Abstract
Four novel quaternary ammonium dimethacrylate monomers named IMQ (side alkyl chain length from 12 to 18) were synthesized with the aim to synthesize dental resin with antibacterial activity. All of IMQs were added into bis-GMA/TEGDMA dental resin system with a series of mass ratio (5, 10, and 20 wt%), double bond conversion (DC), flexural strength (FS), modulus of elasticity (FM) and biofilm formation inhibitory effect were studied. According to the results of DC, FS, FM, and the biofilm inhibitory effect, IMQ-16 containing polymer had the best comprehensive properties, and the optimal concentration of IMQ-16 in bis-GMA/TEGDMA dental resin would be in the range of 5–10 wt%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Resin-based dental composites have been widely used to restore carious teeth because of their superior esthetic quality and excellent adhesive strength to dentin and enamel [1, 2]. However, resin-based dental composites have a limited service life, and secondary (recurrent) caries is found to be the main reason to the restoration failure of resin-based dental composites [3–5]. Secondary caries that often occurs at the interface between the restoration and the cavity preparation is primarily caused by demineralization of tooth structure due to the invasion of plaque bacteria such as Streptococcus mutans in the presence of fermentable carbohydrates [6, 7]. Therefore, to prevent secondary carries, the dental composites and bonding agents could be made antibacterial.
One approach to endow dental composites with an antibacterial activity is incorporating releasable antibacterial agents such as silver ions, zinc ions, chlorhexidine, and cetylpyridinium chloride into the material by physical blending [8–11]. Unfortunately, these agents could bring some inevitable disadvantages, such as a small amount of agent could decrease the mechanical properties of materials [12], and the releasing agent may exert toxic effects as well as cause short-term antibacterial effectiveness [13–15].
In order to achieve long-term antibacterial effectiveness, Imazato introduced a concept of “immobilized bactericide” into dentistry. According to his innovative idea, a quaternary ammonium containing polymerizable antibacterial monomer methacryloyloxy-dodecylpridinium bromide (MDPB) was synthesized and used in dental composite materials as an antibacterial agent [16]. After that, many kinds of quaternary ammonium methacrylate monomers (QAMs) were synthesized and incorporated into dental materials as antibacterial agents [17–22]. However, most of these QAMs have miscible problem with the commercially used dimethacrylate diluent triethylene glycol dimethacrylate (TEGDMA), and only a small amount of QAMs could be added into the resin system, which limited antibacterial activity [19, 23–25].
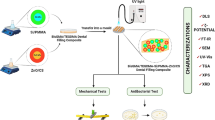
The objective of this study was to design and synthesize a series of novel quaternary ammonium dimethacrylate monomers (IQM) with different alkyl chain length (from 12 to 18). The hypothesis was that the synthesized IQMs could be mixed well with the commonly used bis-GMA/TEGDMA dental resin system. Then, the concentrations of synthesized monomers in the resin system were optimized by using antibacterial activity and mechanical property as two parameters. Double bond conversion of every obtained antibacterial resin system was also measured.
2 Materials and methods
2.1 Materials
2-Isocyanatoethyl methacrylate and TEGDMA were purchased from Tokyo Chemical Industry Co. Dibutyltin dilaurate (DBTDL), bis-GMA, 2-(N,N-dimethylamino)ethyl methacrylate (DMAEMA), camphorquinone (CQ), hydroquinone were obtained from Sigma-Aldrich Co. All of the intermediate products HQAs were synthesized in our lab according to literature [22]. All purchased reagents were used without any purification.
2.2 General procedure for the synthesis of IMQs
Acetone used here was dried over 4 Å molecular sieves for 2 weeks. A mixture of HQA (0.04 mol), MEI (0.08 mol), 40 ml acetone, a small amount of hydroquinone, and two droplets of DBTDL were stirred at 45 °C. The reaction was continued until the infrared absorbance peak of the –NCO group (2,270 cm−1) disappeared in the FTIR spectra of the samples that taken from the reaction medium. After removing the acetone by distillation under vacuum, the product was washed with diethyl ether to remove DBTDL and hydroquinone. Then the light yellow viscose liquid was dried under vacuum at 35 °C for 48 h to obtain IQM. All data of FT-IR and 1H-NMR of each IMQ were shown as below.
2.2.1 N,N-bis[2-(3-(methacryloyloxy)propanamido)ethyl]-N-methyldodecyl ammonium bromide (IQM-12)
FT-IR: v (cm−1) 3360, 2920, 2851, 1720, 1637, 1164, 814, 725. 1H-NMR (CDCl3, 400 MHz): δ 6.61-6.62[2H, 2-NH–], 6.07[2H, 2CH 2=C(CH3) trans], 5.52[2H, 2CH 2=C(CH3) cis], 4.51[4H, 2-NHCH2CH 2O–], 4.15–4.17[4H, N+CH2CH 2O–], 3.87[4H, N+CH 2CH2O–], 3.47–3.51[2H, N+CH 2CH2(CH2)9CH3], 3.36–3.41[7H, N+CH 3 and 2-NHCH 2CH2O–], 1.86[6H, 2CH2=C(CH 3)], 1.66[2H, N+CH2CH 2(CH2)9CH3], 1.18–1.28[18H, N+CH2CH2(CH 2)9CH3], 0.73–0.82[3H, N+CH2CH2(CH2)9CH 3].
2.2.2 N,N-bis[2-(3-(methacryloyloxy)propanamido)ethyl]-N-methyltetradecyl ammonium bromide (IQM-14)
FT-IR: v (cm−1) 3360, 2921, 2857, 1717, 1638, 1159, 814, 721. 1H-NMR (CDCl3, 400 MHz): δ 6.75–6.76[2H, 2-NH–], 6.14[2H, 2CH 2=C(CH3) trans], 5.59[2H, 2CH 2=C(CH3) cis], 4.59[4H, 2-NHCH2CH 2O–], 4.22–4.25[4H, N+CH2CH 2O–], 3.95[4H, N+CH 2CH2O–], 3.56-3.58[2H, N+CH 2CH2(CH2)11CH3], 3.43–3.47[7H, N+CH 3 and 2-NHCH 2CH2O–], 1.93[6H, 2CH2=C(CH 3)], 1.74[2H, N+CH2CH 2(CH2)11CH3], 1.25–1.35[22H, N+CH2CH2(CH 2)11CH3], 0.86–0.90[3H, N+CH2CH2(CH2)11CH 3].
2.2.3 N,N-bis[2-(3-(methacryloyloxy)propanamido)ethyl]-N-methylhexadecyl ammonium bromide (IQM-16)
FT-IR: v (cm−1) 3340, 2925, 2851, 1720, 1637, 1164, 814, 722. 1H-NMR (CDCl3, 400 MHz): δ 6.63–6.66[2H, 2-NH–], 6.15[2H, 2CH 2=C(CH3) trans], 5.60[2H, 2CH 2=C(CH3) cis], 4.60[4H, 2-NHCH2CH 2O–], 4.23–4.26[4H, N+CH2CH 2O–], 3.97[4H, N+CH 2CH2O–], 3.57-3.61[2H, N+CH 2CH2(CH2)13CH3], 3.46–3.49[7H, N+CH 3 and 2-NHCH 2CH2O–], 1.94[6H, 2CH2=C(CH 3)], 1.75[2H, N+CH2CH 2(CH2)13CH3], 1.26–1.36[26H, N+CH2CH2(CH 2)13CH3], 0.87–0.91[3H, N+CH2CH2(CH2)13CH 3].
2.2.4 N,N-bis[2-(3-(methacryloyloxy)propanamido)ethyl]-N-methyloctadectyl ammonium bromide (IMQ-18)
FT-IR: v (cm−1) 3360, 2925, 2851, 1720, 1637, 1164, 814, 720. 1H-NMR (CDCl3, 400 MHz): δ 6.90–6.71[2H, 2-NH–], 6.15[2H, 2CH 2=C(CH3) trans], 5.60[2H, 2CH 2=C(CH3) cis], 4.60[4H, 2-NHCH2CH 2O–], 4.24–4.26[4H, N+CH2CH 2O–], 3.96[4H, N+CH 2CH2O–], 3.54–3.58[2H, N+CH 2CH2(CH2)15CH3], 3.44–3.52[7H, N+CH 3 and 2-NHCH 2CH2O–], 1.95[6H, 2CH2=C(CH 3)], 1.74–1.75[2H, N+CH2CH 2(CH2)15CH3], 1.27–1.36[30H, N+CH2CH2(CH 2)15–CH3], 0.88–0.91[3H, N+CH2CH2(CH2)15CH 3].
2.3 Preparation of experimental resin system
Each IQM was added into bis-GMA/TEGDMA (50/50, wt/wt) resin system with mass ratios of 5, 10, and 20 wt%, CQ (0.7 wt%) and DMAEMA (0.7 wt%) were mixed as a photoinitiator system. bis-GMA/TEGDMA without IMQ was prepared as control group. All of the compounds were well blended to obtain a homogeneous mixture, and stored in dark before use.
2.4 Double bond conversion
The degree of double bond conversion (DC) was determined by using a FTIR spectrometer (Spectrum One, Perkin-Elmer, Waltham, MA, USA) with an attenuated total reflectance accessory. The FTIR spectra were recorded with 16 scans at a resolution of 4 cm−1. All the samples were analyzed in a mold that was 2 mm thick and 6 mm in diameter. First, the spectrum of the unpolymerized sample was measured. Then, the sample was irradiated for 60 s with a visible light-curing unit (450 mW cm−2, QHL750, Dentsply International, USA) at room temperature. The sample was scanned for its FTIR spectrum 15 min after the beginning of irradiation.
To determine the percentage of reacted double bonds, the absorbance intensities of the methacrylate C=C absorbance peak at 1,636 cm−1, which were decreased after being irradiated, and an internal phenyl ring standard peak at 1,608 cm−1, were calculated using a baseline method. The ratios of absorbance intensities were calculated and compared before and after polymerization. The DC was calculated by using the equation
where A c=c and A ph are the absorbance intensity of methacrylate C=C at 1,636 cm−1 and phenyl ring at 1,608 cm−1, respectively. (A C=C/A ph)a and (A C=C/A ph)b are the normalized absorbance of functional group after and before being irradiated, respectively.
2.5 Three point bending test
Eight specimens were prepared for every sample formulation (size 2 × 2 × 25 mm3). Three point bending test (span 20 mm) was carried out to evaluate the flexural strength and modulus according ISO 4049:1988 (E) standard with a material testing machine (model LRX, Lloyd Instrument Ltd., Fareham, England), at a cross-head speed of 1.00 mm min−1. Test was carried out in air for dry specimens.
2.6 Biofilm Inhibition test
Disc shaped samples (2 mm thick and 8 mm in diameter) for each resin formulation were prepared for a biofilm inhibition test. Each disc was polished with 4,000 grit (FEPA) grinding paper and soaked in distilled water for 24 h to remove the unreacted monomers.
The inhibition of biofilm formation reflecting plaque accumulation was tested by using a modification of the method originally presented by Ebi et al. [26]. The microorganism we used was the reference strain S. mutans Ingbritt. It was first grown overnight in Brain Heart Infusion medium (BHI; Becton–Dickinson and Company, Sparks, MD, USA). In the morning the cells were washed with phosphate-buffered saline (5,000×g, 10 min) and then they were suspended in BHI containing 1 % sucrose (A 550 = 0.05). This suspension (500 μl) was pipetted onto the experimental discs placed in the wells of 24-well cell culture plates. The plates were incubated anaerobically (90 % N2, 5 % CO2, 5 % H2) at 37 °C for 24 h.
The biofilms were collected with microbrushes (Quick-Stick®, Dentsolv AB, Saltsjö-Boo, Sweden) from the disc surface exposed to the medium to test tubes containing Tryptic Soy Broth (Becton–Dickinson and Company). The tubes were vortexed and mildly sonicated and then serially diluted for plate culturing of S. mutans. The plates were grown for 3 days anaerobically (80 % N2, 10 % CO2, 10 %H2) at +37 °C on Mitis salivarius agar (Becton–Dickinson and Company), the colonies were counted under a stereomicroscope and results expressed as colony-forming units (CFU)/disc surface. The biofilm collection method has been tested in our earlier studies and it is highly reproducible [27, 28].
2.7 Statistical analysis
All the results were statistically analyzed with analysis of variance (ANOVA) at the P < 0.05 significance level. Subsequent multiple comparisons were conducted using Tukey’s post hoc analysis.
3 Results
Figure 1 is the synthesis route of IMQs, all of IMQs were successfully synthesized through a two-steps route and their structures were confirmed by FT-IR and 1H-NMR spectra.
Results of the DC are shown in Fig. 2. There was no significant difference between the DC of control resin and the DC of every IMQ containing resin (P > 0.05). The concentration of IMQ and the alkyl chain length of IMQ had no influence on the DC in this work (P > 0.05).
Figures 3 and 4 are for the flexural strength (FS) and modulus (FM) of control and IMQ containing polymers. From Figs. 3 and 4, it could be seen that only polymers, which contained 5 wt% of IMQ, had nearly the same FS and FM as the control polymer (P > 0.05), all of the other IMQ containing polymers had lower FS and FM than the control polymer (P < 0.05). With the same content of IMQ, the alkyl chain length of IMQ had no influence on the FS and FM of relevant polymers (P > 0.05).
Results of biofilm inhibition test are summarized in Tables 1, 2, and 3. When content of IMQ was 5 wt%, only IMQ-16 containing polymer accumulated less bacteria on its surface than control polymer did (Table 1, P < 0.05). After increasing the content of IMQ to 10 and 20 wt%, amount of bacteria recovered from the surfaces of IMQ containing polymers were all less than the amount of bacteria recovered from the surface of control polymer (Tables 2, 3, P < 0.05), and the amount of bacteria recovered from the surfaces of IMQ-12, IMQ-14, and IMQ-16 containing polymers were nearly the same (P > 0.05), which were less than the amount of bacteria recovered from the surface of IMQ-18 containing polymer (P < 0.05).
4 Discussion
Incorporation of QAM into dental materials is an effective way to endow dental materials with long-lasting antibacterial activity [29, 30]. However, an appropriate concentration of QAM in the dental materials is very important, because insufficient QAM would limit the antibacterial activity of dental materials [20, 23, 24], and excess QAM would disrupt the mechanical properties of dental materials [20, 22, 25]. In this work, we synthesized a series of novel QAMs as antibacterial agents of dental materials, and tried to find suitable concentrations of them in the dental resin to keep the balance between antibacterial activity and mechanical properties.
As an crucial factor in determining the mechanical performance of dental materials [31, 32], the degree of DC of experimental resins was studied. The results of DC (Fig. 2) showed that all of IMQ containing polymers had nearly the same DC as control polymer (P > 0.05), which means that all of IMQs have no negative effect on the photopolymerizablity of the relevant dental resin within the concentration range of 5–20 wt%.
Even having the same DC, most of the IMQ containing polymers had a lower FS and FM than the control polymer (P < 0.05) except for the polymers containing 5 wt% of IMQ which had nearly the same FS and FM as the control polymer (P > 0.05). It has been already reported that when the concentration of QAMs was beyond a certain limit, mechanical strength could be decreased significantly [5, 6, 20]. In this work, 10 wt% has already exceeded the concentration limit for the synthesized IMQs. Upon the current work, only in the terms of FS and FM, the optimal concentration of IMQs in bis-GMA/TEGDMA dental resin will be a value between 5 and 10 wt%.
Even though the present ecological plaque hypothesis emphasizes that non-mutans bacteria may be the key microorganisms responsible for maintaining dynamic stability on tooth surface, S. mutans has a central role in the initiation of dental caries on enamel and root surface [33]. Therefore, a single-species biofilm model with S. mutans as the testing organism was used to evaluate the antibacterial property of the IMQ containing polymers. It is well known that QAM containing antimicrobial polymers kill bacteria on contact by causing the bacterial cell to burst and there are four main elementary processes for antibacterial polymer to kill bacteria: (1) adsorption onto the negatively charged bacterial cell surface; (2) diffusion through the cell wall; (3) binding to the cytoplasmic membrane; (4) disruption of the cytoplasmic membrane, release of cytoplasmic constituents and cell death [18, 34, 35]. Because the step (3) and (4) can be promoted when the length of the alkyl side chain increases [34, 36], lots of works have found that antibacterial activity of QAM containing dental polymer was enhanced when the alkyl side chain was increased [6, 18, 37]. However, in this work, the IMQ-18 containing polymer, which had the longest alkyl side chain, had the weakest antibacterial activity, and the IMQ-16 containing polymer was found to have the strongest antibacterial activity. It is noteworthy that in most of the recent works about QAM containing dental polymers [6, 18, 37], the longest alkyl side chain length of studied QAM was only 16, so the conclusion about the influence of the alkyl side chain length on antibacterial activity was only suitable for the length equal to or less than 16. In some other studies, the investigators have shown that the antibacterial activity of QAMs will decrease when the alkyl side chain length reaches 18 or more [38, 39]. Moreover, the second step of the elementary processes for antibacterial polymer to kill bacteria will be weakened when the alkyl chain length become longer [36]. Therefore, the IMQ-18 containing polymer had the weakest antibacterial activity in this research.
In the aspect of antibacterial activity, IMQ-16 might be the best antibacterial agent because 5 wt% of IMQ-16 in the dental polymer has already contributed to a polymer with certain antibacterial activity.
5 Conclusion
Four quaternary ammonium dimethacrylate monomers were successfully synthesized and applied into a bis-GMA/TEGDMA dental resin as antibacterial agents. All of the IMQs may be used in dental resin composites with an antibacterial activity after their concentration increased to 10 wt% or more. According to the results of mechanical and antibacterial properties, IMQ-16 should be the best choice in this research and its optimal concentration in dental resin would be in the range of 5–10 wt%.
References
He J, Liu F, Luo Y, Jia D. Synthesis and characterization of a dimethacrylates monomer with low shrinkage and water sorption of dental application. J Appl Polym Sci. 2012;125:114–20.
He J, Söderling E, Vallittu PK, Lassila LVJ. Investigation of double bond conversion, mechanical properties, and antibacterial activity of dental resins with different alkyl chain length quaternary ammonium methacrylate monomers (QAM). J Biomater Sci Polym Ed. 2013;24:565–73.
Kidd EAM. Caries diagnosis within restored teeth. Adv Dent Res. 1990;4:10–3.
Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. J Biomed Mater Res B. 2012;100B:1151–62.
Weng Y, Guo X, Chong VJ, Howard L, Gregory RL, Xie D. Synthesis and evaluation of a novel antibacterial dental resin composite with quaternary ammonium salts. J Biomed Sci Eng. 2011;4:147–57.
Weng Y, Howard L, Guo X, Chong VJ, Greogry RL, Xie D. A novel antibacterial resin composite for improved dental restoratives. J Mater Sci. 2012;23:1553–61.
Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Prim Dent Care. 2001;8:5–11.
Yamamoto K, Ohashi S, Aono M, Kokubo T, Yamada I, Yamauchi J. Antibacterial activity of silver ions implanted in SiO2 filler on oral Streptococci. Dent Mater. 1996;12:227–9.
Osinaga PW, Grande RH, Ballester RY, Simonato MR, Delgado Rodrigues CR, Muench A. Zinc sulfate addition to glass-ionomer-based cements: influence on physical and antibacterial properties, zinc and fluoride release. Dent Mater. 2003;19:212–7.
Shay DE, Allen TJ, Mantz RF. The antibacterial effects of some dental restorative materials. J Dent Res. 1956;35:25–32.
Al-Musallam TA, Evans CA, Drummond JL, Matasa C, Wu CD. Antimicrobial properties of an orthodontic adhesive combined with cetylpyridinium chloride. Am J Orthod Dentofac Orthop. 2006;129:245–51.
Jedrychowski JR, Caputo AA, Kerper S. Antibacterial and mechanical properties of restorative materials combined with chlorhexidines. J Oral Rehabil. 1983;10:373–81.
Wilson SJ, Wilson HJ. The release of chlorhexidine from modified dental acrylic resin. J Oral Rehabil. 1993;20:311–9.
Waltimo T, Luo G, Samaranayake LP, Vallittu PK. Glass fiber-reinforced composite laced with chlorhexidine digluconate and yeast adhesion. J Mater Sci. 2004;15:117–21.
Lahdenperä MS, Puska MA, Alander PM, Waltimo T, Vallittu PK. Release of chlorhexidine digluconate and flexural properties of glass fiber reinforced provisional fixed partial denture polymer. J Mater Sci. 2004;15:1349–53.
Imazato S, Torii M, Tsuchitani Y. Immobilization of an antibacterial component in composite resin. Dent Jpn. 1993;30:63–8.
Xiao Y-H, Chen J-H, Fang M, Xing X-D, Wang H, Wang Y-J, et al. Antibacterial effects of three experimental quaternary ammonium salt (QAS) monomers on bacteria associated with oral infections. J Oral Sci. 2008;50:323–7.
Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent Mater. 2011;27:487–96.
Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent Mater. 2012;28:219–28.
Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. J Biomed Mater Res. 2012;100B:1151–62.
He J, Söderling E, Österblad M, Vallittu PK, Lassila LVJ. Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules. 2011;16:9755–63.
Liang X, Huang Q, Liu F, He J, Lin Z. Synthesis of novel antibacterial monomers (UDMQA) and their potential application in dental resin. J Appl Polym Sci. 2013;129:3373–81.
Huang L, Xiao YH, Xing XD, Li F, Ma S, Qi LL, et al. Antibacterial activity and cytotoxicity of two novel cross-linking antibacterial monomers on oral pathogens. Arch Oral Biol. 2011;56:267–373.
He J, Söderling E, Lassila LVJ, Vallittu PK. Incorporation of an antibacterial and radiopaque monomer into dental resin system. Dent Mater. 2012;28:e110–7.
He J, Söderling E, Vallittu PK, Lassila LVJ. Preparation and evaluation of dental resin with antibacterial and radio-opaque functions. Int J Mol Sci. 2013;14:5445–60.
Ebi N, Imazato S, Noiri Y, Ebisu S. Inhibitory effects of resin composite containing bactericide-immobilized filler on plaque accumulation. Dent Mater. 2001;17:485–91.
Tanner J, Robinson C, Söderling E, Vallittu P. Early plaque formation on fibre-reinforced composites in vivo. Clin Oral Investig. 2005;9:154–60.
Lassila LV, Garoushi S, Tanner J, Vallittu PK, Söderling E. Adherence of Streptococcus mutans to fiber-reinforced filling composite and conventional restorative materials. Open Dent J. 2009;3:227–32.
Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73:1437–43.
Xiao Y-H, Ma S, Chen J-H, Chai Z-G, Li F, Wang Y-J. Antibacterial activity and bonding ability of an adhesive incorporating an antibacterial monomer DMAE-CB. J Biomed Mater Res. 2009;90(2):813–7.
Alshali RZ, Silikas N, Satterthwaite JD. Degree of conversion of bulk-fill compared to conventional resin-composites at two time intervals. Dent Mater. 2013;29:e213–7.
Ferracane J, Greener E. The effect of resin formulation on the degree of conversion and mechanical properties of dental restorative resins. J Biomed Mater Res. 1986;20:121–31.
Tanzer JM, Livingston J, Thompson AM. The microbiology of primary dental caries in human. J Dent Educ. 2001;65:1028–37.
Lu G, Wu D, Fu R. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React Funct Polym. 2007;67:355–66.
Rawlinson LA, Ryan SM, Mantovani G, Syrett JA, Haddleton DM, Brayden DJ. Antibacterial effects of poly(2-(dimethyamino ethyl)methacrylate) against selected gram-positive and gram-negative bacteria. Biomacromolecules. 2010;11:443–53.
Salahuddin N, Badr B, Abdeen R. Synthesis and antimicrobial activity of biocidal polymer–montorillonite nanocomposites. Polym Int. 2012;61:99–110.
Weng Y, Guo X, Gregory RL, Xie D. Preparation and evaluation of an antibacterial dental cement containing quaternary ammonium salts. J Appl Polym Sci. 2011;122:2542–51.
Jono K, Takayama T, Kuno M, Higashide E. Effect of alky chain length of benzalkonium chloride on the bactericidal activity and binding to organic materials. Chem Pharm Bull. 1986;34:4215–24.
Thorsteinsson T, Másson M, Kristinsson KG, Hjálmarsdóttir MA, Hilmarsson H, Loftsson T. Soft antimicrobial agents: synthesis and activity of labile environmentally friendly long chain quaternary ammonium compounds. J Med Chem. 2003;46:4173–81.
Acknowledgments
We would like to greatly thank biomedical research technician Oona Hällfors for her help in biofilm inhibition testing. We also thank the support supplied by the National Science Foundation of Guangdong Province (8151064101000048, S2011020001452), China, and the Fundamental Research Funds for the Central Universities (2014ZM0006), China. Study belongs to activity of BioCity Turku Biomaterials Research Program (www.biomaterials.utu.fi).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, X., Söderling, E., Liu, F. et al. Optimizing the concentration of quaternary ammonium dimethacrylate monomer in bis-GMA/TEGDMA dental resin system for antibacterial activity and mechanical properties. J Mater Sci: Mater Med 25, 1387–1393 (2014). https://doi.org/10.1007/s10856-014-5156-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5156-x