Abstract
This paper presents novel and distinctive Copper(I) iodide polymers with intriguing structure motifs, constructed from 1, 4-bis(imidazole-1-ylmethyl)benzene (bix). Treatment of bix with CuI generated [(Cu4I4)(bix)2] n 1, whose X-ray single crystal structural determination revealed a novel 2-D battlement-like sheet structure with square grids based on distorted cubane-like Cu4I4 clusters. The structural features and photoluminescence of the complexes are discussed. The present finding may provide insight into the coordination versatility of copper(I) halide clusters with N-donor ligands and an inspiration for the self-assembly of novel supramolecular networks based on copper(I) halide clusters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although inorganic supramolecular networks and coordination polymers have received ever-increasing interest not only because of their potentials as functional materials but also of their intriguing variety of architectures and topologies [1], generating polymetallic coordination networks with interesting supramolecular solid-state architectures remains a great challenge. Recently, numerous studies have been concerned on the polynuclear Cu(I)-halide discrete complexes containing organic ligands, such as pyridine, substituted pyridine, piperidine, benzimidazole, 4,4′-diamino-diphenylmethane, 4,4′-diaminodiphenyl ether [2]. However, few difunctional ligands have been adopted to construct polymeric architectures based on Cu(I)-halide clusters particularly Cu4I4 cluster dimer [3].
Numerous polymeric compounds containing grid-like sheets with (4,4) or (6,3) topology have been reported over recent years [1b, 4]. It is a tradition that the design of the grid-like sheets rests on the directing coordination instructions, which are based on the coordination geometry of the metal ion and the structure of the ligand’s coordination sites. The polymeric compounds with clusters acting as nodes of the grids and the bidentate pyridyl-based ligands as the edges are rare. In particular, those involving Cu4I4 clusters have not been seen before. Our research interest is to construct new complexes with larger grid frameworks supported by Cu(I)-halide clusters and N-donor ligands and then stake the grid sheets together through the interlayer π–π interactions to form 3-D networks. In the previous literatures, bix ligands have shown to be able to coordinate to metal centers in various configurations providing remarkable polyrotaxane-like polymers as well as other interesting networks [5]. Therefore, a highly flexible N-donor ligand bix was employed to react with Cu(I)-iodide under hydrothermal condition. In this reaction, we obtained a novel compound [(Cu4I4)(bix)2] n 1, which has a 2-D battlement-like sheet structure with square grids based on distorted cubane-like Cu4I4 clusters. In this paper we report the syntheses, structures and luminescent properties of compounds 1.
Experimental
Preparation of Polymer [(Cu4I4)(bix)2] n (1). (1)
Compound 1 was hydrothermally synthesized under autogenous pressure. A mixture of CuI (38 mg, 0.2 mmol), bix (55 mg, 0.2 mmol), concentrated KI solution (6 mL) and CH3OH (4 mL) was sealed in a 20 mL Telflon-lined autoclave, which was heated to 160 °C for 3 days. After slow cooling to room temperature, gray yellow crystals of 1 were obtained by filtration. The crystals were washed with distilled water several times and finally dried in air. Yield, 80% (based on Cu). Anal. Calc. For 1: C, 27.16; H, 2.28; N, 9.05%; Found: C, 27.24; H, 2.20; N, 9.75%. IR (KBr pellet, cm−1): 3117(w), 2924(w), 1615(w), 1516(s), 1432(m), 1352(w), 1278(m), 1231(s), 1105(s), 1080(m), 1029(m), 938(w), 825(m), 746(s), 656(s), 627(w).
Characterization
All chemical reagents were commercially available. Ligand bix was prepared as described in the literature [5a]. Elemental analyses of C, H and N were carried out by Elemental Vario EL III microanalyzer. The IR spectra were recorded on a PerkinElmer Spectrum One FT-IR spectrometer using KBr pellet technique in the range of 3200–4000 cm−1. Thermogravimetric analyses were carried out on a NETZSCH STA 449C unit at a heating rate of 15 °C/min under a nitrogen atmosphere. Fluorescent analyses were performed on an Edinburgh Instruments analyzer model FLS920.
Determination of Crystal Structure
Suitable crystals of 1 (0.20 × 0.15 × 0.10 mm) was used for X-ray analyse. Data collections were performed on a Rigaku mercury CCD diffractometer with graphite-monochromated MoKα radiation (λ = 0.71073 Å). Empirical absorption corrections were applied by using the SADABS program [6] for the Siemens area detector. The structures were solved by direct methods, refined by full-matrix least-squares methods. The Cu and I atoms were located from the E-map and all non-hydrogen atoms were derived from the successive difference Fourier syntheses. All the hydrogen atoms were calculated on the ideal positions and refined isotropically and all non-hydrogen atoms were refined anisotropically. The programs for structure solution and refinement are SHELXS-97 [7] and SHELXL-97 [8], respectively. The crystallographic data, selected bond lengths and angles for 1 is listed in the Tables 1 and 2.
Results and Discussion
Synthesis
Complexes 1 was synthesized in high yield as gray yellow crystals from the hydrothermal reactions of the bix ligands and CuI with a metal-to-ligand ratio of 1:1. Concentrated KI solution was used to increase the solubility of CuI. The crystals are stable in air for a long period and insoluble in water and common organic solvents.
Structure Description
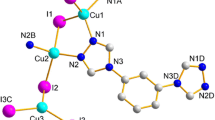
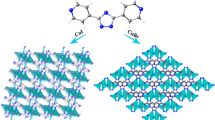
Single crystal X-ray diffraction analysis reveals that 1 is a non-interpenetrated 2D grids complex in which bix acts as a μ2-bridging ligand to link the distorted cubane-like Cu4I4 clusters. As depicted in Fig. 1, the repeating tetrameric skeletal core brings together four Cu+ cations and four I− anions in a distorted cubane arrangement. Coordination of the copper atoms in 1 is a distorted NI3 tetrahedral geometry with each copper atom bonding to three iodide atoms and completed by a nitrogen atom of the bix ligand. All the bond lengths and bond angles of the Cu4I4 units in 1 are normal [2b]. The Cu–Cu bond lengths, ranging from 2.6488(2) to 2.7188(1) Ǻ (average value 2.678 Ǻ), are within the range suggested by Mehrotra and Hoffman [9]. Each cubane-like Cu4I4 unit is linked by four bix ligands in trans conformation to its four neighboring clusters, thus affording a 2D sheet with (4,4) grid units. The grid-like [(Cu4I4)4(bix)4] units can be viewed as the basic building blocks of the structure, in which the apices are occupied by the Cu4I4 clusters and the sides by the bix ligands. The grid motif has the dimensions of 14.726 × 14.075 Ǻ (based on the distances of Cu4I4 centers), the diagonals are 19.953 and 20.780 Å, and the interior angles are 87.7 and 92.3°, respectively (Fig. 2). Although the square grid network is one of the most frequently encountered types of coordination networks with ditopic spacers, there are a limited number of multidimensional examples containing linked cubane tetramers [3a, b]. Contrary to the fruitful productions containing the bix ligand [5a, b, c, 10] there are no interlocks exhibit in compound 1. Probably the steric hindrance of the Cu4I4 clusters prevent the interpenetrating in the compound.
It should be noted that the actual structure of the 2-D layer in 1 is battlement-like (Fig. 3a), which is different from the previous complexes with coplanar, quasi-coplanar and wavelike basic grids [11]. The convex surface of one layer is immersed into the concave surface of an adjacent layer to form a striking 3-D brick-wall configuration from b or c-direction via strong π–π interactions between the parallel aromatic imidazole rings of the bix ligands that belong to adjacent sheets (the offset face to face separation of ca. 3.3 Å, the centroid–centroid distance of ca.3.5 Å) [12]. As a result, the grid layers are staked in an offset fashion along a-direction with the cavity of each layer being blocked by the neighboring layers (Fig. 3b).
Thermogravimetric and Luminescent Analysis
The thermogravimetric analyses showed drastic decompositions in the temperature range 339–392 °C for 1. It exhibited no obvious weight loss up to ca. 340 °C, indicating good thermal stability for 1 (Fig. 4). Such characteristic may due to its topological structure.
Among earlier studies, photophysical properties of discrete Cu(I)-halide complexes have been reported [2a, 13], but few luminescence based on multidimensional Cu(I)-halide clusters were studied. Figure 5 depicts solid-state luminescent spectra of 1 at room temperature. Emission spectrum of 1 shows strong photoluminescence, with emission maximum at 604 nm [λex = 396 nm, τ = 8.129 μs (τ is lifetime)]. Compared to other Cu(I) halide compounds [14], from the structural profile, energy, and lifetime of the emissions of 1, the emission can be assigned to phosphorescence and may be originate from a cluster-centered (CC) excited state and CuI d-s[14]. The free ligands bix (λex, max = 350 nm, λem, max = 392 nm) are strongly photoluminescent, but they do not display any significant photoluminescent behavior in 1. This phenomenon may be attributed to the effect of the metal centers on the ligands [15].
Conclusion
In our research, we have first synthesized by hydrothermal reactions the novel supramolecular complexes of Cu(I) clusters and imidazole-containing ligands with (4,4) topology. The results are significant not only for producing the novel architecture of CuI clusters but also for further exploration of Cu(I) halide clusters through introducing new synthetic route.
Supporting Information
Supplementary data associated with this article are included. Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number: CCDC 294081 (1). Copies of the data can be obtained free of charge on application to CCDC, 12, Union Road, Cambridge CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk).
References
(a) D. B. Amabilino, and J. F. Stoddart (1995). Chem. Rev. 95, 2725. (b) B. Moulton and M. J. Zaworotko (2001). Chem. Rev. 101, 1629. (c) J. H. Yu, J. Q. Xu, L. Ye, H. Ding, J. N. Xu, H. B. Jia, T. G. Wang, Z. C. Mu, and G. D. Yang (2003). J. Cluster. Sci 14, 1. (d) J. Zhou, G. Q. Bian, J. Dai, Y. Zhang, Q. Y. Zhu, and W. Lu (2006). Inorg. Chem. 45, 8486.
(a) K. R. Kyle, C. K. Ryu, P. C. Ford, and J. A. DiBenedetto (1991). J. Am. Chem. Soc. 113, 2954. (b) V. Schramm (1978). Inorg. Chem. 17, 714. (c) A. Toth, C. Floriani, A. Chiesi-Villa, and C. Guastini (1987). Inorg. Chem. 26, 3897. (d) R. H. Wang, M. C. Hong, J. H. Luo, R. Cao, and J. B. Weng (2002). Eur. J. Inorg. Chem. 3097.
(a) X. Xue, X. S. Wang, R. G. Xiong, X. Z. You, B. F. Abrahams, C. M. Che, and H. X. Ju (2002). Angew. Chem. Int. Ed. Engl. 41, 2944. (b) A. J. Blake, N. R. Brooks, N. R. Champness, M. Crew, D. H. Gregory, P. Hubberstey, M. Schröder, A. Deveson, D. Fenske and L. R. Hanton (2001). Chem. Commun. 1432. (c) C. Nätherand and I. Jeß (2002). J. Solid. State. Chem. 169, 103. (d) S. Muthu, Z. Ni, and J. J. Vittal (2005). Inorg. Chim. Acta. 358, 595.
S. A. Barnett, and N. R. Champness (2003). Coord. Chem. Rev. 246, 145.
(a) B. F. Hoskins, R. Robson, and D. A. Slizys (1997). J. Am. Chem. Soc. 119, 2952. (b) B. F. Hoskins, R. Robson, and D. A. Slizys (1997). Angew. Chem. Int. Ed. Engl. 36, 2336. (c) L. Carlucci, G. Ciani, and D. M. Proserpio (2004). Chem. Comm. 380. (d) E. Q. Gao, Y. X. Xu, and C. H. Yan (2004). CrystEngComm. 6, 298. (e) L. L. Wen, D. B. Dang, C. Y. Duan, Y. Z. Li, Z. F. Tian, and Q. J. Meng (2005). Inorg. Chem. 44, 7161.
G. M. Sheldrick, A Program for the Siemens Area Detector ABSorption Correction (University of Göttingen, 1997).
G. M. Sheldrick, SHELXS97 Program for Solution of Crystal Structures (University of Göttingen, Germany, 1997).
G. M. Sheldrick, SHELXL97 Program for Refinement of Crystal Structures (University of Göttingen, Germany, 1997).
P. K. Mehrotra, and R. Hoffmann (1978). Inorg. Chem. 17, 2187.
L. Carlucci, G. Ciani, D. M. Proserpio, and L. Spadacini (2004). CrystEngComm. 6, 96.
(a) K. Biradha, and M. Fujita (2000) J. Chem. Dalton. Trans. 3805. (b) K. Biradha and M. Fujita (2001). Chem. Commun. 15. (c) K. Biradha, Y. Hongo, and M. Fujita (2000). Angew. Chem. Int. Ed. Engl. 39, 3843. (d) M. J. Plater, M. R. St. J. Foreman, T. Gelbrich, S. J. Coles, and M. B. Hurst-house (2000). J. Chem. Soc. Dalton Trans. 3065.
C. Janiak (2000). J. Chem. Soc. Dalton Trans. 3885.
P. C. Ford, and A. Vogler (1993). Acc. Chem. Res. 26, 220.
(a) V. W. W. Yam, and K. K. W. Lo (1999). Chem. Soc. Rev. 28, 323. (b) P. C. Ford, E. Cariati, and J. Bourassa (1999). Chem. Rev. 99, 3625.
O. Horváth (1994). Coord. Chem. Rev. 135–136, 303.
Acknowledgements
The work was supported by the grants from the State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences (CAS), the Ministry of Science and Technology of China (001CB108906) and the National Science Foundation of China (20333070).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, T., Du, SW. Syntheses, Crystal Structure and Luminescent Properties of Co-ordination Networks Based on Copper(I) Iodide Clusters from 1, 4-Bis(imidazole-1-ylmethyl)benzene. J Clust Sci 19, 323–330 (2008). https://doi.org/10.1007/s10876-007-0172-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-007-0172-3