Abstract
A new copper(I) iodide coordination polymer, [(CuI)3(dtb)] n (1) (dtb = 1,3-di-(1,2,4-triazole-4-yl)benzene) has been synthesized solvothermally and structurally characterized by single crystal and powder X-ray diffractions, elemental analysis, IR, and thermogravimetric analysis. Overall, 1 exhibits a 2D hybrid structure containing dtb as structure-directing agents (SDAs) and 1D Cu3I3 chain as inorganic moiety. The copper-iodide chain can be regarded as two Cu2I2 rhomboids are connected by CuI fragments via Cu–I bonds. Dtb act as bridging ligands regularly link the Cu3I3 chains along both sides through Cu–N bonds to give the final 2D network. Moreover, solid state luminescent property of 1 has been investigated at room temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coordination polymer (CPs) or organic–inorganic hybrid materials, constructed by inorganic secondary building units (SBUs) and organic bridging ligands has taken wide attention in recent years owing to their intriguing structures as well as distinctive properties such as catalytic, luminescence, gas adsorption, and magnetism [1,2,3,4,5,6]. It is well known that copper(I) halide culsters, particularly copper(I) iodide motifs have been employed widely as inorganic components in the constructions of CPs since their rich coordinate sites as well as their excellent photophysical properties [7,8,9,10]. Indeed, copper iodide clusters not only defined the frameworks of the CPs but also impart their distinctive properties to the frameworks. Various copper(I) iodide clusters with the formulae (CuxIy)x–y have been reported in the past decades such as lower nuclearity or higher nuclearity clusters, discrete oligomers, polymeric chains or layers, cationic, anionic or neutral species [11,12,13,14,15]. These copper iodide hybrid clusters often constructed by so called structure-directing agents (SDAs) organic ligands (L) that contain N, P, S coordination atoms to give diverse copper iodide based CPs of the general formula CuxIyLz [16,17,18]. Copper iodide clusters based luminescent CPs have gained considerable interest for their structural diversity and high emission quantum yields [19,20,21,22]. The luminescence properties of these CPs can arise from different mechanisms, for example metal-to-ligand charge transfer (MLCT) [23], halide-to-ligand charge transfer (XLCT) [24], halide to metal charge transfer (XMCT) [25], cluster centered (CC) charge transfer [26], and so on. Moreover, short Cu–Cu interactions may be capable of influencing the emission behavior [27,28,29,30]. Inspired by the above-mentioned opinions and as our continuous work on copper(I) CPs [31], herein we report the preparation and characterization of a new 2D coordination polymer based on Cu3I3 chains connected via 1,3-di-(1,2,4-triazole-4-yl)benzene (dtb), [Cu3I3(dtb)] n (1).
Experimental
Materials and Methods
All reagents and solvents for synthesis were purchased from commercial sources and used directly without further purifications. Infrared spectra (4000–600 cm−1) were recorded on a Nicolet Avatar-360 spectrometer with KBr pellets. C, H, N analyses were carried out on a Flash 1112 elemental analyzer. The thermogravimetric measurement was carried out on a Perkin-Elmer TGA7 thermal analysis instrument at a heating rate of 10 °C min−1 under a nitrogen atmosphere. Powder X-ray diffraction data were collected on a Bruker AXS D8-Advanced diffractometer with Cu–Kα radiation (λ = 1.5406 Å). The solid-state luminescent spectra were performed on a FL-4500 fluorescent spectrometer using Xe lamp as the light source.
Synthesis of Compound [Cu3I3(dtb)] n (1)
A mixture of CuI (0.1 mmol, 19 mg), dtb (0.05 mmol, 11 mg), H2O (6 mL) and acetonitrile (4 mL) was sealed in a 25 mL Teflon-lined stainless steel vessel and heated at 140 °C for 3 days. The reaction mixture was slowly cooled to room temperature at a rate of 3 °C/h, and the pink block crystals were obtained. Yield (based on Cu): 6.8 mg, 26%. Elemental analysis (%) for C10H8Cu3I3N6, Found (calcd): C, 15.21(15.33); H, 1.09(1.03); N, 10.67(10.73). IR (KBr, cm−1): 3066(w), 1601(m), 1537(s), 1361(w), 1290(m), 1100(m), 869(m), 791(m), 684(s).
X-ray Crystallography
Crystal structure determination of 1 by X-ray diffractions was carried on a Bruker SMART APEX II CCD diffractometer equipped with a graphite-monochromated MoΚα radiation (λ = 0.71073 Å) at room temperature. The structure was solved by direct methods and refined by the full-matrix least-squares techniques on F2 using SHELXTL-97 [32]. All non-hydrogen atoms were assigned anisotropic displacement parameters in the refinement. Hydrogen atoms were treated using a riding model. The crystallographic data for 1 are summarized in Table 1. The selected bond lengths and angles are listed in Table 2.
Results and Discussion
Crystal Structure
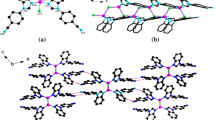
Single crystal X-ray diffraction study demonstrates that 1 crystallizes in the monoclinic space group C2/m, and shows a 2-D network with 1D Cu3I3 cluster chain. The asymmetric unit of 1 contains a Cu3I3 cluster and a dtb ligand (Fig. 1). Cu1 and Cu2 are both four-coordinated with distorted tetrahedral coordination environments. And the coordination spheres are completed by two iodine atoms and two nitrogen atoms from different dtb ligands. The τ4 parameter for Cu1 and Cu2 is 0.85 and 0.80, respectively, showing the distortion of the geometry [33]. Cu3 are surrounded by three iodine atoms in a planar triangle arrangement. The Cu–I distances are in the range of 2.5056(8)–2.7264(8) Å and the Cu–N lengths are 2.028(3) and 2.007(3) Å (Table 2), respectively.
In 1, iodine atoms show two different coordination modes, µ2- and µ3-bridging modes. Two Cu1 atoms are linked by two µ3-I (I1) to form a rhombus Cu2I2 dimer, another Cu2I2 rhombic cluster is composed by two Cu3 atoms and two µ3-I (I3). These two rhomboid cores are connected together by µ2-I (I2) and Cu2 to give a 1D wavelike chain (Fig. 2). The distance of Cu1···Cu1 and Cu3···Cu3 are 2.6507(3) and 2.5517(10) Å, respectively, which are shorter than the sum of Cu···Cu Van der Waals radii (2.8 Å) and is within the range of other CuI clusters [28]. The dtb adopt a µ4-η2N,N, η2N’,N’ fashion and connect the 1D chains into a 2D sheet along ab plane (Fig. 3).
Powder X-ray Diffraction (PXRD) and Thermogravimetric Analysis (TGA)
The PXRD analysis was performed for 1 at room temperature (Fig. S1). All major peaks of the experimental pattern are in agreement with the simulated patterns, showing the purity of the crystals. The thermal behavior of 1 was evaluated by thermogravimetric analysis (TGA) from 30 to 900 °C (Fig. S2). The TGA curve of 1 displays a weight loss of 26.31% from 235 to 368 °C, which corresponds to the loss of dtb ligand (calc. 26.80%). Then the framework begins to collapse. The thermal decomposition temperature of 1 is similar to that of the coordination polymer we reported before [31].
Luminescent Property
The luminescent property of complex 1 was investigated in the solid state at room temperature (Fig. 4). Complex 1 exhibits a broad green emission band at 557 nm with excitation at 360 nm. The emission is reminiscent of other CuI cluster-containing coordination polymers with different N-heterocyclic ligands [34, 35]. The luminescence is might be derived from a cluster-centered excited state (CC) of Cu3I3, which is supported by the short Cu···Cu distances [16, 17]. The emission maximum observed for 1 is within the range of similar CC emission [26].
Conclusions
In summary, a unique 2D CP based on 1D Cu3I3 chain and 1,3-di-(1,2,4-triazole-4-yl)benzene has been synthesized under solvothermal condition. Moreover, 1 displays green solid luminescence at room temperature which can be assigned to CC transitions. Concerning the special structures and properties of copper(I) iodide clusters based CPs, further studies are currently in progress.
References
M. D. Allendorf, C. A. Bauer, R. K. Bhakta, and R. J. T. Houk (2009). Chem. Soc. Rev. 38, 1330.
M. Kurmoo (2009). Chem. Soc. Rev. 38, 1353.
J. R. Li, R. J. Kuppler, and H. C. Zhou (2009). Chem. Soc. Rev. 38, 1477.
M. E. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O’Keeffe, and O. M. Yaghi (2002). Science 295, 469.
S. Kitagawa, R. Kitaura, and S. Noro (2004). Angew. Chem. Int. Ed. 43, 2334.
J. J. Perry IV, J. A. Perman, and M. J. Zaworotko (2009). Chem. Soc. Rev. 38, 1400.
J. P. Zhang, Y. Y. Lin, X. C. Huang, and X. M. Chen (2005). J. Am. Chem. Soc. 127, 5495.
Y. H. Liu, J. F. Zhang, L. P. Gong, and C. Zhang (2016). J. Clust. Sci. 27, 1353.
M. A. Tershansy, A. M. Goforth, L. Peterson Jr., M. C. Burns, M. D. Smith, and H. C. zur Loye (2009). Solid State. Sci. 9, 895.
J. He, Y. G. Yin, T. Wu, D. Li, and X. C. Huang (2006). Chem. Commun. 27, 2845.
S. Hu, F. Y. Yu, Y. Yan, Z. F. Hao, L. Yu, and M. L. Tong (2011). Inorg. Chem. Commun. 14, 622.
H. H. Li, Z. R. Chen, Y. Liu, K. N. Ding, J. Q. Li, C. C. Huang, and L. Q. Guo (2007). J. Cluster. Sci. 18, 817.
T. Wu, M. Li, D. Li, and X. C. Huang (2008). Cryst. Growth. Des. 8, 568.
M. H. Bi, G. H. Li, J. Hua, Y. L. Liu, X. M. Liu, Y. W. Hu, Z. Shi, and S. H. Feng (2007). Cryst. Growth. Des. 7, 2066.
L. Maini, D. Braga, P. P. Mazzeo, L. Maschio, M. Rérat, I. Manet, and B. Ventura (2015). Dalton. Trans. 44, 13003.
J. Conesa-Egea, J. Gallardo-Martínez, S. Delgado, J. I. Martínez, J. Gonzalez-Platas, V. Fernández-Moreira, U. R. Rodríguez-Mendoza, P. Ocón, F. Zamora, and P. Amo-Ochoa (2017). Small 13, 1700965.
X. C. Shan, F. L. Jiang, D. Q. Yuan, H. B. Zhang, M. Y. Wu, L. Chen, J. Wei, S. Q. Zhang, J. Pan, and M. C. Hong (2013). Chem. Sci. 4, 1484.
M. S. Deshmukh, A. Yadav, R. Pant, and R. Boomishankar (2015). Inorg. Chem. 54, 1337.
M. A. Tershansy, A. M. Goforth, J. M. Ellsworth, M. D. Smith, and H. C. zur Loye (2008). CrystEngComm 10, 833.
H. Park, E. Kwon, H. Chiang, H. Im, K. Y. Lee, J. Kim, and T. H. Kim (2017). Inorg. Chem. 56, 8287.
A. Bonnot, C. Strohmann, M. Knorr, and P. D. Harvey (2014). J. Clust. Sci. 25, 261.
L. Li, H. Y. Li, Z. G. Ren, and J. P. Lang (2014). Eur. J. Inorg. Chem. 5, 824.
S. L. Li, J. Wang, F. Q. Zhang, and X. M. Zhang (2017). Cryst. Grwoth Des. 17, 746.
F. De Angelis, S. Fantacci, A. Sgamellotti, E. Cariati, R. Ugo, and P. C. Ford (2006). Inorg. Chem. 45, 10576.
F. S. Wu, H. B. Tong, Z. Y. Li, W. Lei, L. Liu, W. Y. Wong, W. K. Wong, and X. J. Zhu (2014). Dalton. Trans. 43, 12463.
D. Braga, F. Grepioni, L. Maini, P. P. Mazzeo, and B. Ventura (2011). New. J. Chem. 35, 339.
J. A. Tompkins, J. L. Maxwell, and E. M. Holt (1987). Inorg. Chim. Acta. 127, 1.
D. Sun, S. Yuan, H. Wang, H. F. Lu, S. Y. Feng, and D. F. Sun (2013). Chem. Commun. 49, 6152.
H. Araki, K. Tsuge, Y. Sasaki, S. Ishizaka, and N. Kitamura (2005). Inorg. Chem. 44, 9667.
W. V. Taylor, U. H. Soto, V. M. Lynch, and M. J. Rose (2016). Inorg. Chem. 55, 3206.
S. B. Miao, Z. H. Li, B. M. Ji, D. S. Deng, C. Y. Xu, and L. Zhou (2014). J. Clust. Sci. 25, 1137.
G. M. Sheldrick SHELXS-97 and SHELXL-97, Programs for Crystal Structure Refinement (University of Göttingen, Germany, 1997).
L. Yang, D. R. Powell, and R. P. Houser (2007). Dalton. Trans. 9, 955.
T. Li and S. W. Du (2008). J. Clust. Sci. 19, 323.
S. Hu and M. L. Tong (2005). Dalton. Trans. 7, 1165.
Acknowledgements
We are grateful to the Natural Science Foundation of China (Grant No. 21372112) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10876_2018_1333_MOESM1_ESM.pdf
CCDC 1583584 contains the supplementary crystallographic data for 1. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. (PDF 54 kb)
Rights and permissions
About this article
Cite this article
Miao, SB., Xu, CY., Deng, DS. et al. Synthesis, Crystal Structure, and Properties of a 2D Cu(I) Coordination Polymer Based on Cu3I3 Chains Linked by 1,3-Di-(1,2,4-Triazole-4-yl)Benzene. J Clust Sci 29, 313–317 (2018). https://doi.org/10.1007/s10876-018-1333-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-018-1333-2