Abstract
Rutaceae are widely recognized for their toxic and repellent activity exerted against mosquitoes. In our research, the essential oils extracted from fresh leaves of wild and cultivated plants of Ruta chalepensis L. (Rutaceae) were evaluated for larvicidal and repellent activity against the Asian tiger mosquito, Aedes albopictus Skuse (Diptera: Culicidae), currently the most invasive mosquito worldwide. In this research, gas chromatography and gas chromatography–mass spectrometry analyses of the essential oils from wild and cultivated plants showed only quantitative differences, in particular relatively to the amounts of ketone derivatives, while the qualitative profile evidenced a similar chemical composition. Both essential oils from wild and cultivated R. chalepensis plants were able to exert a very good toxic activity against A. albopictus larvae (wild plants, LC50 = 35.66 ppm; cultivated plants, LC50 = 33.18 ppm), and mortality was dosage dependent. These data are the first evidence of the toxicity of R. chalepensis against mosquitoes. Furthermore, the R. chalepensis essential oil from wild plants was an effective repellent against A. albopictus, also at lower dosages: RD50 was 0.000215 μL/cm2 of skin, while RD90 was 0.007613 μL/cm2. Our results clearly evidenced that the larvicidal and repellent activity of R. chalepensis essential oil could be used for the development of new and safer products against the Asian tiger mosquito.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In tropical countries, mosquitoes serve as vectors of very dangerous diseases, such as malaria, filariasis, yellow fever, dengue, and other viral infections, which contribute consistently to poverty and social debility (James 1992). Mosquitoes are important human pests also in Europe since their bites cause local skin reactions, as well as serious allergic and systemic responses such as angioderma and urticaria (Peng et al. 1999). They are still considered the most important group of insects in terms of public health, and further research is needed to improve their control strategies.
The Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae) was first described in India by Skuse (1894) as the “banded mosquito of Bengali”. In the 1960s, the distribution area of A. albopictus was restricted to the tropical and subtropical forests of some parts of Asia, India, and a few Pacific Islands, where it was likely zoophilic. Thus, it progressively adapted to anthropogenic environments, which provide alternative food sources and larval habitats (Paupy et al. 2009). In recent decades, A. albopictus has invaded many countries globally, spreading rapidly to Europe, North and South America, the Caribbean, Africa, and the Middle East (for a recent review see Caminade et al. 2012). Moreover, further efforts should be made to conduct surveys for A. albopictus in countries at high risk for its future establishment, with particular emphasis to the coastal harbor areas of Cyprus, Bulgaria, Portugal, Turkey, the Benelux, Germany, and the United Kingdom (Caminade et al. 2012). Overall, A. albopictus is currently ranked as one of the world’s 100 most invasive species, and it is retained the most invasive mosquito species in the world (Reiter and Sprenger 1987; Benedict et al. 2007). This seems to be due to its huge ecological plasticity that allows for its rapid adaptation to a wide range of habitats. Indeed, A. albopictus is able to displace other different mosquito species from their original habitats (Juliano and Lounibos 2005). Moreover, the Asian tiger mosquito has long-lived eggs that possess the ability to adapt to cold temperatures by becoming dormant during the winter of temperate regions (Estrada-Franco 1995). This physiological plasticity is likely linked to its ability to synthesize a high amount of lipid in cold temperatures (Paupy et al. 2009; Yamani et al. 2012).
The medical importance of A. albopictus is mainly due to the aggressive daytime human-biting behavior (Gratz 2004) and to its ability to serve as a vector and transmit many viruses, including dengue, yellow fever, West Nile, Japanese encephalitis, and St. Louis encephalitis viruses (Flaviridae, genus Flavivirus); chikungunya, Eastern equine encephalitis, Venezuelan equine encephalitis, Western equine encephalitis, Ross River, Sindbis, Mayaro, and Getah viruses (Togaviridae, genus Alphavirus); Potosi, San Angelo, La Crosse, and Jamestown Canyon viruses (Bunyaviridae, genus Bunyavirus); and Rift Valley fever (Bunyaviridae, genus Phlebovirus) and Orungo viruses (Reoviridae, genus Orbivirus) (Gratz 2004; Benedict et al. 2007; Paupy et al. 2009). Furthermore, A. albopictus is also the vector of different filariasis, such as Dirofilaria immitis Leidy, Dirofilaria repens Railliet and Henry, and Setaria labiatopapillosa Perroncito (Paupy et al. 2009).
Since there are no vaccines or drugs against the main pathogens transmitted by the Asian tiger mosquito (Paupy et al. 2009), the vector control remains the key tool for the prevention of these diseases. A. albopictus larvae located in natural and/or peridomestic sites can be treated with organophosphates and insect growth regulators, such as diflubenzuron and methoprene (Rai 1991), but repeated use of these chemicals can lead to the development of resistant strains (Robert and Olson 1989) or to undesirable effects on non-target organisms and to human health (Severini et al. 1993). In the same context, the treatments with Bacillus thuringiensis (var. israeliensis) are not suitable against A. albopictus larvae (Kamgang et al. 2011), and alternative biological control strategies based on the release of larvivorous organisms still require further research (Lapied et al. 2009). Against adults, the applications of skin repellents and/or insecticide-treated materials are the two oldest and commonest tools for personal protection (Fradin and Day 2002). Among repellent molecules, N,N-diethyl-3-methylbenzamide (DEET) is acknowledged as one of the most efficient chemicals, giving long lasting protection against many mosquitoes (Klun et al. 2006). However, this compound is irritating for mucous membranes (Koren et al. 2003; Klun et al. 2006), and there have been in the literature case reports of DEET toxicity, especially among children and elderly (Sudakin and Trevathan 2003). In summary, only few studies have showed an effective and sustainable control of A. albopictus through the above reported methods (Paupy et al. 2009). On this basis, there is a worldwide need to find alternatives to synthetic insecticides.
Botanical pesticides are environmentally friendly, easily biodegraded, and often less expensive than the synthetic ones. In the latest years, renewed efforts to investigate their efficacy against an impressive range of insect pests (Elango et al. 2011; Govindarajan et al. 2011; Benelli et al. 2012a, b; Govindarajan and Sivakumar 2012). Among these products, many essential oils are known as toxic against larvae of different mosquito species (Kamsuk et al. 2007; Mathew et al. 2009; Conti et al. 2010; Hafeez et al. 2011). Some oils are also recognized as ovicidal, oviposition deterrents, growth and/or reproduction inhibitors (Rajkumar and Jebanesan 2005; Pushpanathan et al. 2006), and adult repellents (Gleiser et al. 2011; Koliopoulos et al. 2010). In some cases, the repellent activity of these compounds is higher or it has longer duration than synthetic chemicals (Moore et al. 2002; Omolo et al. 2004). Nowadays, the number of essential oils showing larvicidal and repellent properties against mosquitoes continues to grow (Amer and Mehlhorn 2006a, b; Gillij et al. 2008; Pitarokili et al. 2011; Conti et al. 2012b).
On this purpose, Rutaceae are largely studied in many countries for their larvicidal and repellent properties against several Culicidae (Fradin and Day 2002; Rahuman et al. 2000; Kovendan et al. 2012). For instance, the essential oil from seeds and fruits of Zanthoxylum limonella Alston is effective as repellent against three medically important mosquito species, A. aegypti (L.), Culex quinquefasciatus Say, and Anopheles dirus Peyton and Harrison (Trongtokit et al. 2005), while the essential oils from the seeds of Z. armatum DC and Clausena anisata (Willd.) Hook. f. ex Benth exert good toxicity against the larvae of A. aegypti, Anopheles stephensi (Liston) and C. quinquefasciatus (Tiwary et al. 2007; Govindarajan 2010). It was also proved that Ruta graveolens essential oil is a good repellent and larvicide against A. aegypti (Tabanca et al. 2012). Very recently, good larvicidal toxicity and repellence rates of sweet orange (Citrus sinensis L.) and lemon (Citrus limon L.) essential oils were found against A. albopictus (Giatropoulos et al. 2012).
Even if many studies investigated the anti-mosquito properties of Rutaceae, only little insights are achieved on the mosquito repellent activity of Ruta chalepensis L. (Figure 1) (Guarrera 1999; Hadis et al. 2003), and nothing is known about the R. chalepensis toxicity on mosquito larvae. This study investigates the chemical composition of R. chalepensis essential oil, extracted from wild and cultivated plants from Tunisia and its larvicidal and repellent activity against the Asian tiger mosquito, A. albopictus.
Materials and methods
Plant material and essential oil extraction
Wild R. chalepensis plants used in this research were collected in a fruit orchard located in El Ala, Tunisia (35°36′5,782′′N latitude, 9°33′34′′E longitude, 151.80-km altitude). Aerial parts were collected in May 2012 at the flowering stage, air-dried at room temperature and then ground with a blade-carbide grinding (IKA-WERK, Type:A:10). Cultivated R. chalepensis plants were grown in Tunisi (Tunisia) starting from local seeds. Irrigation and mechanical weed control, but no fertilizers, were used for the entire cultivation period. The biomass was collected in June 2012. Thus, aerial parts (100 g) of wild and cultivated R. chalepensis plants were subjected to hydro-distillation for 2 h in a Clevenger-type apparatus. The essential oils, collected over water, were dried over anhydrous sodium sulfate and stored at 5 °C until analysis.
GC and GC–MS analysis of the R. chalepensis essential oils
The gas chromatography (GC) analysis was accomplished with an HP-5890 Series II instrument equipped with a HP-Wax and HP-5 capillary columns (both 30 m × 0.25 mm, 0.25-μm film thickness), working with the following temperature program: 60 °C for 10 min, rising at 5 °C/min to 220 °C. The injector and detector temperatures were maintained at 250 °C; carrier gas, nitrogen (2 mL/min); detector, dual FID; and split ratio, 1:30. The volume injected was 0.5 μL. The relative proportions of the oil constituents were percentages obtained by FID peak area normalization without the use of a response factor. Gas chromatography–mass spectrometry (GC–MS) analyses were performed with a Varian CP3800 gas chromatograph equipped with a HP-5 capillary column (30 m × 0.25; coating thickness, 0.25 μm) and a Varian Saturn 2000 ion trap mass detector. Analytical conditions were as follows: injector and transfer line temperature, 220 and 240 °C, respectively; oven temperature, programmed from 60 to 240 °C at 3 °C/min; carrier gas, helium at 1 mL/min; injection, 0.2 μL (10 % hexane solution); and split ratio, 1:30. Identification of the constituents was based on comparison of the retention time with those of authentic samples, comparing their linear indices relative to a series of n-hydrocarbons and on computer matching against commercial ones (National Institute of Standards and Technology 1999) and also made possible by the use of a homemade library of mass spectra built up from pure substances and components of known oils and mass spectra literature data (Lafferty and Stauffer 1994; Adams 1995; König et al. 2001). Moreover, the molecular weights of all the identified substances were confirmed by GC–CIMS, using methanol as CI ionizing gas.
A. albopictus rearing conditions
Adults of A. albopictus originated from field-collected eggs, deposited by wild females on bars of masonite placed outdoors in dark vases containing water. Egg batches were collected daily and kept moist for 24 h. Then, they were placed in laboratory conditions (25 ± 1 °C, 45 ± 5 % relative humidity (RH), natural summer photoperiod) in 250-cc beakers and submerged in mineral water for hatching. Newly emerged larvae were reared in groups of 150 specimens in 500 cc beakers, with mineral water and a small amount of cat food until they reached the prepupal stage, when they were introduced in the bioassay cage. Emergent adults were maintained (300 specimens per cage, sex ratio 1:1) at 25 ± 1 °C, 65 ± 5 % RH, natural summer photoperiod, and supplied with 10 % sucrose solution on a cotton wick (Conti et al. 2012a, b).
R. chalepensis larvicidal activity
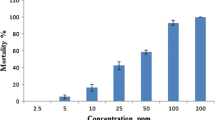
Three groups of 20 fourth-instar larvae were isolated in 250-ml beakers and exposed to dosages of 10, 20, 25, 30, 35, 40, 50, 100, and 150 ppm of wild and cultivated R. chalepensis essential oil in mineral water with 0.1 % of Tween® 80 for 24 h; 250-ml beakers with the same number of larvae (for three replicates) and mineral water with 0.1 % of Tween® 80 were used as control. Mortality was recorded after 24 h, at the end of the test, during which no food was given to the larvae (WHO 1981; Conti et al. 2010, 2012a). Larval mortality was reported as an average of three replicates; mortality percentage rates were corrected using Abbott’s formula (Abbott 1925), and they were used to calculate the LC50 and LC90 values.
R. chalepensis repellent activity
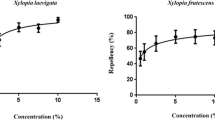
The repellence of wild R. chalepensis essential oil was evaluated using the human-bait technique to simulate the condition of human skin on which repellents will be applied (Gleiser et al. 2011; Conti et al. 2012a, b). Experiments were conducted during the summer of 2012. Groups of 150 nulliparous, nonblood-fed, starved female of A. albopictus (8–12 days old) were placed into Plexiglas cylindrical laboratory cages (diameter 35 cm, length 60 cm). Each cage had a cotton stockinet access sleeve on the front. Since A. albopictus is a day-biting mosquito, the testing period was between 9:00 a.m. and 4:00 p.m. Ten volunteers were chosen among susceptible to mosquito bites and non-allergic subjects. They had no contact with lotions, perfumes, oils, or perfumed soaps on the day of the bioassay. After cleaning their hands in distilled water, they protected their forearms with a thick fabric sleeve and wore a latex surgical glove, in which a dorsal square area 5 × 5 cm was cut open. Mosquito-exposed skin was treated with 100 μL of ethanol as a negative hand control. The other hand was treated with 100 μL of essential oil in ethanol solution (dosages ranging from 0.004 to 0.08 μL/cm2, refer to Table 6). All concentrations were replicated five times on different volunteers. Firstly, the control hand was exposed in the cage for 3 min, during which the number of probing mosquitoes was recorded. Immediately after, the other hand was withdrawn and treated with repellent formulation; then it was exposed to mosquitoes in the same test cage. The number of probing mosquitoes in a 3-min exposure period was recorded. The percentage of repellence obtained from five replicates—expressed as percentage protective efficacy (PE%)—was calculated at each dosage using this formula: PE% = [(number probing untreated hand − number probing treated hand) / number probing untreated hand] × 100 (Fradin and Day 2002).
During each test, the control and the treated hand were regularly interchanged to verify the mosquitoes’ readiness to bite. On rare occasions, when no mosquito attempted to bite the untreated hand, trial was discarded and test repeated with a new mosquito’s cage to ensure that the lack of bites was due to repellence and not to mosquitoes being unwilling to have a blood meal at the time. To calculate the RD50 and RD90 values, the essential oil was tested at dosages of 0.000005, 0.00001, 0.00002, 0.0002, 0.0004, 0.008, 0.016 and 0.024 μL cm−2 (WHO 2009).
Statistical analysis
Larvicidal activity data were transformed into arcsine√proportion values before statistical analysis. Data were processed by JMP® using a general linear model (GLM) with two factors, oil and dosage: y j = μ + O j + D j + O j *D j + e j , in which y j is the observation, μ is the overall mean, O j is the oil (j = 1–2), D j is the dosage (j = 1–9), O j *D j is the interaction between oil and dosage, and e j is the residual error. Averages were separated by Tukey–Kramer HSD test. Only the probability level P < 0.05 was used, for the significance of differences between means, to simplify statistical analysis.
Repellency data was analyzed using a GLM (JMP® SAS, 1999) with two factors with interactions, dosage and time: y j = μ + D j + T j + D j *T j + e j in which y j is the observation, μ is the overall mean, D j is the dosage (j = 1–8), T j is the time (j = 1–6), D j *T j is the interaction between dosage and time, and e j is the residual error in the interaction between oil and dosage.
LC50, LC90, RD50, and RD90 were calculated using Prism 5 (GraphPad© software, San Diego, CA, USA). Bottom and top parameters were fixed to 0 (0 % mortality and no repellence, respectively) and 100 (100 % mortality and full repellence, respectively).
Results and discussion
The GC and GC–MS investigations conducted on the essential oils obtained from the aerial parts of wild and cultivated R. chalepensis plants collected in Tunisia led to the identification of 46 compounds in the wild specimens and 37 molecules in the cultivated ones, representing 97.1 and 92.7 % of the whole oils, respectively (Tables 1 and 2). Both essential oils were characterized by high amounts of non-terpenoid derivatives (90.3 % in the wild R. chalepensis, 83.2 % in the cultivated plants). Ketones were present in percentages higher than 50 % (wild plants 59.4 %, cultivated plants 63.7 %). In both essential oils, the main constituents were 2-nonanone and 2-undecanone. The relative amount of these latter chemicals was quite different among wild and cultivated plants (wild R. chalepensis 37.4 and 20.5 %, cultivated R. chalepensis 20.5 and 39.3 %). Also, 2-methyl octyl acetate was present in appreciable amounts in both essential oils, but its relative amount differed among the analyzed oils (wild R. chalepensis 19.0 %, cultivated R. chalepensis 7.6 %). These differences may be a function of the plant’s origin and cultivation, as already observed for different plant species (Tchoumbougang et al. 2005; Noudjou et al. 2007).
Concerning the larvicidal activity of R. chalepensis essential oil, the obtained results demonstrated that both wild and cultivated R. chalepensis oils exerted toxic activity against A. albopictus larvae. LC50 was 35.66 ppm for the essential oil from wild plants and 33.18 ppm for the oil extracted from cultivated specimens (Table 3). There were significant differences in mortality rates, as a function of the tested essential oil (F = 16.05, df = 1, P = .0003) and the dosage (F = 108.60, df = 8, P < .0001). No differences in larval mortality were detected as a function of the interaction oil × dosage (F = 1.47, df = 8, P = 0.209) (Table 4). At the highest dosages, 150 and 100 ppm, no significant differences on mortality were highlighted, with rates ranging from 100 and 96.67 %, respectively. At dosages ranging from 30 to 40 ppm, mortality rates were lower and did not differ from each other. To our knowledge, these data are the first evidence of the efficacy of the R. chalepensis essential oil against mosquitoes. However, the insecticidal properties of R. chalepensis oil are well recognized. Indeed, it achieves more than 80 % of mortality in toxicity trials against two serious coffee pests, the coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae) and the antestia bug, Antestiopsis intricata (Ghesquiere and Carayon) (Hemiptera: Pentatomidae) (Mendesila et al. 2012). Furthermore, treatments with R. chalepensis essential oil result in 73.5 % of mortality when tested against the maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) (Mendesila et al. 2012). Also, the aqueous extract of R. chalepensis is able to extert good mortality rates against second and third instars of Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) (67 % and 60 %, respectively), while it is not effective against whitefly eggs, pupae and adults (Al-mazra’awi and Ateyyat 2009). This latter extract gives 24 % of mortality against the B. tabaci parasitoid Eretmocerus mundus (Mercet) (Hymenoptera: Aphelinidae) (Al-mazra’awi and Ateyyat 2009). More generally, insecticidal properties against Culicidae larvae are already reported for essential oils of other species of the genus Ruta. For instance, the R. graveolens oil is a good toxic against larvae of the yellow fever mosquito, A. aegypti L. (LD50 = 21.25 ppm) (Tabanca et al. 2012). We believe that the toxicity exerted by the R. chalepensis essential oil may be mainly due to its major constituents: 2-nonanone, 2-undecanone, and 2-methyl octyl acetate (they represent more than the 60 % of the whole oil composition). Indeed, these molecules are widely acknowledged as toxic for several other arthropod pests (Kauffman and Kennedy 1989 and references therein).
Results from the repellence assays highlighted that R. chalepensis essential oil from wild plants was a good repellent against A. albopictus, also at lower dosages. RD50 was 0.000215 μL/cm2 of the skin, while RD90 was 0.007613 μL/cm2 (Table 5). Significant differences in repellence rates were detected as a function of dosage (F = 64.05, df = 4, P < .0001), time (F = 250.16, df = 6, P < .0001), and their interaction (F = 6.83, df = 24, P < .0001). At the highest dosage tested (0.08 μL/cm2 of skin), R. chalepensis essential oil can repel the 50 % of mosquitoes for at least 45 min (Table 6). In agreement with our observations, R. chalepensis fresh plants are traditionally used in Central Italy (e.g., Marche, Abruzzo, and Lazio) to repel mosquitoes and other insects. On this purpose, an infusion of leaves picked in spring was rubbed vigorously onto the skin (Guarrera 1999). In addition, field trials conducted in Western Ethiopia with R. chalepensis oil (concentration, 40 % of essential oil in coconut oil) show a noticeable repellent activity (78 %) against a mixed population of mosquitoes, the majority of which were Mansonia species (Diptera: Culicidae) (Hadis et al. 2003). By contrast, the aqueous extract of R. chalepensis does not seem able to repel insect pests, such as the whitefly B. tabaci (Al-mazra’awi and Ateyyat 2009). The lack of efficacy found in this latter study can be due both to physiological differences in the target pest, as well as to the variations in the chemical composition of aqueous extract over the essential oil (e.g., polar constituents, such as alcohols and esthers). Furthermore, also other Ruta species have been tested for their repellent efficacy against other mosquitoes, such as A. aegypti. Against this latter species, the R. graveolens oil shows a repellent activity down to a minimum effective dosage of 0.187 mg/cm2, using cloth patch assay (Tabanca et al. 2012). It must be also noted that some common ingredients in natural repellents, such as essential oils, may be hazardous. For instance, some R. chalepensis oils, at concentration higher than 0.15 %, may act as phototoxic skin irritants due to the presence of psoralenes (Strickman et al. 2009). However these problems have never been observed in other researches carried out with R. chalepensis essential oils or plant parts (Guarrera 1999; Hadis et al. 2003). On this basis, a dedicated research is still in progress to evaluate the real potential of the tested oil to cause allergic reactions and/or contact dermatitis on healthy volunteers.
Conclusions
This study improves the knowledge about the composition and the main constituents of the essential oil extracted from R. chalepensis. Essential oils from wild and cultivated plants showed only quantitative differences, in particular, related to the amounts of ketone derivatives, while the qualitative profile evidenced a similar chemical composition. Larvicidal assays clearly demonstrated the toxicity of both R. chalepensis essential oils against A. albopictus, even at very low dosages. Furthermore, the essential oil from wild R. chalepensis plants was able to exert noticeable repellency against the Asian tiger mosquito. On this basis, we believe that the insecticidal and repellent properties of R. chalepensis could be used for the development of new and safer products against A. albopictus.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Adams RP (1995) Identification of essential oils components by gas chromatography/mass spectroscopy. Carol Stream, Allured
Al-mazra’awi MS, Ateyyat M (2009) Insecticidal and repellent activities of medicinal plant extracts against the sweet potato whitefly, Bemisia tabaci (Hom.: Aleyrodidae) and its parasitoid Eretmocerus mundus (Hym.: Aphelinidae). J Pest Sci 82:149–154
Amer A, Mehlhorn H (2006a) Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol Res 99:466–472
Amer A, Mehlhorn H (2006b) Repellency effect of forty-one essential oils against Aedes, Anopheles and Culex mosquitoes. Parasitol Res 99:478–490
Benedict MQ, Levine RS, Hawley WA, Lounibos LP (2007) Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vect Bor Zoon Dis 7:76–85
Benelli G, Flamini G, Canale A, Cioni PL, Conti B (2012a) Toxicity evaluation of different essential oil formulations against the Mediterranean fruit fly Ceratitis capitata (Wiedemann) (Diptera Tephritidae). Crop Protect 42:223–229
Benelli G, Flamini G, Canale A, Molfetta I, Cioni PL, Conti B (2012b) Repellence of Hyptis suaveolens L. (Lamiaceae) whole essential oil and major constituents against adults of the granary weevil Sitophilus granarius (L.) (Coleoptera: Dryophthoridae). Bull Insectol 65:177–183
Caminade C, Medlock JM, Ducheyne E, McIntryre KM, Leach S, Baylis M, Morse A (2012) Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J R Soc Interface 9:2708–2717
Conti B, Canale A, Bertoli A, Gozzini F, Pistelli L (2010) Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol Res 107:1455–1462
Conti B, Benelli G, Flamini G, Cioni PL, Profeti R, Ceccarini L, Macchia M, Canale A (2012a) Larvicidal and repellent activity of Hyptis suaveolens (Lamiaceae) essential oil against the mosquito Aedes albopictus Skuse (Diptera: Culicidae). Parasitol Res 110:2013–2021
Conti B, Benelli G, Leonardi M, Afifi UF, Cervelli C, Profeti R, Pistelli L, Canale A (2012b) Repellent effect of Salvia dorisiana, S. longifolia and S. sclarea (Lamiaceae) essential oils against the mosquito Aedes albopictus Skuse (Diptera: Culicidae). Parasitol Res 111:291–299
Elango G, Rahuman AA, Kamaraj C, Bagavan A, Zahir AA (2011) Efficacy of medicinal plant extracts against malarial vector, Anopheles subpictus Grassi. Parasitol Res 108:1437–1445
Estrada-Franco J (1995) Biology, disease relationship, and control of Aedes albopictus. PAHO technical paper p. 42
Fradin MS, Day JF (2002) Comparative efficacy of insect repellents against mosquito bites. New Engl J Med 347:13–18
Giatropoulos A, Papachristos DP, Kimbaris A, Koliopoulos G, Polissiou MG, Emmanouel N, Michaelakis A (2012) Evaluation of bioefficacy of three Citrus essential oils against the dengue vector Aedes albopictus (Diptera: Culicidae) in correlation to their components enantiomeric distribution. Parasitol Res. doi:10.1007/s00436-012-3074-8
Gillij YG, Gleiser RM, Zygadlo JA (2008) Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Bioresour Technol 99:2507–2515
Gleiser RM, Bonino MA, Zygadlo JA (2011) Repellence of essential oils of aromatic plants growing in Argentina against Aedes aegypti. Parasitol Res 108:69–78
Govindarajan M (2010) Chemical composition and larvicidal activity of leaf essential oil from Clausena anisata (Willd.) Hook. f. ex Benth (Rutaceae) against three mosquito species. Asian Pacif J Tropic Med 2010:874–877
Govindarajan M, Sivakumar R (2012) Adulticidal and repellent properties of indigenous plant extracts against Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). Parasitol Res 110:1607–1620
Govindarajan M, Mathivanan T, Elumalai K, Krishnappa K, Anandan A (2011) Mosquito larvicidal, ovicidal, and repellent properties of botanical extracts against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 109:353–367
Gratz NG (2004) Critical review of the vector status of Aedes albopictus. Med Vet Entomol 18:215–227
Guarrera PM (1999) Traditional antihelmintic, antiparasitic and repellent uses of plants in Central Italy. J Ethnopharmacol 68:183–192
Hadis LMM, Meknonnen V, Asfar T (2003) Field trials on repellent activity of four plant products against mainly Mansonia population in Western Ethiopia. Phytother Res 17:202–205
Hafeez F, Akram W, Shaalan EA (2011) Mosquito larvicidal activity of citrus limonoids against Aedes albopictus. Parasitol Res 109:221–229
James AA (1992) Mosquito molecular genetics: the hands that feed bite back. Science 257:37–38
Juliano SA, Lounibos LP (2005) Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett 8:558–574
Kamgang B, Marcombe S, Chandre F, Nchoutpouen E, Nwane P, Etang J, Corbelle V, Paupy C (2011) Insecticide susceptibility of Aedes aegypti and Aedes albopictus in Central Africa. Par Vect 4:79
Kamsuk K, Choochote W, Chaithong U, Jitpakdi A, Tippawangkosol P, Riyong D, Pitasawat B (2007) Effectiveness of Zanthoxylum piperitum-derived essential oil as an alternative repellent under laboratory and field application. Parasitol Res 100:339–345
Kauffman WC, Kennedy GG (1989) Toxicity of allelochemicals from wild insect-resistant tomato Lycopersicon hirsutum f. glabratum to Campoletis sonorensis, a parasitoid of Heliothis zea. J Chem Ecol 15:2051–2060
Klun JA, Khrimian A, Debboun M (2006) Repellent and deterrent effects of SS220, Picaridin, and Deet suppress human blood feeding by Aedes aegypti, Anopheles stephensi, and Phlebotomus papatasi. J Med Entomol 43:34–39
Koliopoulos G, Pitarokili D, Kioulos E, Michaelakis A, Tzakou O (2010) Chemical composition and larvicidal evaluation of Mentha, Salvia, and Melissa essential oils against the West Nile virus mosquito Culex pipiens. Parasitol Res 107:327–335
Konig WA, Hochmuth DH, Joulain D (2001) Terpenoids and related constituents of essential oils. Library of Mass Finder 2.1, Institute of Organic Chemistry, Hamburg, Germany
Koren G, Matsui D, Bailey B (2003) DEET-based insect repellents: safety implications for children and pregnant and lactating women. Canad Med Assoc J 169:209–212
Kovendan K, Arivoli S, Maheshwaran R, Baskar K, Vincent S (2012) Larvicidal efficacy of Sphaeranthus indicus, Cleistanthus collinus and Murraya koenigii leaf extracts against filarial vector, Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 111:1025–1035
Lafferty FW, Stauffer DB (1994) Wiley Registry of Mass Spectral Data, 6th edn. Mass Spectometry Library Search System Bench-Top/PBM, Version 3.10d. Palisade, Newfield
Lapied B, Pennetier C, Apaire-Marchais V, Licznar P, Corbel V (2009) Innovative applications for insect viruses: towards insecticide sensitization. Trends Biotechnol 4:190–198
Mathew N, Anitha MG, Bala TSL, Sivakumar SM, Narmadha R, Kalyanasundaram M (2009) Larvicidal activity of Saraca indica, Nyctanthes arbor-tristis and Clitoria ternatea extracts against three mosquito vector species. Parasitol Res 104:1017–1025
Mendesila E, Tadesseb M, Negashc M (2012) Efficacy of plant essential oils against two major insect pests of coffee (Coffee berry borer, Hypothenemus hampei, and antestia bug, Antestiopsis intricata) and maize weevil, Sitophilus zeamais. Archiv Phytopathol Plant Protect 45:366–372
Moore SJ, Lenglet A, Hill N (2002) Field evaluation of three plant-based insect repellents against malaria vectors in Vaca Diez Province, the Bolivian Amazon. J Am Mosq Control Assoc 18:107–110
National Institute of Standards and Technology (1999) NIST/EPA/NIH Mass Spectral Library, Pc Version 1.7. Perkin Elmer, Norwalk
Noudjou F, Kouninki H, Ngamo LST, Maponmestsem PM, Ngassoum M, Hance T, Haubruge E, Malaisse F, Marlier M, Lognay GC (2007) Effect of site location and collecting period on the chemical composition of Hyptis spicigera Lam. An insecticidal essential oil from North-Cameroon. J Essent Oil Res 19:597–601
Omolo MO, Okinyo D, Ndiege IO, Lwande W, Hassanali A (2004) Repellency of essential oils of some Kenyan plants against Anopheles gambiae. Phytochem 65:2797–2802
Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D (2009) Aedes albopictus, an arbovirus vector: from the darkness to the light. Microb Infect 11:1177–1185
Peng Z, Yang J, Wang H, Simons FER (1999) Production and characterisation of monoclonal antibodies to two new mosquito Aedes aegypti salivary protein. Insect Biochem Mol Biol 29:909–914
Pitarokili D, Michaelakis A, Koliopoulos G, Giatropoulos A, Tzakou O (2011) Chemical composition, larvicidal evaluation, and adult repellency of endemic Greek Thymus essential oils against the mosquito vector of West Nile virus. Parasitol Res 109:425–430
Pushpanathan T, Jebanesan A, Govindarajan M (2006) Larvicidal, ovicidal and repellent activities of Cymbopogan citrates Stapf (Graminae) essential oil against the filarial mosquito Culex quinquefasciatus (Say) (Diptera: Culicidae). Tropical Biomed 23:208–212
Rahuman AA, Gopalakrishnan G, Ghouse BS, Arumugama S, Himalayan B (2000) Effect of Feronia limonia on mosquito larvae. Fitoterapia 71:553–555
Rai KS (1991) Aedes albopictus in the Americas. Annu Rev Entomol 36:459–484
Rajkumar S, Jebanesan A (2005) Repellency of volatile oils from Moschosma polystachyum and Solanum xanthocarpum against filarial vector Culex quinquefasciatus Say. Tropical Biomed 22:139–142
Reiter P, Sprenger D (1987) The used tire trade: a mechanism for the worldwide dispersal of container breeding mosquitoes. J Am Mosq Control Assoc 3:494–501
Robert LL, Olson JK (1989) Susceptibility of female Aedes albopictus from Texas to commonly used adulticides. J Am Mosq Control Assoc 5:251–253
Severini C, Romi R, Marinucci M, Rajmond M (1993) Mechanism of insecticide resistance in field populations of Culex pipiens from Italy. J Am Mosq Control Assoc 9:164–168
Skuse F (1894) The banded mosquito of Bengal. Indian Mus Notes 3:20
Strickman D, Frances SP, Debboun M (2009) Put on something natural. In: Prevention of bugs, bites, stings and disease. Oxford University Press, New York
Sudakin DL, Trevathan WR (2003) DEET: a review and update of safety and risk in the general population. J Toxicol Clin Toxicol 41:831
Tabanca N, Demirci B, Kiyan HT, Ali A, Bernier UR, Wedge DE, Khan IA, Başer KHC (2012) Repellent and larvicidal activity of Ruta graveolens essential oil and its major individual constituents against Aedes aegypti. Planta Med 2012:78–90
Tchoumbougang F, Amvam Zollo PH, Fecam Boyom F, Nyegue MA, Bessière JM (2005) Aromatic plants of Tropical Central Africa. XLVIII. Comparative study of the essential oils of four Hyptis species from Cameroon: H. lanceolata Poit., H. pectinata (L.) Poit., H. spicigera Lam. and H. suaveolens Poit. Flavour Fragr J 20:340–343
Tiwary M, Naik SN, Tewary DK, Mittal PK, Yadav S (2007) Chemical composition and larvicidal activities of the essential oil of Zanthoxylum armatum DC (Rutaceae) against three mosquito vectors. J Vect Borne Dis 44:198–204
Trongtokit Y, Rongsriyam Y, Komalamisra N, Apiwathnasorn C (2005) Comparative repellency of 38 essential oils against mosquito bites. Phytother Res 19:303–309
WHO (1981) Instruction for determining the susceptibility or resistance of mosquito larvae to insecticide. WHO/VBC/81.807. Control of tropical diseases. World Health Organization, Geneva
WHO (2009) Guidelines for efficacy testing of mosquito repellents for human skin. WHO/HTM/NTD/WHOPES/2009.4. Control of neglected tropical diseases. World Health Organization, Geneva
Yamani AS, Mehlhorn H, Adham FK (2012) Yolk protein uptake in the oocyte of the Asian tiger mosquito Aedes albopictus (Skuse) (Diptera: Culicidae). Parasitol Res 111:1315–1324
Acknowledgments
We would like to thank Angelo Canale (University of Pisa) for his insightful critical comments on an earlier version of the manuscript, Helen Romito (Sant’Anna School of Advanced Studies, Pisa) for the language proofreading, Alessandra Francini (Institute of Life Sciences, Sant’Anna School of Advanced Studies, Pisa) for the statistical advices, Pancrazio Campagna for providing the pictures of R. chalepensis, Giulia Fiore, Isaia Nisoli, Francesca Baroncelli, and Lorenzo Rossi for the superb assistance, and the ten patient volunteers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Conti, B., Leonardi, M., Pistelli, L. et al. Larvicidal and repellent activity of essential oils from wild and cultivated Ruta chalepensis L. (Rutaceae) against Aedes albopictus Skuse (Diptera: Culicidae), an arbovirus vector. Parasitol Res 112, 991–999 (2013). https://doi.org/10.1007/s00436-012-3221-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-3221-2