD test [33]. Mosquito mortality data were also subjected to probit analysis. LC50 and LC90, were calculated using the method by Finney [34]. Data were analyzed using the SPSS Statistical Software Package version 17.0. A probability level of P < 0.05 was used for the significance of differences between values.
Results and Discussion
Bio-Physical Characterization of Nanoparticles
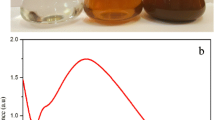
Biofabricated silver nanoparticles were characterized by UV–vis spectrophotometry, which could be used to examine size and shape-controlled nanoparticles in aqueous suspensions [39]. When the AgNO3 solution and leaf extract were mixed, the colour changed from light pale yellow to brownish indicating the reduction from Ag+ to Ag (Fig. 1a-b). The color changes were noted by visual observation in the leaf extracts when incubated with AgNO3 solution. The absorption spectrum of Ag NP showed a maximum absorption peak at 430 nm (Fig. 1c). Manusadianas et al. [35] previously reported that the lethality response of aquatic organisms (Nitellopsis obtusa algae, Thamnocephalus platyurus shrimps, and Brachionus calyciflorus rotifers) induced by sonicated and non-sonicated nano ZnO suspensions with various particle sizes (10 and 20–30 nm) and nano ZnO particles showed LC50 values of 438, 0.21, and 0.6 mg/L for 20–30 nm, respectively. Here the main peak is probably due to the excitation of longitudinal plasmon vibrations of Ag NP in the suspension [36, 37]. The synthesis of Ag NP was confirmed through visual assessment. Similarly, the S. muticum aqueous extract (5 % w/v) changed from yellowish light brown to dark brown after the addition of 1 mM AgNO3. [38].
Representative SEM micrograph (Fig. 2) of synthesized nanoparticles showed spherical and cubic structures with a size range of 30–70 nm. The latter may originate from the capping biomolecules bound to the surface of Ag NP [40, 41]. In agreement with our research, Ankanna et al. [42] reported SEM micrographs of Ag NP indicating that they were well-dispersed and ranged in size 30–40 nm. Also Ag NP produced using Emblica officinalis were predominantly spherical with an average size of 16.8 nm [43]. Murugan et al. [44] reported that Aristolochia indica leaf extract can be used for effective synthesis of Ag NP with 20–35 nm in size. The Sargassum muticum-mediated synthesis led to the production of well dispersed Ag NP with a size range of 43–79 nm [38].
Notably, the Ag NP formed was irregularly cubical or spherical in shape. It is known that the shape of metal nanoparticles considerably changes their optical and electronic properties [45]. The EDX spectrum recorded from B. glabra synthesized Ag NP revealed a distinct signal and high atomic percent values for silver. EDX spectroscopy confirmed the presence of Ag in the analyzed samples, showing a sharp optical absorption band peak at 5 keV (Fig. 3), typical for the absorption of metallic silver nanocrystallites [46–49]. Figure 4 shows TEM of Ag NP synthesized using B. glabra leaf extract. Among shapes, spheres dominated, an average size lower than 20 nm. Most of the Ag NP was roughly circular in shape with smooth edges. In previous findings, Ag NP from Annona squamosa leaf extract were spherical in shape with an average size ranging from 20 to 100 nm [50] while Thirunavokkarasu et al. [51] have reported spherical nanoparticles with size ranging from 8 to 90 nm following one-pot fabrication with Desmodium gangeticum.
Nanoparticles in XRD patterns exhibited different size dependent features leading to anomalous peak position, height and width (Fig. 5). The sharp Bragg’s peaks may be due to the presence of capping agent stabilizing Ag NP [52]. Our findings are in agreement with previous research conducted on Ag NP synthesized using leaf extract of Acalypha indica [53]. The XRD pattern of pure silver ions was known to display peaks at 2θ = 7.9°, 11.4°, 17.8°, 30.38°, and 44° [54]. Dubey et al. [55] showed that the size of silver nanocrystals, as estimated from the full width at half maximum of (111) peak of silver using the Scherrer’s formula, was 20–60 nm. In agreement with our results, a peak with maximum absorption at 410 nm characterized the synthesis of Aloe vera-fabricated Ag NP [37]. The XRD patterns indicated that the structure of Ag NP is face-centered cubic [56]. Overall, from the XRD pattern, it can be noted that Ag NP synthesized using B. glabra leaf extract were essentially crystalline. Recently, XRD analyses aimed at the characterization of Ag NP fabricated using the extract of Mimusops elengi leaves and S. muticum also pointed out the production of silver nanocrystallites [38, 57]. Our XRD results also highlights that the crystallization of the bioorganic phase occurs on the surface of the green-synthesized Ag NP [58].
FTIR spectroscopy was carried out to identify the possible bio-molecules from the B. glabra leaf extract, which may be responsible for the green synthesis and stabilization of Ag NPs. Figure 6 reports that the FTIR spectrum of aqueous Ag NP prepared from the B. glabra leaf extract have major transmittance peaks at 3369.87, 2975.46, 2937.18, 2132.62, 1921.04, 1738.50, 1654.34, 1438.79, 1368.48, 1218.23, 1086.28, 1045.30, 879.68, 655.22, 598.92, and 544.49 cm−1. They indicate that these different functional groups from bio molecules have probably capped Ag NP to prevent agglomeration, thus stabilizing the medium [25, 26, 59]. In detail, the peaks at 1,620–1,636 cm−1 may represent carbonyl groups from polyphenols such as catechin gallate, epicatechin gallate, epigallocatechin, epigallocatechin gallate, gallocatechin gallate and theaflavin; the results suggest that molecules interacting with Ag NP have free and bound amide groups. These amide groups may also be in the aromatic rings [60]. The peak at 1,381 cm−1 is probably due to the C–N stretching of the aromatic amine group [61]. The peaks at 1,027–1,092 cm−1 could correspond to the C–N stretching vibration of aliphatic amines or to alcohols/phenols, representing the presence of polyphenols [62]. The broad intense band close to 3400 cm−1 may be assigned to the N–H stretching arising from peptide linkages present in the proteins of the extract [63], while the peak located close to 1640 cm−1 could be assigned to the C=O stretching in carboxyl groups or C=N bending in amide groups [64, 65].
Ovicidal, Larvicidal and Pupicidal Potential
Egg hatchability of Cx. tritaeniorhynchus post-exposure to different doses of B. glabra extract and Ag NP was showed in Table 1. The percent hatchability was inversely proportional to the concentration of extract. Among the extracts tested for ovicidal activity on C. tritaeniorhynchus, the ovicidal activities of crude benzene and ethyl acetate extracts of Ervatamia coronaria (leaf) and Caesalpinia pulcherrima were highly effective [82]. Also, 100 % mortality was found post-treatment with methanol extract of A. alnifolia (125 ppm) [21].
The leaf extract of B. glabra was effective also against larvae (I-IV) and pupae of Cx. tritaeniorhynchus. LC50 values were 198.93, 234.50, 309.18, 371.69 and 466.09 µg/ml, respectively. LC90 values were 477.28, 563.56, 711.75, 772.26 and 936.36 µg/ml, respectively (Table 2). The potential role of botanical as nove mosquito larvicides and pupicides has been reported for a wide number of species [2, 19, 37, 66, 67]. Kovendan et al. [68] showed that methanolic leaf extract of Jatropha curcas exhibited dose-dependent larval toxicity against Cx. quinquefasciatus. Mahesh Kumar et al. [69] reported that S. xanthocarpum was toxic for larvae and pupae of Cx. quinquefasciatus with LC50 of 155.29, 198.32, 271.12, 377.44 and 448.41 ppm. Results of the present study are consistent with earlier findings [70] where the LC50 values of ethyl acetate extract of Leucas aspera were 75.40, 93.09, 132.20, and 138.60 ppm against the first, second, third, and fourth larval instars of Cx. quinquefasciatus. Also, the leaf extract of A. alnifolia with different solvents (hexane, chloroform, ethyl acetate, acetone and methanol) tested for larvicidal activity C. quinquefasciatus led to LC50 of 198.79, 172.48, 151.06, 140.69 and 127.98 ppm and LC90 = 458.73, 430.66, 418.78, 408.83 and 386.26 ppm, respectively [71]. Unfortunately, most of these researches failed to show insights on toxicity mechanisms exterted by the tested botanicals.
Green-synthesized Ag NP were highly effective in laboratory experiments conducted on Cx. tritaeniorhynchus larvae, with LC50 of 7.77, 9.40, 13.19, 15.67 and 19.44 µg/ml; LC90 were 19.28, 25.12, 30.80, 33.50 and 41.63 µg/ml concentration of 5 to 25 µg/ml, respectively (Table 3). Recently, the larvicidal activity of Ag NP synthesized using an aqueous extract from neem cake were highly effective against A. aegypti LC50 of 3.96, 4.52, 5.42, 6.71, and 8.30 ppm, respectively [28].]. L. aspera-synthesized Ag NP showed LC50 ranging from 13.06 (I) to 25.54 ppm (pupae) towards A. aegypti [77]. Besides the effectiveness of green-synthesized Ag NP, they are often eco-friendly. Nowadays, moderate knowledge is available about the acute toxicity of mosquitocidal nanoparticles towards non-target aquatic species [56]. Notably, Subarani et al. [72] did not reported toxicity effects of Vinca rosea-synthesized Ag NP against P. reticulata, after 72 h of exposure to dosages toxic against A. stephensi and C. quinquefasciatus. Similarly, Haldar et al. [73] did not detected toxicity of Ag NP produced using dried green fruits, D. roxburghii against P. reticulata, after 48 h exposure to LC50 of IV instars larvae of A. stephensi and C. quinquefasciatus. Similarly, the larvicidal effect of aqueous crude leaf extracts, silver nitrate solution, and Ag NP of Mimosa pudica showed that the highest mortality was achieved by synthesized Ag NP both against the larvae of A. subpictus (LC50 = 8.89, 11.82, and 0.69 ppm) and C. quinquefasciatus (LC50 = 9.51, 13.65, and 1.10 ppm) [74]. High mortality was reported for treatments on A. subpictus using Nelumbo nucifera plant extract methanol extract, aqueous extract, and green-synthesized Ag NP (LC50 = 8.89, 11.82, and 0.69 ppm; LC90 = 28.65, 36.06, and 2.15 ppm) and also against the larvae of C. quinquefasciatus (LC50 = 9.51, 13.65, and 1.10 ppm; LC90 = 28.13, 35.83, and 3.59 ppm) [75]. As regards to other arthropods, ZnO nanoparticles showed the LC50 and r 2 values against Rhipicephalus microplus (13.41 mg/L; 0.982), Pediculus humanus capitis (11.80; 0.966 mg/L), and larvae of An. subpictus (3.19; 0.945 mg/L) and C. quinquefasciatus (4.87; 0.970 mg/L) [76].
Combined treatments with B. glabra leaf extract plus 5.12.5 µg/ml of Ag NP lowered the botanical LC50 to 66.09 (I), 76.48 (II), 99.02 (III), 133.43 (IV) and 179.74 µg/ml (IV), respectively (Table 4). Similarly, Morinda citrifolia tested in blend with the fungus Metarhizium anisopliae achieved values of LC50 3.71, 16.73, 29.71, 40.60, and 138.10 mg/L; LC90 were 122.29, 150.15, 156.90, 211.99 and 806.67 mg/L at 48 h, respectively [78]. Moreover, the combined treatment of Catharanthus rosesus with Bacillus thuringiensis against the malaria vector An. stephensi obtained LC50 of 2.18, 2.41, 2.76 and 3.22 mg/L, respectively [79]. The larval mortality after a combined treatment of B. thuringiensis subsp. israelensis plus Jatropha curcas leaf extract (JCLE) was found toxic for al mosquito larval instars. At a dose of 14 μg/ml + 1.0 % combined B. thuringiensis subsp. israelensis, the treatment led to IV instar larval mortality of 83 %. The LC50 values were 1.20, 1.29 1.35 and 1.44 %, respectively [80]. The larvicidal activity of aqueous crude bark extracts and synthesized Ag NP from F. racemosa was tested against fourth instars larvae of the filarial vector C. quinquefasciatus and the JE vector C. gelidus showing LC50 of 12.00 and 11.21 mg/l, respectively [81].
Impact on Longevity and Fecundity
Adult longevity and fecundity of Cx. tritaeniorhynchus, after the treatment of B. glabra extract and green synthesized Ag NP, is shown in Table 5. Significant reduction in adult longevity and fecundity was recorded in experiment when compared with the control. Longevity was reduced to 8.1 days post-treatment at 500 µg/ml concentration of aqueous extract of B. glabra, whereas control longevity was 23.2 days. The fecundity was also reduced post-treatment with B. glabra-synthesized Ag NP. One hundred and fifty eggs were recorded in control and the numbers of eggs recorded in treatment were 151.8, 143.4, 135.2, 128.6 and 112.5 eggs when doses of 5, 10, 15, 20, and 25 µg/ml were tested, respectively. Similarly, Naresh Kumar et al. [83] reported a reduction in adult longevity (4.2 in male and 11.7 in female at 10 ppm) in An. stephensi after the treatment with Ag NP fabricated using A. squamosa. Furthermore, Poopathi and Tyagi [84] showed a reduction of adult longevity (17 in male and 27 in female) in C. quinquefasciatus after the treatment with B. sphaericus (GR strain). Also, Makowski et al. [85, 86] noted a reduction in adult longevity (17 days for males and 27 for females) in C. quinquefasciatus after the treatment with B. sphaericus (GR strain).
Adulticidal Potential on Cx. tritaeniorhynchus
In laboratory assays, the adulticidal potential of B. glabra leaf extract and Ag NP against Cx. tritaeniorhynchus showed LC50 and LC90 of 302.54 and 647.70 µg/ml; and 10.87 and 26.83 µg/ml, respectively (Table 6). Concerning other botanicals, the adulticidal activity of the essential oil isolated from Mentha longifolia was screened by fumigant toxicity assay against the house mosquito, C. pipiens by Oz et al. [87]. Also, the bio-surfactant products released by Bacillus subtilis subsp. subtilis (VCRC B471), are a potential bio adulticide for ULV spray against A. stephensi [88]. The adult mortality exterted by ethanol extract of C. sinensis showed LC50 and LC90 of 272.19 and 457.14 ppm, A. stephensi of 289.62 and 494.88 ppm, and A. aegypti of 320.38 and 524.57 ppm, respectively [89]. The methanol extract of Andrographis paniculata on the adults of C. quinquefasciatus and A. aegypti showed LC50 and LC90 of 149.81, 172.37 ppm and 288.12, 321.01 ppm, respectively [90]. The LC50 and LC90 values of A. alnifolia leaf extracts (hexane, benzene, ethyl acetate, acetone and methanol) on C. quinquefasciatus adults were 383.59, 354.13, 327.74, 314.33 and 291.71 ppm, respectively [21]. The LC50 and LC90 values of Cassia tora leaf extracts (hexane, chloroform benzene, acetone, and methanol) against C. quinquefasciatus adults led to LC50 values of 338.81, 315.73, 296.13, 279.23, and 261.03 ppm and LC90 values were 575.77, 539.31, 513.99, 497.06, and 476.03 ppm, respectively [91] .
Effectiveness in the Field
In the field, the Ag NP (10 × LC50) efficacy led to Cx. tritaeniorhynchus larval reduction of 17.8, 63.3, and 91.9 %, while the leaf extract of B. glabra achieved values of 7.84, 56.9, and 100 % of larval reduction after 24, 48, and 72 h, respectively (Table 7). These results are in agreement with previous research [92], analyzing the field efficacy of biopesticides in tsunami-affected areas of India, which reported strong reduction, or even eradication, of larval populations of several mosquito vectors. A total of 1,400 C. quinquefasciatus larvae found were observed in the sewage water systems. After being treated with L. aspera extracts, the C. quinquefasciatus larval density was reduced by 60.4, 81.9, and 99.7 % at 24, 48, and 72 h, respectively; similarly, the reduction of C. quinquefasciatus larval densities after treatment with A. indicum, H. suaveolens, and J. curcas were 51.7, 77.6 and 92 %; and 50, 73.5 and 90.4 %; 46.7, 71.7, and 89.9 % at 24, 48, and 72 h, respectively [93]. More recently, the mosquitocidal efficacy of the leaf extract of E. hirta was investigated in a field condition against A. stephensi, and larval density was reduced by 13.17, 37.64 and 84.00 % after 24, 48, and 72 h, respectively [94]. The larval density decreased rapidly post-treatment with plant extracts and Ag NP at the mosquito breeding sites (sewage water), thus these novel products could be considered as promising alternatives to synthetic insecticides for the mosquito vector management.
Conclusions
To our mind, the plant-mediated reduction of metal ions to nanocomposites could represent a valid tool for the development of clean, nontoxic, and environmentally acceptable control agents. The green-synthesized Ag NP fabricated in this research are hydrophilic in nature, disperse uniformly in water, and highly stable. They also exhibit significant mosquito potential activity against the JE vector Cx. tritaeniorhynchus. Indeed, the effectiveness of green-fabricated Ag NP against the JE vector was confirmed in adulticidal tests, as well as evaluating the impact of Ag NP on fecundity and longevity of adult mosquitoes. Larvicidal effectiveness of Ag NP was confirmed in treated sewage water bodies. Overall, this study suggests that the green-synthesized Ag NP fabricated using B. glabra can be considered a potential mosquito control device against the JE vector, Cx. tritaeniorhynchus in Asian regions.
References
H. Mehlhorn, K. A. S. Al-Rasheid, S. Al-Quraishy, and F. Abdel-Ghaffar (2012). Parasitol. Res. 110, 259.
G. Benelli (2015). Parasitol. Res. 114, 2801.
World Health Organization WHO, Geneva (Japanese encephalitis, Fact sheet No, 2014). 386.
J. Keiser, M. F. Maltese, T. E. Erlanger, R. Bos, M. Tanner, and B. H. Singer (2005). Acta. Trop. 95, 40.
D. J. Lee, M. M. Hicks, M. L. Debenham, M. Griffiths, E. N. Marks, J. H. Bryan, and R. C. Russell The Culicidae of the Australasian region (Australian, Government Publishing Service, Canberra, 1989).
R. A. Bram (1967). Contr. Am. Entomol. Instt. 2, 1–296.
N. Arunachalam, P. Philip Samuel, J. Hiryan, R. Rajendran, and A. P. Dash (2005). Am. J. Trop. Med. 72, 198.
B. P. Das, S. Lal, and V. K. Saxena (2004). J. Vect. Borne Dis. 41, 32–36.
P. C. Kanojia and G. Geevarghese (2004). J. Med. Entomol. 41, 994–996.
A. Umamaheswari, R. Shreevidya, and A. Nuni (2008). Adv. Biol. Res. 2, 1–5.
V. Gupta, M. George, L. Joseph, M. Singhal, and H. P. Singh (2009). J. Chem and Pharm. Res. 1, 233.
A. Aguilar, J. R. Camacho, S. Chino, P. Jacques, and M. E. López (1994). Instituto. Mexicano del Seguro Social 5.
F. Lara-Ochoa and C. Márquez-Alonso Plantas Medi- cinales de México II (Composición (Usos y Actividades Biológicas, Universidad Nacional Autónoma de México, Mexico, 1996), p. 1996.
F. Kugler, F. Stintzing, and R. Carle (2007). Analyt. Bioanalyt. Chem. 387, 637.
S. N. Giri, A. K. Biswas, B. P. Saha, S. P. Pal, and M. Pal (1988). J. Pharm. Sci. 50, 42.
I. Joseph, D. Edwin Chellaiah, and A. J. A. Ranjith Singh (2010). J. Biopest. 3, 553.
P. Rajasekhar reddy and P. Usha Rani (2010). J. Biopest. 3, 586.
A. Kalirajan, R. Mariselvam, J. R. Savarimuthu Michael, K. Narayanan, G. Athi Narayanan, and A. J. A. Ranjit Singh (2012). Int. J. Curr. Res. 4, 009.
G. Benelli (2015). Parasitol. Res. 114, 887.
B. Conti, M. Leonardi, L. Pistelli, R. Profeti, I. Ouerghemmi, and G. Benelli (2013). Parasitol.Res. 112, 991.
K. Kovendan, K. Murugan, P. Mahesh Kumar, P. Thiyagarajan, and S. John William (2013). Parasitol. Res. 112, 1205.
S. Kamalakannan, K. Murugan, A. Naresh Kumar, N. Ramasubramanian, and P. Mathiyazhagan (2008). Afr. J. Biotechnol. 7, 838.
A. Rawani, A. Ghosh, and G. Chandra (2013). Acta. Trop. 128, 613.
D. Amerasan, K. Murugan, C. Panneerselvam, N. Kanagaraju, K. Kovendan, and P. Mahesh Kumar (2015). J. Entomol. Acarol. Res. 47, 31.
K. Murugan, G. Benelli, S. Ayyappan, D. Dinesh, C. Panneerselvam, M. Nicoletti, J. S. Hwang, P. Mahesh Kumar, J. Subramaniam, and U. Suresh (2015). Parasitol. Res. 114, 2243.
K. Murugan, G. Benelli, C. Panneerselvam, J. Subramaniam, T. Jeyalalitha, D. Dinesh, M. Nicoletti, J. S. Hwang, U. Suresh, and P. Madhiyazhagan (2015). Exp. Parasitol. 153, 129.
U. Suresh, K. Murugan, G. Benelli, M. Nicoletti, D. R. Barnard, C. Panneerselvam, P. Mahesh Kumar, J. Subramaniam, D. Dinesh, and B. Chandramohan (2015). Parasitol. Res. 114, 1551.
B. Chandramohan, K. Murugan, C. Panneerselvam, P. Madhiyazhagan, R. Chandirasekar, D. Dinesh, P. M. Kumar, K. Kovendan, U. Suresh, J. Subramaniam, R. Rajaganesh, A. T. Aziz, B. Syuhei, M. S. Alsalhi, S. Devanesan, M. Nicoletti, H. Wei, and G. Benelli (2015). Parasitol. Res. 115, 1015.
World Health Organization (2005). WHO, HO/CDS/WHOPES/GCDPP/1.3.
A. Naresh Kumar, K. Murugan, K. Shobana, and D. Abirami (2014). Afr. J. Mala. Trop. Diseases. 2, 46.
W. R. Almirón and M. E. Brewer (1996). Mem. Inst. Oswaldo. Cruz. 91, 649.
K. Murugan, R. Vahitha, I. Baruah, and S. C. Das (2003). Ann. Med. Entomol. 12, 11.
H. L. Alder and E. B. Rossler 6 th edn (Freeman. San, Francisco, 1977). 246.
D. J. Finney Probit analysis (Cambridge University Press, London, 1971), pp. 68–72.
L. R. Manusadianas, S. Grigutyte, R. Jurkoniene, K. Karitonas, J. F. Sadauskas, S. Férard, L. F. Cotelle (2009). Toxicity of zinc oxide nanoparticle suspensions to aquatic biota, ISTA-14, METZ VIII 30-IX 04, Lithuanian State Science and Studies Foundation Gilibert-France Lithuanian Research Program.
S. S. Shankar, A. Rai, A. Ahmad, and M. Sastry (2004). J. Colloid Interface Sci. 275, 496.
D. Dinesh, K. Murugan, P. Madhiyazhagan, C. Panneerselvam, M. Nicoletti, W. Jiang, G. Benelli, B. Chandramohan, and U. Suresh (2015). Parasitol. Res. 114, 1519.
P. Madhiyazhagan, K. Murugan, A. Naresh Kumar, T. Nataraj, D. Dinesh, C. Panneerselvam, J. Subramaniam, P. Mahesh Kumar, U. Suresh, M. Roni, M. Nicoletti, A. A. Alarfaj, A. Higuchi, M. Nicoletti, M. A. Munusamy, and G. Benelli (2015). Parasitol. Res. 114, 4305.
S. Shrivastava and D. Dash (2010). Nano-Micro. Lett. 2, 164.
K. B. Naryanan and N. Sakthivel (2010). Adv. Colloids. Inter. Sci. 156, 1.
S. Ganesan, K. Sengodan, and S. S. Muthugounder (2014). Parasitol. Res. 113, 875.
S. Ankanna, P. TNVKV, E. K. Elumalai, and N. Savithramma (2010). Dig. J. Nanomater. Biostruct. 5, 369.
B. Ankamwar, M. Chaudhary, and M. Sastry (2005). Nano-Metal. Chem. 35, 19.
K. Murugan, M. Aamina Labeeba, C. Panneerselvam, D. Dinesh, U. Suresh, J. Subramaniam, P. Madhiyazhagan, J. S. Hwang, L. Wang, M. Nicoletti, and G. Benelli (2015). Res. Vet. Sci. 102, 127.
H. Xu and M. Kall (2002). Phys. Rev. Lett. 9, 246802.
P. Magudapathy, P. B. K. Gangopadhyay, K. G. M. Nair, and S. Dhara (2001). Physica. 299, 142.
A. M. Fayaz, K. Balaji, M. Y. R. Girilal, P. T. Kalaichelvan, and R. Venketesan (2010). Nanomed. Nanotechnol. Biol. Med. 6, 103.
N. Soni and S. Prakash (2014). Ann. Microbiol. 64, 1099.
P. Vinayaga Moorthi, C. Balasubramanian, and S. Mohan (2015). Appl. Biochem. Biotechnol. 175, 135.
R. Vivek, R. Thangam, K. Muthuchelian, P. Gunasekaran, K. Kaveri, and S. Kannan (2012). Process Biochem. 47, 2405.
M. Thirunavokkarasu, U. Balaji, S. Behera, P. K. Panda, and B. K. Mishra (2013). Biomol. Spectrosc. 116, 424.
H. Bar, D. H. Bhui, P. G. Sahoo, P. S. Sarkar Pyne, and A. Misra (2009). Colloids. Surf. A. 348, 212.
C. Krishnaraj, E. G. Jagan, S. Rajasekar, P. Selvakumar, P. T. Kalaichelvan, and N. Mohan (2010). Colloids Surf. B Biointerfaces 76, 50.
P. Gong, H. Li, X. He, K. Wang, J. Hu, W. Tan, S. Zhang, and X. Yang (2007). Nanotechnology 18, 7.
S. P. Dubey, M. Lahtinen, and M. Sillanpaa (2009). Process. Biochem. 45, 1065.
K. Shameli, M. B. Ahmad, W. M. Z. W. Yunus, and N. A. Ibrahim (2010). Int. J. Nanomedicine. 5, 743.
J. Subramaniam, K. Murugan, C. Panneerselvam, K. Kovendan, P. Madhiyazhagan, P. Mahesh Kumar, D. Dinesh, B. Chandramohan, U. Suresh, M. Nicoletti, A. Higuchi, J. S. Hwang, S. Kumar, A. A. Alarfaj, M. A. Munusamy, R. H. Messing, and G. Benelli (2015). Environ. Sci. Pollut. Res. 24, 20067.
G. Benelli (2016). Parasitol. Res. 115, 23.
R. Sathyavathi, M. Balamurali Krishna, S. Venugopal Rao, R. Saritha, and D. Narayana Rao (2010). Adv. Sci. Lett. 3, 1.
V. Kumar, S. C. Yadav, and S. K. Yadav (2010). J. Chem. Technol. Biotech. 85, 1301.
B. Mahitha, P. R. Deva, G. R. Dillip, C. Madhukar Reddy, K. Mallikarjuna, L. Manoj, S. Priyanka, R. Jayantha, and N. John Sushma (2011). J. Nanomat. Biostruct. 6, 135.
J. Y. Songa, H. K. Janga, and B. S. Kim (2009). Process. Biochem. 44, 1133.
P. Mukherjee, M. Roy, B. P. Mandal, G. K. Dey, P. K. Mukherjee, J. Ghatak, K. Tyagia, and S. P. Kale (2008). Nanotechnol. 19, 075.
A. Bankara, B. Joshi, A. Ravi Kumar, and S. Zinjardea (2010). Colloids Surf. B: Biointerfaces 80, 45.
P. Nagajyothi, N. Minh, T. Sreekanth, T. An, L. Dong, K. D. Lee, C. V. M. Jae-il Lee, and S. P. Kale (2013). Mat. Lett. 108, 160.
A. Amer and H. Mehlhorn (2006). Parasitol. Res. 99, 466.
G. Benelli (2015). Parasitol. Res. 114, 3201.
K. Kovendan, K. Murugan, S. Vincent, and S. Kamalakannan (2011). Parasitol. Res. 109, 1251.
P. Mahesh Kumar, K. Murugan, K. Kovendan, J. Subramaniam, and D. Amaresan (2012). Parasitol. Res. 110, 2541.
J. Muthukrishnan, E. Pushpalatha, and A. Kasthuribhai (1997). Insect. Sci. Appl. 17, 389.
K. Kovendan, K. Murugan, S. Vincent, and D. R. Barnard (2012). Parasitol. Res. 110, 195.
S. Subarani, S. Sabhanayakam, and C. Kamaraj (2013). Parasitol. Res. 112, 487.
K. M. Haldar, B. Haldar, and G. Chandra (2013). Parasitol. Res. 112, 1451.
S. Marimuthu, A. A. Rahuman, G. Rajukumar, T. Santhosh kumar, V. A. Kirthi, C. Jayaseelan, A. Bagavan, A. A. Zahir, G. Elango, and C. Kamaraj (2011). Parasitol. Res. 108, 1541.
T. Santhoshkumar, A. A. Rahuman, G. Rajakumar, S. Marimuthu, A. Bagavan, C. Jayaseelan, A. A. Zahir, G. Elango, and C. Kamaraj (2011). Parasitol. Res. 108, 693.
A. V. Kirthi, A. A. Rahuman, G. Rajakumar, S. Marimuthu, T. Santhoshkumar, C. Jayaseelan, and K. Velayutham (2011). Parasitol. Res. 109, 461.
S. Sivapriyajothi, P. Mahesh Kumar, K. Kovendan, J. Subramaniam, K. Murugan, C. Jayaseelan, and K. Velayutham (2014). J. Entomol. Acarol. Res. 46, 1787.
K. Kovendan, S. P. Shanthakumar, C. Praseeja, P. Mahesh Kumar, K. Murugan, S. Vincent, and K. Velayutham (2014). Asi. Paci. J. Tro. Dis 4, 173.
C. Panneerselvam, K. Murugan, K. Kovendan, and P. Mahesh Kumar (2012). Parasitol. Res. 111, 2241.
K. Kovendan and K. Murugan (2011). J. Adv. in Environ. Biol. 5, 335.
K. Velayutham, A. A. Rahuman, G. Rajakumar, S. M. Roopan, G. Elango, C. Kamaraj, S. Marimuthu, T. Santhosh kumar, M. Iyappan, and C. Siva (2013). A. Paci.J. Trop. Med. 6, 95.
M. Govindarajan, T. Mathivanan, K. Elumalai, K. Krishnappa, and A. Anandan (2011). Parasitol. Res. 109, 353.
A. Naresh Kumar, K. Murugan, C. Rejeeth, P. Madhiyazhagan, and D. R. Barnard (2012). Vector-Borne. Zoo. Dis. 12, 262.
S. Poopathi and B. K. Tyagi (2002). Appl. Entomol. Zool. 37, 365.
R. Makowski (1993). Ecol. Epidemiol. 83, 1229.
A. Naresh Kumar, K. Murugan, K. Shobana, and D. Abirami (2013). Scient. Res. Essa. 8, 425.
E. Oz, I. Cinbilgel, and H. Cetin The 4th European Mosquito Control Association Workshop (Czech Republic, September, Prague, 2007), pp. 11–14.
I. Geetha, K. P. Paily, and A. M. Manonmani (2011). Pest. Manag. Sci. 68, 1447.
K. Murugan, P. Mahesh Kumar, K. Kovendan, D. Amerasan, and J. Subramaniam (2012). Parasitol. Res. 111, 1757.
M. Govindarajan and R. Sivakumar (2012). Parasitol. Res. 110, 1607.
D. Amerasan, K. Murugan, K. Kovendan, P. Mahesh Kumar, C. Panneerselvam, J. Subramaniam, S. John William, and J. S. Hwang (2012). Parasitol. Res. 111, 1953.
K. Murugan (2006). Tsunami relief work-biopesticide spray operations—a case study. In: Nadim F, Pöttler R, Einstein H, Klapperich H, Kramer S (eds) Geohazards. ECI Symposium Series, vol. P7. http://services.bepress.com/eci/geohazards/39.
K. Kovendan, K. Murugan, C. Panneerselvam, P. Mahesh Kumar, D. Amerasan, J. Subramaniam, S. Vincent, and D. R. Barnard (2012). Parasitol. Res. 110, 210.
C. Panneerselvam, K. Murugan, K. Kovendan, P. M. Kumar, and J. Subramaniam (2013). Asian. Pac. J. Trop. Med. 6, 102.
Acknowledgments
Prof. C. M. Lukehart and three anonymous reviewers kindly improved an earlier version of the manuscript. The authors are thankful to Science Engineering Research Board (SERB), Department of Science and Technology (DST), Govt. of India, New Delhi (SR/FT/LS-156/2012) for providing financial support for the present work. The authors are grateful to Mr. M. Munirathnam, (ICMR, Madurai), Taxonomy and Field Station in Coimbatore, Tamil Nadu, for helping in mosquito collection and identifying mosquito species of samples for the experiment work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vincent, S., Kovendan, K., Chandramohan, B. et al. Swift Fabrication of Silver Nanoparticles Using Bougainvillea glabra: Potential Against the Japanese Encephalitis Vector, Culex tritaeniorhynchus Giles (Diptera: Culicidae). J Clust Sci 28, 37–58 (2017). https://doi.org/10.1007/s10876-016-1038-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1038-3