Abstract
Self-critical perfectionism confers vulnerability for depressive symptoms, but research suggests vulnerability persists after treatment. Dysregulation of physiological stress systems is a potential mechanism for depression vulnerability, and yet it remains under-studied in research on perfectionism, stress, and depression. We aimed to address this gap by testing the influence of self-critical perfectionism, stress generation, and stress reactivity on depressive symptoms and on diurnal cortisol. A sample of undergraduates (N = 127) completed questionnaires and provided samples of salivary cortisol twice daily (morning and evening) over three days. Data were analyzed using path analysis with diurnal cortisol activity modeled using latent growth modeling. People high in self-critical perfectionism showed a greater propensity toward depressive symptoms through stress generation and stress reactivity processes. Although self-critical perfectionism did not directly predict diurnal cortisol, results supported physiological stress reactivity. Specifically, people high in self-critical perfectionism showed increased waking cortisol in high and low stress conditions, whereas people low in this trait showed higher cortisol only in the context of high daily hassles. Results suggest prolonged physiological activity may be an important factor to consider in future research and points toward the development of bio-psycho-social models when understanding how self-critical perfectionism confers vulnerability to depressive symptoms in the context of stress generation and reactivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Perfectionism increases vulnerability to depressive symptoms, even after accounting for other personality traits such as neuroticism (Smith et al. 2016). Highly perfectionistic people remain vulnerable to depressive symptoms even after psychotherapy due, in part, to increased sensitivity to the effects of stress, (Hawley et al. 2014). Models of perfectionism, stress, and depressive symptoms are increasingly sophisticated (Dunkley et al. 2014), yet these models have only recently begun to explore the possible role of physiological stress processes in depression vulnerability (e.g., Mandel et al. 2018). Physiological stress responses can become dysregulated due to to prolonged stress, which increases risk for depressive symptoms over time (Ancelin et al. 2017; Harris et al. 2000; LeMoult et al. 2015). Perfectionism may thus confer vulnerability to depressive symptoms through multiple pathways. The present research used measures of daily cortisol activity to better understand how perfectionism impacts physiological stress and how these effects may be unique from mechanisms linking perfectionism and depressive symptoms.
Perfectionistic Strivings and Self-Critical Perfectionism
Perfectionism is multidimensional and is widely recognized as a stable personality disposition involving striving for flawlessness, setting unrealistically high standards for oneself, and harshly evaluating oneself for perceived shortcomings (Stoeber 2018). Two forms of perfectionism are commonly described: perfectionistic strivings and self-critical perfectionism. Perfectionistic strivings involves holding oneself to lofty, and often unrealistic, standards for performance and striving relentlessly toward them, whereas self-critical perfectionism involves a pre-occupation with mistakes and negative evaluation from others, doubts about performance abilities, and harsh self-evaluation (Blankstein and Dunkley 2002).
Self-critical perfectionism shows a strong, unambiguous link with depressive symptoms (Smith et al. 2016). Perfectionistic strivings confers risk for depressive symptoms through circumscribed mechanisms (e.g., sensitivity to academic failure; Békés et al. 2015; Hewitt and Flett 1993) and can show adaptive benefits when isolated from self-critical perfectionism (Dunkley et al. 2014). Although both forms of perfectionism remain important (Stoeber 2018), self-critical perfectionism plays a more prominent role in depressive symptoms and stress relative to perfectionistic strivings (Dunkley et al. 2014) and is the focus of this research.
Stress Generation and Reactivity
Theoretical models propose personality traits, such as self-critical perfectionism, can lead to psychological distress through stress generation and stress reactivity (Bolger and Zuckerman 1995; Hewitt and Flett 2002). Stress generation involves a tendency to report more frequent stress, most likely due to a proclivity toward perceiving everyday events (past, present, and future) as stressful, whereas stress reactivity involves an amplified, and more negative, response to stressful events (Hewitt and Flett 2002).
Empirical research supports stress generation and stress reactivity in self-critical perfectionism. People high in self-critical perfectionism report greater frequency of daily hassles (Dunkley et al. 2003), with evidence suggesting they may be particularly susceptible to interpersonal stress (Enns and Cox 2005; La Rocque et al. 2016). Such individuals also tend to interpret daily experiences as more unpleasant, persistent, and stressful, although this tends to arise indirectly through a tendency to cope with daily experiences in avoidant ways (Dunkley et al. 2003; Dunkley et al. 2014). People high in self-critical perfectionism (and its constituent components such as socially prescribed perfectionism) are also more vulnerable to depressive symptoms through stress reactivity, such that vulnerability increases in the presence of stressful life events (Hawley et al. 2014; Hewitt and Flett 1993), chronic stress (Békés et al. 2015), and daily hassles (Dunkley et al. 2014). Stress reactivity is most frequently discussed in relation to emotional distress (Dunkley et al. 2014; La Rocque et al. 2016; Mandel et al. 2015) whereas research on self-critical perfectionism and physiological reactivity remains sparse.
Physiological Stress Reactivity: Gaps in Research and Unique Opportunities
The hypothalamic-pituitary adrenal (HPA) axis mobilizes resources necessary to cope with demands from a person’s environment through the release of cortisol (McEwen 2008). Cortisol levels naturally fluctuate over time based on predictable patterns (e.g., diurnal rhythms, cortisol awakening response) and in response to stressful events (Nicolson 2008). Although daily hassles have not demonstrated an association with HPA-axis activity directly (Herane-Vives et al. 2018), genetic vulnerability and chronic stress can result in HPA-axis dysregulation over time, such that cortisol responses are stronger (hyper-activation) or weaker (hypo-activation) than required to maintain optimal functioning (Miller et al. 2007). Depressive symptoms have also been associated with increased morning cortisol levels and decreased evening cortisol levels (O'Connor et al. 2010) and research suggests HPA-axis dysregulation increases risk for depressive symptoms over time (Ancelin et al. 2017; Harris et al. 2000; LeMoult et al. 2015).
Several studies have supported an association between self-critical perfectionism and cortisol patterns. Self-critical perfectionism (and related forms of perfectionism) has been associated with increased cortisol reactivity during lab-based stress induction protocols (Wirtz et al. 2007; Zureck et al. 2014). One study using daily diary methods showed people high in self-critical perfectionism with higher cortisol awakening responses (i.e., a surge of cortisol that occurs approximately 45 min after awakening) were at increased risk of depressive symptoms six months later, whereas people low in self-critical perfectionism showed the opposite pattern (Mandel et al. 2018).
Two of these studies showed no significant relation between self-critical perfectionism and diurnal cortisol patterns in daily life (Mandel et al. 2018; Wirtz et al. 2007), but neither tested stress reactivity explicitly and each involved a notable limitation. Wirtz et al. (2007) measured diurnal cortisol on a single day, despite low reliability for this sampling frequency (Kraemer et al. 2006). Mandel et al. (2018) assessed diurnal cortisol on two non-consecutive days during a 7-day period but used a mixed-gender sample without accounting for potential confounding factors such as gender, hormonal contraceptive use, or menstrual cycle phase. Accounting for these factors has been suggested for the reliable and the valid measurement of cortisol (Page et al. 2018). Both studies also used relatively small sample sizes (N = 60 and N = 43, respectively).
Objectives and Hypotheses
The present research extends previous research by explicitly testing stress reactivity while sampling cortisol over three consecutive days, using a larger sample size, and accounting for potential confounding effects relevant to cortisol. The primary focus involved testing stress reactivity in relation to diurnal cortisol patterns, and the secondary focus involved comparing stress reactivity effects for diurnal cortisol with stress reactivity effects for depressive symptoms.
Our hypothesized model (see Fig. 1) tested stress reactivity using a moderation framework while accounting for the effects of daily hassles and depressive symptoms on diurnal cortisol parameters (i.e., diurnal intercept and slope). The model also accounts for the overlap between self-critical perfectionism and perfectionistic strivings, as shown in past research (Blankstein and Dunkley 2002); however, unique effects of perfectionistic strivings were not a focus of the present research. All hypotheses relevant to cortisol were tested using participant sex and menstrual cycle phase as covariates.
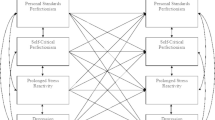
Path analysis with diurnal cortisol patterns (intercept and slope) modeled using latent growth modeling. The latent growth model used individually varying times of observation based on MEMS-reported sampling time relative to self-reported waking time on that day, with time scores indicated by τn. Correlated error terms between cortisol samples account for day-specific variance. Covariates (i.e., menstrual cycle phase and gender) were included in analysis but are not shown to maintain model clarity. Rectangles represent manifest variables and ovals represent latent variables. Single-headed arrows indicate regression paths and double-headed arrows indicate covariance. Grey dashed lines indicate non-significant paths. Parameter estimates are standardized. SCP = Self-critical perfectionism. * = p < .05; ** = p < .01; *** = p < .001
Hypothesis 1
Direct pathways in the model were expected to be consistent with past research. First (Hypothesis 1a), we expected self-critical perfectionism would uniquely predict depressive symptoms and daily hassles beyond perfectionistic strivings (Dunkley et al. 2003; Smith et al. 2016). Second (Hypothesis 1b), we expected depressive symptoms to predict higher a cortisol intercept and steeper slope (Knorr et al. 2010). Research has not demonstrated direct associations between diurnal cortisol and either self-critical perfectionism or daily hassles, and thus we did not expect these effects in our model. All other direct pathways in the model were treated as exploratory.
Hypothesis 2
Based on stress reactivity (Bolger and Zuckerman 1995; Hewitt and Flett 2002) and previous experimental research (Wirtz et al. 2007; Zureck et al. 2014), we hypothesized recent daily hassles would moderate the association between self-critical perfectionism and diurnal cortisol patterns, such that people high in self-critical perfectionism would show increased diurnal cortisol (i.e., higher diurnal intercept and steeper negative slope) in the presence of high daily hassles. We hypothesized these stress reactivity pathways would be unique from the effect of depressive symptoms in the model.
Hypothesis 3
Consistent with past research (Dunkley et al. 2014; Mandel et al. 2015), we hypothesized recent daily hassles would moderate the association between self-critical perfectionism and depressive symptoms, such that people high in self-critical perfectionism would show increased depressive symptoms in the presence of high daily hassles.
Method
Participants
Participants were recruited using the online psychology participant pool and flyers posted around campus. Interested students contacted the lab and completed an online screening questionnaire to determine eligibility. To be eligible, students must have had access to a freezer for sample storage and were excluded if they indicated any of the following: diagnosis of chronic or acute medical or psychiatric conditions, use of psychoactive medication or regular recreational drug use, use of estradiol-based oral contraceptives, or the use of other hormonal treatments. Exclusion criteria were based on recommendations for studies assessing cortisol (Nicolson 2008; Page et al. 2018). Of those who completed the screening (N = 314), 53.8% were eligible to participate and were invited to participate. Oral contraceptive use was the most frequent reason for ineligibility (33.8% of screenings completed).
A total of 129 students attended the lab-based session. Two participants did not complete the sampling protocol. Our final sample included 127 undergraduates (72.4% women) with a mean age of 21.0 years (SD = 4.7 years). Participants were primarily Caucasian (51.2%), Asian (15.7%), Middle Eastern (11.0%), Black (9.4%), or mixed/other ethnicity (12.6%). Most were full-time students (96.9%) and were not employed in addition to their studies (59.8%). The sample included students from first year (30.7%), second year (29.1%), third year (25.2%), and fourth year and above (13.4%). Students were primarily majoring in psychology (22.0%), neuroscience (17.3%), other sciences (26.8%), or were undeclared (21.3%). Women reported being primarily in the follicular phase of their menstrual cycle (80.7%). None of the women indicated being pregnant or nursing.
Measures and Materials
Self-Critical Perfectionism
Consistent with past research (Clara et al. 2007; Dunkley et al. 2003), we measured self-critical perfectionism as a composite of socially prescribed perfectionism, concern over mistakes, doubts about actions, and self-criticism. All scales measured asked participants to respond based on general tendencies over the past several years. We measured socially prescribed perfectionism using the 5-item short-form of Hewitt and Flett’s Multidimensional Perfectionism Scale (HFMPS; Hewitt and Flett 1991; Hewitt et al. 2008). Each item (e.g., “People expect nothing less than perfection from me”) was rated on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree).
We measured concerns over mistakes (e.g., “If I fail partly, it is as bad as being a complete failure”) using the 5-item short-form of Frost et al.’s (1990) Multidimensional Perfectionism Scale (FMPS-SF; Cox et al. 2002) and we measured doubts about actions (e.g., “Even when I do something very carefully, I often feel that it is not quite right”) using the original 4-item subscale from Frost et al.’s (1990) Multidimensional Perfectionism scale. Research demonstrates superior psychometric properties for the original 4-item doubts about actions subscale compared to the 3-item short-form of this scale (Cox et al. 2002). Both concern over mistakes and doubts about actions were rated on a 5-point scale from 1 (strongly disagree) to 5 (strongly agree).
We measured self-criticism using a 5-item short form of the self-criticism subscale of the Reconstructed Depressive Experiences Questionnaire (RDEQ-SC; Bagby et al. 1994; Blatt et al. 1976). Each item (e.g., “I often find that I don’t live up to my own standards or ideals”) was rated on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree). Four items from the original RDEQ-SC do not directly reflect self-criticism (e.g., “I never really feel secure in a close relationship”) and were removed. The 5-item version of this scale shows similar internal reliability to the original 9-item scale (α = .89 vs. .87), with a high correlation between scale versions (r = .92; Nealis and Sherry 2017).
Research supports the reliability and the validity of each scale (Bagby et al. 1994; Cox et al. 2002; Hewitt et al. 2008), and internal reliabilities were adequate for each scale in our data (α = .78–.83). Subscales were standardized, summed, and re-standardized to create the composite. Evidence supports the validity and the reliability for this composite as a whole (Clara et al. 2007), with internal reliability of α = .89, 95% CI [.86, .91], in this study.
Perfectionistic Strivings
Consistent with past research, we measured perfectionistic strivings as a composite of three subscales reflecting self-oriented perfectionism and high standards for performance (McGrath et al. 2012). All scales asked participants to respond based on general tendencies over the past several years. We measured self-oriented perfectionism using the 5-item short-form of the HFMPS (Hewitt and Flett 1991; Hewitt et al. 2008). Each item (e.g., “One of my goals is to be perfect in everything I do”) was rated on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree). We also used the 4-item self-oriented perfectionism subscale from the Eating Disorders Inventory (EDI; Garner et al. 1983). Each item (e.g., “I feel that I must do things perfectly or not do them at all”) was rated on a 6-point scale from 1 (never) to 6 (always). We measured high standards for performance using the 5-item short form of the personal standards subscale from the FMPS (Cox et al. 2002; Frost et al. 1990). Each item (e.g., “I set higher goals than most people”) was rated on a 5-point scale from 1 (strongly disagree) to 5 (strongly agree). Research supports the reliability and the validity of each scale (Cox et al. 2002; Hewitt et al. 2008; McGrath et al. 2012), with adequate internal reliability for individual scales in our data (α = .75–.86). Research supports the validity of the composite as a whole (McGrath et al. 2012). Internal reliability for the composite was α = .91, 95% CI [.89, .94] in our study.
Depressive Symptoms
We measured depressive symptoms with the 10-item (e.g., “I felt that I could not shake off the blues even with the help from friends or family”) short form of the Center for Epidemiological Studies Depression scale (CES-D-SF; Cole et al. 2004). Each item was rated on a 4-point scale from 0 (rarely or none of the time) to 3 (most or all of the time) based on how often participants felt that way the previous two weeks. Research shows the 10-item short form demonstrates similar reliability as the full 20-item version, demonstrates acceptable factor structure, and is correlated highly (r = .75) with other measures of depressive symptoms (e.g., Beck Depression Inventory; see Cole et al. 2004). In this study, internal reliability of this scale was α = .77, 95% CI [.70, .83].
Daily Hassles
We measured recent stressful events using the Inventory of College Student Recent Life Events (ICSRLE; Kohn et al. 1990). The 49 items reflected various domains of life stress including academic (e.g., “finding courses too demanding”), interpersonal (e.g., “conflicts with your family”), and other life stress (e.g., “difficulties with transportation”). Participants responded to each item based on the intensity of their experiences over the past two weeks using a 4-point scale from 1 (not at all part of my life) to 4 (very much part of my life). All items were summed to provide a total daily hassles score with an internal reliability of α = .90, 95% CI [.88, .93].
Diurnal Cortisol
Diurnal cortisol patterns were estimated as a linear function using data from all six saliva samples completed during the three-day sampling period. Saliva samples were obtained using Salivettes™ (Sarstedt, Germany), which are commonly used in the collection of salivary cortisol (Nicolson 2008). Participants stored completed samples in opaque plastic bottles fitted with Medication Event Monitoring System (MEMS®) caps to electronically log each time participants opened the bottles, which provided electronically verified sample completion time. The MEMS system is regarded as best practice for measuring protocol adherence for daily cortisol sampling (Kudielka et al. 2003). Participants received an ice pack to keep samples cold during transportation back to the lab.
Salivary cortisol concentrations were measured from saliva samples using enzyme-linked immunosorbent assay (ELISA) kits (high sensitivity salivary cortisol ELISA, no. 1–3002; Salimetrics™, USA). Assay kits used a competitive binding technique and have a published sensitivity of <0.007 μg/dL. Samples were analyzed in duplicate and showed low intra-assay variability (CV% < 6). Standard curves using 4-parameter non-linear regression curve fit showed high reliability (r > .99). Assays were performed according to the protocol provided by the manufacturer. Values reflect cortisol concentrations in μg/dL unless stated otherwise. Cortisol concentration values were used in latent growth modeling (see Section 2.4 for details) to provide a latent diurnal cortisol intercept (i.e., average waking cortisol concentration across the three-day sampling period) and a latent diurnal cortisol slope (i.e., average within-day rate of change in cortisol concentration during the three-day sampling period). These latent variables were modeled in relation to other study variables in the hypothesized model (see Fig. 1).
Procedure
A research ethics board approved our study, which involved an initial lab-based session and cortisol sampling at home over the following three-day period. Initially, participants attended a research lab at Dalhousie University where they provided informed consent, and completed self-report questionnaires of personality, recent daily stress, depressive symptoms, and other measures not used in the current study. Participants also received instructions regarding the saliva sampling protocol and were assigned cortisol sampling materials at this time.
During the cortisol sampling period, participants were asked to complete two saliva samples each day (at waking and in the evening evening) for three consecutive days. To reduce cost and participant burden, empirical research demonstrates as little as two daily cortisol samples are sufficient to model diurnal cortisol patterns, and this sampling protocol yields estimates of diurnal cortisol slope that correlate highly (r = .92) with more frequent measurement (e.g., five daily samples; Kraemer et al. 2006). These authors state the number of sampling days is a more important contributor to reliable diurnal cortisol estimation than the number of samples per day, with three sampling days being optimal (Kraemer et al. 2006). Participants were instructed to complete the morning sample within 15 min of waking to avoid capturing the cortisol awakening response and to complete the evening sample 12 h after the morning sample to capture the diurnal nadir (Kraemer et al. 2006). Before completing samples, participants were instructed to avoid eating or drinking anything other than water within one hour prior to a sample, brushing their teeth within 30 min of a sample, engaging in vigorous exercise within one hour of completing a sample, and drinking alcohol within 12 h of completing a sample. These instructions were provided according to published guidelines reflecting prior research and best practices for salivary cortisol collection (Nicolson 2008).
Participants were instructed in how to provide saliva samples and completed a practice sample during the session to ensure participants completed samples as required. The collection protocol for Salivettes™ was based on recommendations from the manufacturer (Sarstedt n.d.). Participants were instructed to store their samples in the provided containers and to keep the containers frozen until samples were returned to the lab. Participants were also provided with a tracking sheet to record self-reported time of awakening each morning, time each sample was completed, and the duration of each sample collection (i.e., length of time the cotton roll was in their mouth), and any deviations from protocol (e.g., consumption of alcohol prior to the sample). Detailed printed instructions for completing samples were also provided to participants.
After the three-day sampling period was complete, participants returned their saliva samples to the lab. Participants were then debriefed about the study and received their compensation for participating (2.5 credits and $5 cash or $30 cash). Samples were promptly labeled and transferred to a laboratory freezer for storage at −20 °C until analysis.
Data Analytic Plan
We tested hypotheses using path analysis with latent growth modeling to estimate diurnal cortisol intercept and slope (see Fig. 1). We combined all available cortisol samples across the three-day sampling period when modeling latent growth curves to reflect a single aggregated diurnal cortisol pattern during the sampling period rather than modeling day-specific cortisol trajectories. This method of modeling diurnal cortisol has been used in research in naturalistic settings (Adam 2006; Adam and Gunnar 2001). Diurnal cortisol and slope are strongly and negatively related in naturalistic research (r = −.96), with these two indices likely reflecting complementary, rather than distinct, aspects of diurnal cortisol (Adam & Gunner, 2001). In contrast to other approaches, such as calculating area under the curve with respect to ground (AUCg; Pruessner et al. 2003), latent growth modeling allows use of all available cortisol samples rather than requiring complete data on each sampling day. To account for variable sampling times, we modeled latent growth curves with individually varying times of observation. Time scores (τn) reflected the MEMS-reported sampling time relative to self-reported waking time that day (in hours). We specified correlated errors between cortisol samples taken on the same day (see Fig. 1) to account for day-specific variance (Adam 2006). Problems with model convergence arising from modeling random slopes with very low variance values were addressed by multiplying cortisol concentrations by a factor of 100 prior to inclusion in the growth model. We included gender and menstrual cycle phase as covariates based on published recommendations (Page et al. 2018) and used self-reported time (in days) since the start of the last menstrual cycle to classify women into follicular phase (≤ 14 days) or luteal phase (> 14 days). Predictors and mediators were standardized.
Indirect effects were tested using a Monte Carlo technique (Preacher et al. 2010) with 20,000 samples. One-tailed confidence intervals (90%) not including zero indicate a significant indirect effect (Efron and Tibshirani 1985). Analyses were conducted in Mplus 7.0 (Muthén and Muthén 2012) using robust maximum likelihood estimation. Simple intercepts and simple slopes were calculated for significant interactions with high vs. low groups defined using conditional values of ±1 standard deviation from the mean (Preacher et al. 2006). We used Cohen’s (1992) criteria to evaluate effect sizes, with correlations and path coefficients of .10 to .30 indicating small effect sizes, correlations of .30 to .50 indicating medium effect sizes, and correlations greater than .50 indicating large effect sizes.
Results
Missing Data and Protocol Compliance
No item-level or scale-level data were missing on self-report questionnaires. Participants provided a total of 729 saliva samples (95.7% compliance), with 95.3% of participants (n = 121) providing complete samples at all six sampling times. One sample did not contain sufficient saliva for analysis. The cortisol awakening response (CAR) peaks between 30 to 45 min post waking and reflects a distinct physiological process from diurnal cortisol patterns (Fries et al. 2009). Self-reported sampling time and MEMS sampling time data were highly correlated (r = .97). Based on MEMS-reported sampling time, samples provided between 15 and 60 min after self-reported waking time (n = 82) were considered to reflect the CAR and were removed from analysis. We used multiple regression to test the potential impact of food and alcohol consumption, brushing teeth, and vigorous exercise prior to sampling. After controlling for the time of sampling (i.e., morning or evening), these factors did not significantly affect cortisol concentration, F(4, 727) = .39, p = .82, and were retained for analysis. Time of sampling could not be determined for 27 samples (3.8%) because MEMS data indicated failure to follow protocol (e.g., no samples completed within the expected time period) and these samples were excluded from analysis. Complete MEMS data were available for a majority of participants (n = 117; 92.1%). Final analyses included 619 cortisol samples (84.9% of provided samples), with an average of 5.3 samples (SD = 0.9) per person. Path analysis used full information maximum likelihood in Mplus (Muthén and Muthén 2012) to address missing data. This method provides less biased estimates than other methods (e.g., listwise deletion) when all available data are included in analysis (Acock 2005).
Path Analysis
Table 1 shows descriptive statistics and bivariate correlations and Fig. 1 shows results of the path model. Menstrual cycle phase and gender were included as covariates in the path model but were not shown in Fig. 1 to aid clarity of presentation. Menstrual cycle phase did not show a significant relation with diurnal cortisol intercept (β = 7.94, p = .05) or slope (β = −0.36, p = .24), although the former trended toward significance. Gender did not show unique significant effects on diurnal cortisol intercept (β = −0.13, p = .98) or slope (β = 0.48, p = .15).
Direct Pathways
Model results showed a positive association between self-critical perfectionism and perfectionistic strivings (large effect). As hypothesized, self-critical perfectionism was positively and significantly associated with depressive symptoms (large effect) and recent hassles (large effect) when accounting for covariance with perfectionistic strivings. Perfectionistic strivings demonstrated a negative and significant association with depressive symptoms (small effect) but showed no unique relation with daily hassles. Daily hassles demonstrated a positive and significant unique association with depressive symptoms (small effect) but no unique effect on diurnal cortisol parameters. Associations between self-critical perfectionism and diurnal cortisol parameters were not significant when accounting for covariates and all other effects in the model. As hypothesized, recent depressive symptoms were significantly associated with diurnal cortisol intercept and slope over the three-day sampling period (medium effect sizes); however, higher depressive symptoms predicted lower diurnal intercept and higher slope rather than the opposite pattern.
Stress Reactivity and Diurnal Cortisol
Hypothesis 2 was partially supported. The interaction between self-critical perfectionism and recent daily hassles significantly predicted diurnal cortisol intercept and slope. Both effect sizes were small. However, the direction of effects was opposite to hypotheses. Path estimates from the model were used to plot the interaction of self-critical perfectionism, recent daily hassles, and time on cortisol levels (see Fig. 2) and to calculate simple intercepts and slopes. Calculations used a latent growth curve analysis framework for three-way interactions (see Preacher et al. 2006) and 95% confidence intervals indicated patterns of significance.
Interaction plot of self-critical perfectionism (low vs. high) by daily hassles (high vs. low) by time (hours after waking) on estimated diurnal cortisol trajectories. Cortisol trajectories were calculated using latent diurnal intercept and latent diurnal slope derived from latent growth analysis (see Fig. 1). Conditional values for self-critical perfectionism and daily hassles were defined as ±1 standard deviation from the mean and conditional values of time on the x-axis were chosen to reflect cortisol concentrations at waking and 12 h post waking (i.e., evening levels). Error bars are 95% confidence intervals
Contrary to predictions, diurnal cortisol trajectories were not significantly different between people high in self-critical perfectionism (simple intercept = 0.315, 95% CI [0.242, 0.388], z = 8.47, p < .001; simple slope = −0.019, 95% CI [−0.024, −0.013], z = 6.90, p < 001) and people low in self-critical perfectionism (simple intercept = 0.304, 95% CI [0.204, 0.404], z = 5.96, p < .001; simple slope = −0.019, 95% CI [−0.027, −0.011], z = −4.76 p < .001) who also reported high recent daily hassles (see Fig. 2b). Instead, differences were evident for those reporting low levels of recent daily stress (see Fig. 2a). People high in self-critical perfectionism showed significantly higher intercept and slope (simple intercept = 0.358, 95% CI [0.258, 0.458], z = 7.02, p < .001; simple slope = −0.024, 95% CI [−0.031, −0.016], z = −5.52, p < .001) compared to people low in self-critical perfectionism (simple intercept = 0.247, 95% CI [0.164, 0.330], z = 5.87, p < .001; simple slope = −0.016, 95% CI [−0.022, −.009], z = −4.73, p < .001).
Stress Reactivity and Depressive Symptoms
Hypothesis 3 was fully supported. The interaction between self-critical perfectionism and recent daily hassles was positively and significantly associated with recent depressive symptoms (small effect size). Path estimates from the model were used to plot the interaction between self-critical perfectionism and recent daily hassles on depressive symptoms (see Fig. 3) and to calculate simple intercepts and slopes. Calculations were conducted consistent with Hypothesis 2 but using a two-way interaction framework (Preacher et al. 2006).
Interaction plot showing the effect of self-critical perfectionism (high vs. low) and daily hassles (high vs. low) on depressive symptoms. Depressive symptoms reflect standardized (Z) scores. Conditional values for self-critical perfectionism and daily hassles were defined as ±1 standard deviation from the mean. Error bars are 95% confidence intervals
Results indicated significantly higher depressive symptoms for people reporting high levels of recent daily stressors (simple intercept = 0.29, 95% CI [−0.06, 0.65], t = 1.60, p = .11) compared to people reporting low levels of recent daily stressors (simple intercept = −0.27, 95% CI [−0.65, 0.12], t = −1.35, p = .18) when aggregating across level of self-critical perfectionism. For people who reported low daily hassles, depressive symptoms were significantly higher for people higher in self-critical perfectionism than people lower in this trait (simple slope = 0.35, 95% CI [0.15, 0.56], t = 3.40, p < .001). As hypothesized, people who reported high daily hassles and had high levels of self-critical perfectionism showed significantly higher depressive symptoms than people who reported high daily hassles and had low levels of self-critical perfectionism (simple slope = 0.70, 95% CI [0.40, 1.01], t = 4.49, p < .001).
Discussion
Our primary objective was to extend prior research by testing patterns of stress reactivity in relation to self-critical perfectionism, daily hassles, and depressive symptoms. While ample literature exists focusing on emotional vulnerability (e.g., depressive symptoms), stress reactivity in regard to physiological processes (e.g., HPA-axis dysregulation) has been sparse. We tested a model of stress reactivity including both depressive symptoms and diurnal cortisol patterns while accounting for methodological limitations of past research. Overall, results supported stress reactivity in regard to both depressive symptoms and diurnal cortisol patterns, although in somewhat unexpected ways.
Although stress reactivity was the primary focus on this research, direct effects of our model provided the necessary context for interpreting stress reactivity effects. Results supported self-critical perfectionism as uniquely associated with depressive symptoms and daily hassles (Hypothesis 1a), which is broadly consistent with extant literature on self-critical perfectionism and vulnerability to stress and emotional distress. Consistent with past work (Mandel et al. 2018; Wirtz et al. 2007), results showed no direct effect of self-critical perfectionism on diurnal cortisol patterns despite a larger sample size, use of multiple sampling days, and accounting for potential confounding factors (e.g., gender, oral contraceptive use, menstrual period phase). This was not taken to suggest self-critical perfectionism is unrelated to HPA-axis functioning, but rather that the association may manifest only under certain conditions.
Results were also consistent with past research showing no apparent association between recent daily hassles and diurnal cortisol activity (Herane-Vives et al. 2018). Higher daily stress may trigger elevated cortisol responses; however, coping adaptively to those demands could result in a “net zero” effect on physiological stress (Drake et al. 2016) and therefore negate any direct impact of stress generation on physiological stress processes.
Contrary to Hypothesis 1b, depressive symptoms predicted blunted morning cortisol levels rather than elevated morning levels and decreased evening levels. This discrepancy reflects a broader contradiction in extant literature; meta-analysis suggests substantial variability in effects (Knorr et al. 2010). Timing has been suggested to explain these equivocal findings, with recent distress linked to elevations in daily cortisol levels and more chronic and pervasive distress linked to decreased levels (Miller et al. 2007). Blunting effects are also more prominent with specific manifestations of depressive symptoms including increased mood reactivity and interpersonal sensitivity (Herane-Vives et al. 2018). Self-critical perfectionism is frequently associated with these characteristics (Flett et al. 2014; Mandel et al. 2015), making the interactions between self-critical perfectionism, depressive symptoms, and HPA-axis functioning an important area for further research.
The primary objective of this research, and the most compelling results from this study, relate to stress reactivity. Most previous research in perfectionism and stress reactivity (e.g., Mandel et al. 2015; Hawley et al. 2014) pertains to emotional reactivity, which was clearly demonstrated in the present study as predicted in Hypothesis 2. Specifically, results suggest people high in self-critical perfectionism are most vulnerable to depressive symptoms in the context of high daily hassles. These findings support previously demonstrated diathesis-stress models of perfectionism (Chang and Rand 2000; O’Connor, Rasmussen, & Hawton, 2010). Most interestingly, the pathways reflecting emotional reactivity were distinct from those reflecting physiological reactivity and may represent distinct forms of stress vulnerability.
In terms of physiological reactivity, recent daily hassles moderated the relation between self-critical perfectionism and diurnal cortisol patterns as predicted (Hypothesis 3). However, rather than people high in self-critical perfectionism demonstrating amplified physiological stress in response to recent hassles, they showed increased diurnal cortisol when accompanied by low daily hassles. Several possibilities could explain this pattern. First, people high in both self-critical perfectionism and recent daily hassles showed the greatest levels of depressive symptoms, and depressive symptoms were associated with blunted morning cortisol (see Hypothesis 1b). This blunting effect may have suppressed diurnal cortisol activity when self-critical perfectionists when physiological stress is normally highest. Second, people high in self-critical perfectionism may be prone to chronically high diurnal cortisol, even during periods of low stress, due to a prolonged stress response (Brosschot et al. 2006). Rather than people high in self-critical perfectionism showing more stress reactivity than people low on this trait, they seemingly show less reactivity because their HPA-axis may be chronically activated. Research suggests self-critical perfectionists are vulnerable to both chronic stress (Békés et al. 2015) and negative repetitive thinking patterns (Macedo et al. 2015) that could perpetuate the stress response. Chronic activation of the HPA-axis and the blunting effects of depressive symptoms during periods of high stress could therefore obscure direct effects of self-critical perfectionism on the HPA-axis, both in past research and the current study.
Overall, results point toward a potential quagmire for someone high in self-critical perfectionism. Such a person may experience their day-to-day lives as more stressful than others, and could be particularly vulnerable to depressive symptoms during periods of high stress. Together with patterns of rumination, the physiological effects of stress could be prolonged over time, so physiological stress would remain high even when daily demands are relatively low. With abnormal HPA-functioning linked to increased risk of depressive symptoms over time (e.g., Harris et al. 2000), this person may even become more vulnerable to depressive symptoms over time. This represents one potential scenario, although not all people high in self-critical perfectionism may be vulnerable to depressive or HPA-axis dysregulation in the same ways or at the same time. Further research is needed better understand how these processes unfold, and influence each other, over time.
Limitations and Future Directions
This research provides insight into self-critical perfectionism and stress reactivity, and these results require replication. Methodological improvements would provide opportunities for increasingly robust tests of these effects. A multi-wave longitudinal study would allow differentiation between the effects of recent depressive symptoms and daily hassles versus those occurring concurrently with cortisol sampling. Temporal separation between measurement of personality, stress, and emotional distress would also allow investigation of meditational processes that could not be tested in this study. We used MEMS caps to record sampling time to overcome limitations of self-report, but we relied on self-report to assess waking time and menstrual cycle phase. Future research might use objective measures of waking (e.g., actigraph watches) and assessment of salivary hormone levels to improve accuracy. Although research supports the cortisol sampling strategy and analytic approach used in this study (Adam 2006; Kraemer et al. 2006), results should be replicated with more frequent daily measurement.
Replication with more diverse samples would aid with generalizability. We excluded students taking oral contraceptives and psychoactive medications to reduce confounds for cortisol measurement (Nicolson 2008), although this also reduces generalizability. The experience of perfectionistic people may be variable across developmental periods, and the pattern of HPA-axis changes could reflect the high academic demands placed on university students. Mid-life adults could be vulnerable to HPA-axis dysregulation in a different way, reflecting the chronic “wear and tear” of daily life. Testing this model in community and clinical samples would be needed to demonstrate generalizability of results beyond a student sample and better understand temporal persistence of these effects in other populations.
Finally, we focused on psychological and physiological aspects of stress and vulnerability to depressive symptoms, but interpersonal aspects and coping patterns were not included in this study. Research is needed to integrate these findings with other frameworks (e.g., trigger and maintenance models; Dunkley et al. 2014).
Concluding Remarks
This research points toward the importance of a bio-psycho-social model of perfectionism and stress reactivity. Extant research focuses predominantly on psychological and interpersonal factors that increase susceptibility to depressive symptoms, but the present study highlights the unique importance of considering physiological stress. Physiological measures are sometimes included as a means for overcoming self-report bias, yet the real strength of such measures is that they capture distinct processes that are uniquely and incrementally important for understanding psychological phenomena (Semmer et al. 2004). Results also highlight the importance of thorough investigation of stress reactivity when attempting to understand the relation between perfectionism and physiological measures. Researchers are encouraged to more carefully consider physiological processes to support development of a bio-psycho-social framework of perfectionism, stress, and depression.
References
Acock, A. C. (2005). Working with missing values. Journal of Marriage and Family, 67, 1012–1028.
Adam, E. K. (2006). Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology, 31, 664–679.
Adam, E. K., & Gunnar, M. R. (2001). Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology, 26, 189–208.
Ancelin, M., Scali, J., Norton, J., Ritchie, K., Dupuy, A., Chaudieu, I., & Ryan, J. (2017). Heterogeneity in HPA axis dysregulation and serotonergic vulnerability to depression. Psychoneuroendocrinology, 77, 90–94.
Bagby, R. M., Parker, J. D. A., Joffe, R. T., & Buis, T. (1994). Reconstruction and validation of the depressive experiences questionnaire. Assessment, 1, 59–68.
Békés, V., Dunkley, D. M., Taylor, G., Zuroff, D. C., Lewkowski, M., Foley, J. E., et al. (2015). Chronic stress and attenuated improvement in depression over 1 year: The moderating role of perfectionism. Behavior Therapy, 46, 478–492.
Blankstein, K. R., & Dunkley, D. M. (2002). Evaluative concerns, self-critical, and personal standards perfectionism: A structural equation modeling strategy. In G. L. Flett & P. L. Hewitt (Eds.), Perfectionism: Theory, research, and treatment (pp. 285–315). Washington: American Psychological Association.
Blatt, S. J., D’Afflitti, J. P., & Quinlan, D. M. (1976). Experiences of depression in normal young adults. Journal of Abnormal Psychology, 85, 383–389.
Bolger, N., & Zuckerman, A. (1995). A framework for studying personality in the stress process. Journal of Personality and Social Psychology, 69, 890–902.
Brosschot, J. F., Gerin, W., & Thayer, J. F. (2006). The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research, 60, 113–124.
Chang, E. C., & Rand, L. M. (2000). Perfectionism as a predictor of subsequent adjustment: Evidence for a specific diathesis-stress mechanism among college students. Journal of Counselling Psychology, 47, 129–137.
Clara, I. P., Cox, B. J., & Enns, M. W. (2007). Assessing self-critical perfectionism in clinical depression. Journal of Personality Assessment, 88, 309–316.
Cohen, J. (1992). A power primer. Psychological Bulletin, 112, 155–159.
Cole, J. C., Rabin, A. S., Smith, T. L., & Kaufman, A. S. (2004). Development and validation of a Rasch-derived CES-D short form. Psychological Assessment, 16, 360–372.
Cox, B. J., Enns, M. W., & Clara, I. P. (2002). The multidimensional structure of perfectionism in clinically distressed and college student samples. Psychological Assessment, 14, 365–373.
Drake, E. C., Sladek, M. R., & Doane, L. D. (2016). Daily cortisol activity, loneliness, and coping efficacy in late adolescence: A longitudinal study of the transition to college. International Journal of Behavioral Development, 40, 334–345.
Dunkley, D. M., Zuroff, D. C., & Blankstein, K. R. (2003). Self-critical perfectionism and daily affect: Dispositional and situational influences on stress and coping. Journal of Personality and Social Psychology, 84, 234–252.
Dunkley, D. M., Ma, D., Lee, I. A., Preacher, K. J., & Zuroff, D. C. (2014). Advancing complex explanatory conceptualizations of daily negative and positive affect: Trigger and maintenance coping action patterns. Journal of Counseling Psychology, 61, 93–109.
Efron, B., & Tibshirani, R. (1985). The bootstrap method for assessing statistical accuracy. Behaviormetrika, 17, 1–35.
Enns, M. W., & Cox, B. J. (2005). Perfectionism, stressful life events, and the 1-year outcome of depression. Cognitive Therapy and Research, 29, 541–553.
Flett, G. L., Besser, A., & Hewitt, P. L. (2014). Perfectionism and interpersonal orientations in depression: An analysis of validation seeking and rejection sensitivity in a community sample of young adults. Psychiatry: Interpersonal and Biological Processes, 77, 67–85.
Fries, E., Dettenborn, L., & Kirschbaum, C. (2009). The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology, 72, 67–73.
Frost, R. O., Marten, P., Lahart, C., & Rosenblate, R. (1990). The dimensions of perfectionism. Cognitive Therapy and Research, 14, 449–468.
Garner, D. M., Olmstead, M. P., & Polivy, J. (1983). Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. International Journal of Eating Disorders, 2, 15–34.
Harris, T. O., Borsanyi, S., Messari, S., Stanford, K., Cleary, S. E., Shiers, H. M., Brown, G. W., & Herbert, J. (2000). Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. The British Journal of Psychiatry, 177, 505–510.
Hawley, L. L., Zuroff, D. C., Brozina, K., Ho, M. R., & Dobson, K. S. (2014). Self-critical perfectionism and stress reactivity following cognitive behavioral therapy for depression. International Journal of Cognitive Therapy, 7, 287–303.
Herane-Vives, A., Angel, V., Papadopoulos, A., Wise, T., Chua, K., Strawbridge, R., et al. (2018). Short-term and long-term measures of cortisol in saliva and hair in atypical and non-atypical depression. Acta Psychiatrica Scandinavica, 37, 216–230.
Hewitt, P. L., & Flett, G. L. (1991). Perfectionism in the self and social contexts. Journal of Personality and Social Psychology, 60, 456–470.
Hewitt, P. L., & Flett, G. L. (1993). Dimensions of perfectionism, daily stress, and depression: A test of the specific vulnerability hypothesis. Journal of Abnormal Psychology, 102, 58–65.
Hewitt, P. L., & Flett, G. L. (2002). Perfectionism and stress processes in psychopathology. In G. L. Flett & P. L. Hewitt (Eds.), Perfectionism: Theory, research, and treatment (pp. 255–284). Washington: American Psychological Association.
Hewitt, P. L., Habke, A. M., Lee-Baggley, D. L., Sherry, S. B., & Flett, G. L. (2008). The impact of perfectionistic self-presentation on the cognitive, affective, and physiological experience of a clinical interview. Psychiatry, 71, 93–122.
Knorr, U., Vinberg, M., Kessing, L. V., & Wetterslev, J. (2010). Salivary cortisol in depressed patients versus control persons: A systematic review and meta-analysis. Psychoneuroendocrinology, 35, 1275–1286.
Kohn, P. M., Lafreniere, K., & Gurevich, M. (1990). The inventory of college Student's recent life experiences: A decontaminated hassles scale for a special population. Journal of Behavioral Medicine, 13, 619–630.
Kraemer, H. C., Giese-Davis, J., Yutsis, M., Neri, E., Gallagher-Thompson, D., Taylor, C. B., & Spiegel, D. (2006). Design decisions to optimize reliability of daytime cortisol slopes in an older population. American Journal of Geriatric Psychiatry, 14, 325–333.
Kudielka, B. M., Broderick, J. E., & Kirschbaum, C. (2003). Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol profiles in noncompliant subjects. Psychosomatic Medicine, 65, 313–319.
La Rocque, C. L., Lee, L., & Harkness, K. L. (2016). The role of current depression symptoms in perfectionistic stress enhancement and stress generation. Journal of Social and Clinical Psychology, 35, 64–86.
LeMoult, J., Ordaz, S. J., Kircanski, K., Singh, M. K., & Gotlib, I. H. (2015). Predicting first onset of depression in young girls: Interaction of diurnal cortisol and negative life events. Journal of Abnormal Psychology, 124, 850–859.
Macedo, A., Soares, M. J., Amaral, A. P., Nogueira, V., Madeira, N., Roque, C., et al. (2015). Repetitive negative thinking mediates the association between perfectionism and psychological distress. Personality and Individual Differences, 72, 220–224.
Mandel, T., Dunkley, D. M., & Moroz, M. (2015). Self-critical perfectionism and depressive and anxious symptoms over 4 years: The mediating role of daily stress reactivity. Journal of Counseling Psychology, 62, 703–717.
Mandel, T., Dunkley, D. M., Lewkowski, M., Zuroff, D. C., Lupien, S. J., Juster, R., Ng Ying Kin, N. M. K., Foley, J. E., Myhr, G., & Westreich, R. (2018). Self-critical perfectionism and depression maintenance over one year: The moderating roles of daily stress-sadness reactivity and the cortisol awakening response. Journal of Counseling Psychology, 65, 334–345.
McEwen, B. S. (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology, 583, 174–185.
McGrath, D. S., Sherry, S. B., Stewart, S. H., Mushquash, A. R., Allen, S. L., Nealis, L. J., & Sherry, D. L. (2012). Reciprocal relations between self-critical perfectionism and depressive symptoms: Evidence from a short-term, four-wave longitudinal study. Canadian Journal of Behavioural Science, 44, 169–181.
Miller, G. E., Chen, E., & Zhou, E. S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45.
Muthén, L., & Muthén, B. O. (2012). Mplus (version 7) [computer software]. Los Angeles: Muthén & Muthén.
Nealis, L. J. & Sherry, S. B. (2017). [Psychometric data]. Unpublished raw data.
Nicolson, N. A. (2008). Measurement of cortisol. In L. J. Luecken & L. C. Gallo (Eds.), Handbook of physiological research methods in health psychology (pp. 37–74). Thousand Oaks: Sage.
O'Connor, R. C., Rasmussen, S., & Hawton, K. (2010). Predicting depression, anxiety and self-harm in adolescents: The role of perfectionism and acute life stress. Behaviour Research and Therapy, 48, 52–59.
Page, M. J., Hill, A. P., Kavanagh, O., & Jones, S. (2018). Multidimensional perfectionism and cortisol stress response in non-clinical populations: A systematic review and evaluation. Personality and Individual Differences, 124, 16–24.
Preacher, K. J., Curran, P. J., & Bauer, D. J. (2006). Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31, 437–448.
Preacher, K. J., Zyphur, M. J., & Zhang, Z. (2010). A general multilevel SEM framework for assessing multilevel mediation. Psychological Methods, 15, 209–233.
Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time dependent change. Psychoneuroendocrinology, 28, 916–931.
Sarstedt (n.d.). Salivette® hygienic saliva collection for diagnostics and monitoring. Retrieved from: https://www.sarstedt.com/fileadmin/user_upload/99_Broschueren/Englisch/ 156_Salivette_GB_0813.pdf.
Semmer, N. K., Grebner, S., & Elfering, A. (2004). Beyond self-report: Using observational, physiological, and situation-based measures in research on occupational stress. In P. L. Perrewé & D. C. Ganster (Eds.), Emotional and physiological processes and positive intervention strategies (pp. 205–263). New York: Elsevier.
Smith, M. M., Sherry, S. B., Rnica, K., Saklofske, D. H., Enns, M. W., & Gralnick, T. M. (2016). Are perfectionism dimensions vulnerability factors for depressive symptoms after controlling for neuroticism? A meta-analysis of 10 longitudinal studies. European Journal of Personality, 30, 201–212.
Stoeber, J. (2018). The psychology of perfectionism: Critical issues, open questions, and future directions. In J. Stoeber (Ed.), The psychology of perfectionism: Theory, research, applications (pp. 333–352). London, England: Routledge.
Wirtz, P. H., Elsenbruch, S., Emini, L., Rüdisüli, K., Groessbauer, S., & Ehlert, U. (2007). Perfectionism and the cortisol response to psychosocial stress in men. Psychosomatic Medicine, 69, 249–255.
Zureck, E., Altstötter-Gleich, C., Wolf, O. T., & Brand, M. (2014). It depends: Perfectionism as a moderator of experimentally induced stress. Personality and Individual Differences, 63, 30–35.
Funding
This research is part of the doctoral dissertation of Logan J. Nealis and was funded by a Dalhousie University Department of Psychiatry Research Fund grant awarded to Logan J. Nealis, Simon B. Sherry, and Sanjay Rao. Logan J. Nealis was supported by a Canada Graduate Scholarship from the Social Sciences and Humanities Research Council (SSHRC) and a Nova Scotia Health Research Foundation (NSHRF) Scotia Scholarship. We thank Lauren Matheson for her research assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Logan Nealis, Simon Sherry, Tara Perrot, and Sanjay Rao declare that they have no conflict of interest.
Ethical Approval
Ethical approval for this study was granted by the Dalhousie University Health Sciences Research Ethics Board on July 15, 2014 (Reference number 2014-3304).
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Experiment Participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nealis, L.J., Sherry, S.B., Perrot, T. et al. Self-Critical Perfectionism, Depressive Symptoms, and HPA-Axis Dysregulation: Testing Emotional and Physiological Stress Reactivity. J Psychopathol Behav Assess 42, 570–581 (2020). https://doi.org/10.1007/s10862-020-09793-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10862-020-09793-9