Abstract
Four new metal–organic supramolecular networks, namely, [Zn(H2pdc)2(H2O)2]·2H2O·bbi (1), {[Cd(Hpdc)2]·2H2O2·H2bbi}n (2), [Zn(BA)2(bbi)]n (3), and {[Cd(BA)2(bbi)]·H2O}n (4) (H3pdc = 3,5-pyrazoledicarboxylic acid, HBA = 3-hydroxybenzoic acid and bbi = 1,1′-(1,4-butanediyl)bis(imidazole)) have been synthesized under hydrothermal conditions and characterized by IR spectra, elemental analyses, and single-crystal X-ray diffraction analyses. Compound 1 possesses zero-dimensional (0D) structure, which is finally extended into a two-dimensional (2D) supramolecular network via O–H···O and C–H···O hydrogen bonds. Complex 2 displays a 2D network structure built from Cd2+ atoms interconnected by Hpdc2− ligands. The adjacent networks are further assembled into three-dimensional (3D) supramolecular structure through O–H···O hydrogen bonds. Compounds 3 and 4 show similar one-dimensional (1D) chains, in which four-coordinated Zn(II) atoms and six-coordinated Cd(II) atoms are bridged by bbi ligands. Through O–H···O and C-H···O hydrogen bonding interactions, the 1D chains are further packed into 2D and 3D supramolecular frameworks for 3 and 4, respectively. Obviously, the structural differences among compelxes 1–4 are attributed to the different central metal atoms and organic ligands. In addition, compounds 1–4 exhibit blue fluorescent emission in the solid state at room temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The recent surge of research interest in the area of supramolecular chemistry has been prompted by the ability of metal–ligand coordination and/or hydrogen bonding and π–π stacking interactions, providing a facile approach aimed at controlling the self-assembly of one-, two-, or three-dimensional supramolecular architectures [1, 2]. The crystal engineering principles have been used as a powerful tool in the rational design and synthesis of metal–organic supramolecular network with intriguing topologies as well as potential application in the fields of catalysis, separation and optics [3–5]. On the basis of supramolecular networks (especially metal–organic frameworks, MOFs), it is noteworthy that a lot of investigates have brought about nanostructured materials with controlled particle size and morphology, some of which showed enhanced adsorption and catalytic properties [6]. For example, Shekhah et al. have reported the step-by-step seeding method to prepare [Cu3(btc)2] (btc = 1,3,5-benzenetricarboxylic acid) films on a gold substrate [7]. This strategy offers the possibility to control the composition of [Cu3(btc)2] in each layer, resulting in porous architectures for gas adsorption. Due to their high BET surface area and large pore volume, MOFs have also been employed as templates or precursors for the preparation of nanoarchitectured materials with a wide range of morphologies [8]. Xu et al. have demonstrated the synthesis of nanoporous carbons (NPCs) by infiltrating furfuryl alcohol into the micropores of MOF-5 (Zn4O(OOCC6H4COO)3). After treatment at 1000 °C, a NPC with BET surface of 2872 m2 g−1 was obtained [9]. Morsali et al. and other researches have described that thermal decomposition of Zn-based MOFs, such as {[Zn(bpcdp)2(DMF)4](ClO4)2·(H2O)2}n (bpcdp = 2,6-bis(4-pyridinecarboxamide)pyridine), [Zn2(btec)(DMF)2]n (btec = 1,2,4,5-benzenetetracarboxylate), and [Zn2(BDC)2(H2O)2·(DMF)2]n (BDC2− = benzene-1,4-dicarboxylate) under various conditions, afforded ZnO nano-materials with different sizes and morphologies [10–12].
In the exploration of supramolecular networks, rigid organic ligands like 1,10-phenanthroline, pyrazine, flexible organic ligands such as 1,1′-(1,4-butanediyl)bis(imidazole) (bbi), 1,3-bis(4-pyridyl)propane, and anionic ligands have been frequently involved [13–16]. Among various anionic ligands, different carboxylic acids, including aromatic and aliphatic acids, such as benzene-, pyridine-, pyraole-, and imidazole-carboxylate [17–19], have been investigated in the construction of supramolecular network for their versatile coordination modes by the N and/or O atoms. Moreover, the O and N atoms can also act as proton donors and acceptors, affording intermolecular hydrogen bonds for connecting the complexes into high-dimensional supramolecular architectures. In early efforts, we used 2,3-pyridinecarboxylic acid or 2,6-pyridinecarboxylic acid and bbi to react with metal salts under hydrothermal conditions, forming a series of novel metal–organic supramolecular systems [20–22]. As part of our ongoing efforts in the exploration of novel supramolecular crystalline materials with beautiful architecture and good physical properties, herein we report four new metal–organic supramolecular frameworks, [Zn(H2pdc)2(H2O)2]·2H2O·bbi (1), {[Cd(Hpdc)2]·2H2O2·H2bbi}n (2), [Zn(BA)2(bbi)]n (3), and {[Cd(BA)2(bbi)]·H2O}n (4) (H3pdc = 3,5-pyrazoledicarboxylic acid and HBA = 3-hydroxybenzoic acid) constructed from H3pdc/HBA and bbi ligands. Moreover, the fluorescence properties of 1–4 at room temperature have been investigated.
2 Experimental
2.1 Materials and Physical Measurements
Reagents and solvents employed were purchased from ALADDIN and used as received without further purification. The ligand bbi was prepared according to the method reported previously [23]. Elemental analyses for C, H, and N were carried out with a Vario EL III elemental analyzer. Infrared spectra using the KBr discs were recorded on a Nicolet 170SX FT-IR spectrophotometer in the 4000–400 cm−1 region. Thermogravimetric analyses were performed on a NETZSCH STA 449C microanalyzer heated from 25 to 700 °C under nitrogen atmosphere. The emission spectra were obtained with a RF-5301PC fluorescence spectrometer, and emission lifetimes in solid states were measured on a FLS920 fluorescence spectrometer.
2.2 Synthesis of [Zn(H2pdc)2(H2O)2]·2H2O·bbi (1)
A mixture of Zn(CH3COO)2·2H2O (0.110 g, 0.5 mmol), H3pdc (0.078 g, 0.5 mmol),bbi (0.095 g, 0.5 mmol), NaOH (0.020 g, 0.5 mmol) and H2O (10 ml) was stirred for 1 h at room temperature and then sealed in a 25 mL Teflon-lined stainless steel container. The container was heated at 393 K for 3 days. After slow cooling to room temperature, colorless block crystals of 1 were obtained with yield of 53 %. Anal. Calc. for C20H28N8O12Zn (%): C, 37.62, H, 4.39, N, 17.56. Found (%): C, 37.66, H, 4.42, N, 17.60. IR (KBr, cm−1): 3415 s, 3136 m, 1689 versus, 1616 versus, 1450 versus, 1360 m, 1200 w, 1029 m, 780 m, 629 m.
2.3 Synthesis of {[Cd(Hpdc)2]·2H2O·H2bbi}n (2)
Complex 2 was synthesized by similar procedure used for 1 except Cd(CH3COO)2·2H2O (0.133 g, 0.5 mmol) was used instead of Zn(CH3COO)2·2H2O. Yield: 55 %. Anal. Calcd for C20H24N8O10Cd (%): C, 36.99, H, 3.70, N, 17.26. Found (%): C, 37.03, H, 3.66, N, 17.22. IR (KBr, cm−1): 3451 m, 3115 w, 1610 versus, 1532 w, 1395 versus, 1225 s, 1105 m, 1083 m, 1012 w, 861 w, 725 w, 655 w.
2.4 Synthesis of [Zn(BA)2(bbi)]n (3)
Complex 3 was synthesized by similar procedure used for 1 except that HBA (0.069 g, 0.5 mmol) was used instead of H3pdc. Yield: 38 %. Anal. Calcd for C24H24N4O6Zn (%): C, 54.34, H, 4.52, N, 10.57. Found (%): C, 54.37, H, 4.50, N, 10.61. IR (KBr, cm−1): 3449 m, 3118 w, 1615 versus, 1536 w, 1398 versus, 1229 s, 1110 m, 1089 m, 1020 w, 859 w, 727 w, 658 w.
2.5 Synthesis of {[Cd(BA)2(bbi)]·H2O}n (4)
Complex 4 was synthesized by similar procedure used for 1 by using HBA (0.069 g, 0.5 mmol) and Cd(CH3COO)2·2H2O (0.133 g, 0.5 mmol) instead of H3pdc and Zn(CH3COO)2·2H2O. Yield: 46 %. Anal. Calcd for C24H26N4O7Cd (%): C, 48.40, H, 4.37, N, 9.41. Found: C, 48.43, H, 4.33, N, 9.45. IR (KBr, cm−1): 3455 m, 3125 w, 1622 versus, 1545 w, 1343 versus, 1235 s, 1119 m, 1085 m, 1021 w, 861 w, 735 w, 660 w.
2.6 Crystal Data Collection and Refinement
Single-crystal X-ray diffraction data for complexes 1–4 were collected at room temperature on a Bruker SMART Apex II CCD diffractometer equipped with a graphite monochromatized Mo Ka radiation (λ = 0.71073 Å) by using an ω scan mode. The structures were solved by direct methods and refined by full-matrix least-squares based on F 2 using SHELXS-97 and SHELXTL-97 programs [24, 25]. All non-hydrogen atoms were refined with anisotropic thermal parameters, whereas the hydrogen atoms of the complexes were placed in geometrically calculated positions. Experimental details for X-ray data collection of 1–4 are summarized in Table 1. Selected bond distances and angles are listed in Table 2.
3 Results and Discussion
3.1 Structure of [Zn(H2pdc)2(H2O)2]·2H2O·bbi (1)
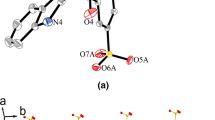
Complex 1 crystallizes in triclinic with space group P-1. As shown in Fig. 1, the Zn(II) atom displays a slightly distorted octahedral geometry coordinated by two oxygen atoms from two water molecules [Zn–O5(O5A) = 2.189(3) Å], two nitrogen atoms (N1, N1A) and two oxygen atoms (O1, O1A) from the H2pdc− ligands [Zn–N1(N1A) = 2.096(3) Å, and Zn–O1(O1A) = 2.103(3) Å]. The O1, O1A, O5 and O5A atoms comprise the base plane, whereas N1 and N1A atoms occupy the axial positions of the octahedron [O1A–Zn1–O5A = 90.48(11)°, O5A–Zn–O1 = 89.52(11)°, O1–Zn1–O5 = 90.48(11)°, O5–Zn1–O1A = 89.52(11)°, and total = 360.00(11)°]. One nitrogen atom and one oxygen atom of H2pdc− coordinate with the Zn(II) atom in the bidentate chelating mode, resulting in mononuclear 0D structure. The mononuclear motifs are bridged through strong hydrogen bonds (O5–H5D···O3) between the coordinated water molecules and the carboxyl groups, forming a 1D chain. Furthermore, the 1D chains are extended into a 2D network via C–H···O (C8–H8···O2 and C6–H6···O3) hydrogen bonding interactions between the bbi molecules and the carboxyl groups (Fig. 2; Table 3). So far, there have been some reports on Zn(II)–H3pdc systems, such as [Zn(Hpdc)(H2O)2]n (I) and [Zn3(pdc)2(H2O)5]n (II) [26]. It is interesting to note that the coordination modes of H3pdc in 1 are different from those in I and II. In I, the Zn(II) atom is coordinated with three Hpdc2− ligands, and the Hpdc2− ligand adopts chelating and monodentate fashion, bridging Zn(II) atoms to form a 1D double chain. In II, three five-coordinated Zn(II) atoms bind with two pdc3− ligands, and each pdc3− ligand chelates to Zn(II) atoms with three oxygen and two nitrogen atoms.
3.2 Structure of {[Cd(Hpdc)2]·2H2O2·H2bbi}n (2)
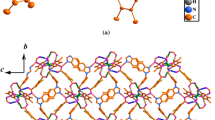
When Zn(CH3COO)2·2H2O was replaced by Cd(CH3COO)2·2H2O, complex 2 was obtained. The crystal analysis reveals that complex 2 crystallizes in monoclinic space group Cc. As shown in Fig. 3, the Cd(II) atom with distorted octahedral geometry is six-coordinated by four oxygen atoms and two nitrogen atoms from four different Hpdc2− ligands. The Cd–N and Cd–O distances in 2 are comparable with related Cd(II) coordination polymers [15]. The Hpdc2− ligands display one coordination mode in 2: one oxygen atom of 3-carboxyl group and one nitrogen atom bind to Cd1 with bidentate chelating coordination mode, while the oxygen atom of the 5-carboxyl group coordinates to Cd1C with monodentate fashion. So each Hpdc2− ligand serves as bis-connector, bridging Cd(II) atoms to form 1D chains along a and b axes, respectively. These chains are further arranged with Cd(II) atoms as hinges, affording a 2D layer pattern with the grid size of 8.9 × 8.9 Å2 (based on the distances of Cd) (Fig. 4). The lattice water molecules form O–H···O hydrogen bonds (O5–H5C···O1 and O5–H5D···O2) with the carboxyl oxygen atoms from the layers, giving rise to the formation of 3D supramolecular network, which further stabilizes the structure (Fig. 5; Table 3).
3.3 Structure of [Zn(BA)2(bbi)]n (3)
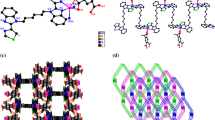
Complex 3 falls in monoclinic with space group P2(1)/c, and the asymmetric unit contains one Zn(II) ion, two BA− anion and one bbi ligand. As shown in Fig. 6, two imidazolyl nitrogen donors from two bbi ligands and two carboxyl oxygen atoms from two different BA− anions, affording the [ZnO2N2] coordination sphere. The Zn–O [1.944(2)–1.950(2) Å] and Zn–N [2.005(3)–2.020(3) Å] bond distances are within the normal ranges and comparable to those of reported Zn(II) complexes [24]. The O(1)–Zn–O(4) and N(2)–Zn–N(4) bond angles are 119.20(10)° and 107.53(11)°, respectively, while the N–Zn–O bond angles are in the range of 101.94(10)–114.21(11)°. Thus, the coordination geometry of Zn(II) center can be portrayed as s distorted tetrahedron with N2O2 donor set. Analogous configuration can also be found in [Zn(L)(BPDA)] (L = 1,4-di(1H-imidazol-4-yl)benzene, H2BPDA = 4,4′-biphenyldicarboxylic acid) [27], [Zn(2,4-D)2(BPP)]n (2,4-DH = 2,4-dichlorophenoxyacetic acid, BPP = 1,3-bis(4-pyridyl)propane) [28] and {[Zn2(BMB)(5-AIPA)2]·2(H2O)}n (5-AIPA = 5-aminoisophthalic acid, BMB = 1,4-bis[(2-methyl-imidazol-1-yl)methyl]benzene) [29].
There exist two types of bbi ligands with trans conformation. The N···N distances are 10.1450 and 8.9084 Å for the bbi ligands containing N2 and N4 atoms, respectively, while the imidazolyl rings are parallel to each other. The bbi ligands acting as bis-connector, link Zn(II) atoms to form a 1D chain with the Zn···Zn separations of 12.2480 and 13.5282 Å alternatively (Fig. 7). These 1D chains are further extended to a 2D network through O3–H···O2 and O6–H6···O5 interactions between the hydroxyl groups and carboxyl groups (Fig. 8). In addition, multiple C–H···O interactions involving the benzene rings, imidazole rings, carboxyl and hydroxyl groups are found between the chains (Table 3).
3.4 Structure of {[Cd(BA)2(bbi)]·H2O}n (4)
When Zn(CH3COO)2·2H2O was replaced by Cd(CH3COO)2·2H2O in 3, complex 4 was isolated. Compound 4 crystallizes in orthorhombic with space group Pbca. The asymmetry unit consists of one Cd(II) atom, two BA− anions, one bbi ligand and one lattice water molecule. As dicipted in Fig. 9, the Cd(II) center lies in a distorted octahedral sphere which is completed by three carboxyl oxygen atoms (O1, O2 and O5) from two different BA− anions and one bbi nitrogen donor (N4) occupying the equatorial plane, while the axial position are furnished by one nitrogen atom (N2) from another bbi ligand and one carboxyl oxygen atom (O4) from the BA− anion. The Cd–O bond lengths are in the range of 2.341(3)–2.399(3) Å and the Cd–N bond lengths are 2.259(4) Å, which are all similar to other Cd(II) complexes [24]. The N2–Cd–O4 bond angle is 134.16(15)°, while the other bond angles around the Cd(II) center range from 54.02(11)° to 145.28(15)°. Each bbi ligand possesses trans conformation with two imidazole rings being twisted by 9.6° and the N···N separation being 10.0795 Å. The bidentate bbi ligands link Cd(II) atoms to afford a 1D chain running along the b axis with the Cd···Cd distance of 13.8422 Å (Fig. 10). The Cd···Cd distance is slightly shorter than those observed in [Cd2(PDA)2(bbi)2(H2O)2]·10H2O (H2PDA = 2,6-pyridinedicarboxylic acid) [24] and {[Cd(bbi)3]·(NO3)2·(H2O)2}n [30] with bbi acting as spacers. Furthermore, the phenolic hydroxyl groups donate H atoms to carboxylate oxygen atoms to form intermolecular O–H···O hydrogen bonding interactions. Besides, the carbon atoms from bbi ligands donate H atoms to the hydroxyl oxygen atoms to give C–H···O hydrogen bonding interactions. These interactions extend the 1D chain into a 3D supramolecular network (Fig. 11; Table 3).
3.5 Structural Comparison for Complexes 1–4
The structural differences of complexes 1–4 imply the role of central ions in constructing metal–organic system. Zinc and cadmium possess similar coordination nature due to they belong to the identical periodic group. In complexes 1 and 2, the Zn(II) and Cd(II) atoms are both six-coordinated by four oxygen atoms and two nitrogen atoms. In 1, one carboxyl oxygen atom and pyrazolyl nitrogen atom are coordinated to Zn(II) atom in bidentate chelating fashion, while another carboxyl group remains idle. These block the extension of the complex to higher dimensional network, resulting in the 0D mononuclear structure. However, in 2, each pdc2− ligand coordinates to two Cd(II) ions through its two monodentate carboxylate and thereby serves as a bis-connector, affording the 2D framework. From the structures of 3 and 4, in can be found that the coordination numbers of the Zn(II) atom in 3 is smaller than that of the Cd(II) atom in 4, which results in different coordination geometries. Four-coordinated Zn(II) atoms in 3 and six-coordinated Cd(II) atoms in 4 are bridged by bbi ligands to generate 1D chains. However, these 1D chains are further extended into 2D and 3D frameworks through O–H···O and C–H···O hydrogen bonding interactions for 3 and 4, respectively. On the other hand, it is well known that the coordination nature, sharp and functionality of the carboxylic acid ligands play important roles on the complexes’ structures. Complexes 1–4 were obtained under similar reaction conditions except that different aromatic acids were used. Both complexes 3 and 4 are 1D chain, in which the metal-carboxylate units are bridged by bbi ligands. However, in 1 and 2, the bbi ligands don’t participate in the coordination, just acting as lattice molecules. The results of present study demonstrate that the carboxylic acid ligands play significant role in determining the final structures.
3.6 Thermogravimetric Analyses and Fluorescent Properties
Thermogravimetic analyses (TG) were performed for compounds 1–4 to investigate their thermal stability. The experiments were carried out for crystalline samples of 1–4 under flowing nitrogen in the temperature range 20–700 °C (Fig. 12). For 1, the first weight loss between 70 and 135 °C (observed 10.9 %, calculated 11.3 %) corresponds to the release of two free and two coordinated water molecules. The second weight loss occurs from 235 °C, corresponding to the decomposition of all organic components (observed 75.5 %, calculated 76.0 %). The remaining weight of 13.6 % makes clear the final residual is ZnO. For 2, the TG study shows that it loses two water molecules between 50 and 182 °C (observed 5.1 %, calculated 5.6 %), then follows a consecutive step of weight loss. the residual weight of 20.5 % (calculated 19.9 %) indicates that the final residue is CdO. Compound 3 is stable up to 205 °C, then it collapse gradually in the temperature 205–520 °C, before the final formation of ZnO (observed 16.1 %, calculated 15.4 %). For compound 4, the weight loss occurring between 85 and 135 °C (observed 3.5 %, calculated 3.0 %), corresponds to the release of one lattice water molecule. The weight loss from 272 to 452 °C is assigned to the decomposition of the network. The remaining residue corresponds to the formation of CdO (observed 20.8 %, calculated 21.6 %).
The solid state emission spectra of complexes 1–4, together with those of the free H3pdc, HBA and bbi ligands were investigated at room temperature (Fig. 13). Emission peaks were found at 434 nm (λex = 365 nm), 436 nm (λex = 295 nm) and 438 nm (λex = 252 nm) for free H3pdc, HBA and bbi, respectively. These emissions may originate from the π* → n or π* → π transitions as previously reported [31, 32]. The emission spectra for complexes exhibit emission peaks at 432 nm (λex = 335 nm) for 1, 422 nm (λex = 330 nm) for 2, 405 nm (λex = 362 nm) for 3, and 417 nm (λex = 325 nm) for 4, respectively. The emissions can probably be attributed to intraligand fluorescent emission due to their similarity. Comparing with the free ligands, the blue-shifts of 1–4 may be ascribed to the coordination of H3pdc or HBA to the metal atoms, which enhance the conformational rigidity of the carboxylic acid ligands and decrease the loss of energy via non-radiative relaxation of the intraligand excited states [33]. The emission difference of 1 and 2 may be related to their different coordination environments of central metal atoms and structural motifs (0D vs. 2D). Additionally, time-resolved luminescence study for the four complexes was carried out. The emission decay lifetimes of 1.1, 1.5, 2.1 and 2.5 ns were observed for 1–4, respectively.
4 Conclusions
Four Zn/Cd-containing supramolecular frameworks based on H3pdc/HBA and bbi ligands have been successfully synthesized under similar conditions. These complexes adopt versatile coordination features with 0D, 1D and 2D frameworks. The structural diversities demonstrate that the organic anions have critical effects on the structures of the resultant products. Furthermore, the central metal atoms also play significant role on the construction of diverse structures of the final products. Additionally, complexes 1–4 show blue luminescence at room temperature, and they may be potential candidates for blue-fluorescent materials.
References
C.D. Si, D.C. Hu, Y. Fan, X.Y. Dong, X.Q. Yao, Y.X. Yang, J.C. Liu, Cryst. Growth Des. 15, 5781 (2015)
V. Valderrey, G. Aragay, P. Ballester, Coord. Chem. Rev. 258, 137 (2014)
A.G. Slater, A.I. Cooper, Science 348, 988 (2015)
A. Dhakshinamoorthy, A.M. Asiric, H. Garcia, Chem. Soc. Rev. 44, 1922 (2015)
D.S. Li, J. Zhao, Y.P. Wu, B. Liu, L. Bai, K. Zou, M. Du, Inorg. Chem. 52, 8091 (2013)
K. Ariga, Y. Yamauchi, G. Rydzek, Q.M. Ji, Y. Yonamine, K.C.W. Wu, J.P. Hill, Chem. Lett. 43, 36 (2014)
O. Shekhah, H. Wang, S. Kowarik, F. Schreiber, M. Paulus, M. Tolan, C. Sternemann, F. Evers, D. Zacher, R.A. Fischer, C. Wöll, J. Am. Chem. Soc. 129, 15118 (2007)
V. Malgras, Q.M. Ji, Y. Kamachi, T. Mori, F.K. Shieh, K.C.W. Wu, K. Ariga, Y. Yamauchi, Bull. Chem. Soc. Jpn 88, 1171 (2015)
B. Liu, H. Shioyama, T. Akita, Q. Xu, J. Am. Chem. Soc. 130, 5390 (2008)
Z.R. Ranjbar, A. Morsali, J. Inorg. Organomet. Polym. 21, 421 (2011)
F.Z. Karizi, V. Safarifard, S.K. Khani, A. Morsali, Ultrason. Sonochem. 23, 238 (2015)
M. Moeinian, K. Akhbari, J. Solid State Chem. 225, 459 (2015)
M.M. Dong, L.L. He, Y.J. Fan, S.Q. Zang, H.W. Hou, T.C.W. Mak, Cryst. Growth Des. 13, 3353 (2013)
R.P. Ye, J.X. Yang, X. Zhang, L. Zhang, Y.G. Yao, J. Mol. Struct. 1106, 192 (2016)
Y.H. Zhou, Y.P. Tian, J. Chem. Crystallogr. 43, 31 (2013)
Y.H. Zhou, J. Inorg. Organomet. Polym. 25, 535 (2015)
Z.Y. Xiao, X. Yang, S.W. Zhao, D.B. Wang, Y. Yang, L. Wang, J. Solid State Chem. 234, 36 (2016)
G.Q. Shi, B.B. Shi, Q. Wang, G. Li, Polyhedron 92, 137 (2015)
B. Li, S.Q. Zang, L.Y. Wang, T.C.W. Mak, Coord. Chem. Rev. 308, 1 (2016)
Y.H. Zhou, Y.P. Tian, Bull. Korean Chem. Soc. 34, 2800 (2013)
Y.H. Zhou, J. Inorg. Organomet. Polym. 23, 458 (2013)
Y.H. Zhou, J. Inorg. Organomet. Polym. 23, 1189 (2013)
J. Yang, J.F. Ma, Y.Y. Liu, J.C. Ma, H.Q. Jia, N.H. Hu, Eur. J. Inorg. Chem. 23, 1208 (2006)
G.M. Sheldrick, SHELXS 97 Program for the solution of crystal structure (University of Göttingen, Göttingen, 1997)
G.M. Sheldrick, SHELXTL 97 program for crystal structure refinement (University of Göttingen, Göttingen, 1997)
C.B. Liu, L. Liu, S.S. Wang, X.Y. Li, G.B. Che, H. Zhao, Z.L. Xu, J. Inorg. Organomet. Polym. 22, 1370 (2012)
S.S. Chen, Y. Zhao, J. Fan, T.A. Okamura, Z.S. Bai, Z.H. Chen, W.Y. Sun, CrystEngComm 14, 3564 (2012)
Y.H. Zhou, Z.Y. Wang, Trans. Met. Chem. 40, 89 (2015)
J.T. Shi, C.S. Zhou, Y.L. Liu, Z.G. Fang, R.L. Zhao, L.L. Xu, K.F. Yue, Z. Anorg, Allg. Chem. 639, 187 (2013)
Y.H. Zhou, Bull. Korean Chem. Soc. 34, 1278 (2013)
H.Y. Liu, H. Wu, J.F. Ma, Y.Y. Liu, B. Liu, J. Yang, Cryst. Growth Des. 10, 4795 (2010)
X.J. Zheng, L.P. Jin, S. Gao, S.Z. Lu, New J. Chem. 29, 798 (2005)
J.G. Lin, S.Q. Zang, Z.F. Tian, Y.Z. Li, Y.Y. Xu, H.Z. Zhu, Q.J. Meng, CrystEngComm 9, 915 (2007)
Acknowledgments
This work was financially supported by Scientific Research Foundation of Anhui Provincial Education Department (KJ2014A228).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, YH., Wang, ZY. Four New Metal–Organic Supramolecular Networks Based on Aromatic Acid and Flexible Bis(imidazole) Ligand: Synthesis, Structures and Luminescent Properties. J Inorg Organomet Polym 26, 648–659 (2016). https://doi.org/10.1007/s10904-016-0353-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-016-0353-3