Abstract
This paper focuses on the analysis and synthesis of two new gemini or bis-quaternary ammonium dimethacrylate compounds (bis-QAC) with different spacer lengths (DMBB and DMBH) for the first time. These innovative quaternary ammonium-based resin monomers can be employed as effective dental adhesives, due to their antibacterial properties against the Streptococcus mutans bacteria (bacteria in dental plaque). In this research, this blend of monomers is incorporated into a commercial adhesive (1 wt% bis-QAC and 99 wt% Tetric N-Bond). The chemical structures and thermal behavior of all prepared compounds are confirmed by FTIR, 1H-NMR, 13C-NMR and TGA/DTG. Moreover, mechanical properties, degree of conversion (monomer to polymer) and cell cytotoxicity of the samples containing an antibacterial agent are compared to commercial adhesives. Finally, experimental results illustrate that the minimum inhibitory concentration of adhesives with new bis-QAC is significantly lower than samples without an antimicrobial monomer. Moreover, the mixture of gemini QAC with adhesive does not demonstrate any adverse effect on the degree of conversion and bond strength of the experimental adhesive. Furthermore, specimens of DMBH indicate a higher antibacterial characteristic, despite the reduction in the cell viability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been a growing interest in the safe examination of microorganisms as a part of human healthcare studies. This is due to several serious infections which are also reported [1,2,3]. Dental caries is an infectious disease caused by cariogenic bacteria. Therefore, attempts to produce restorative materials with antibacterial effects have been seriously researched in dental materials science [4, 5]. Currently, utilization of composite resins to treat dental caries is dramatically increasing by virtue of their esthetic standards and appropriate performance [6, 7]. Nevertheless, these restorative materials store more dental plaque in comparison with other compounds, leading to secondary caries; thus, this is an important challenging issue in dental treatment. This problem is partly resolved by the use of a combination of fillers and antibacterial material. A conventional technique for providing antibacterial dental materials has been developed based on enriching them with antibacterial agents, such as silver ion and fluoride. However, applying these agents has been accompanied by unfavorable consequences such as toxicity and short-term effects. Thus nowadays, scientific researchers have become focused on the production of compounds that possess special advantages, such as good antibacterial effects, along with maintaining the mechanical properties of resin, low cytotoxicity, economy and being easily available [8, 9]. Quaternary ammonium compounds (QAC) have been found to have broad applications in medicine [10], in dental materials [11,12,13] and in industry, on account of their low toxicity and a wide range of antimicrobial activities [14]. They are salts of quaternary ammonium cations with an anion. These compounds consist of four organic groups attached to a central nitrogen atom that form the cationic part, coupled with an anionic moiety which is usually chloride or bromide [15]. The QAC structure is composed of a hydrophobic section with an oily and water-repellent character. This oily part has some special characteristics, such as the capability of surface neutralization, good wetting and adhesive behavior [2]. The QAC produce a stable antibacterial agent because of a strong covalent bond with the resin. Hence, they are capable of effective immobilization in resins without a decrease in their antibacterial nature and mechanical features over time [4, 16, 17]. Previous studies have revealed that the addition of antibacterial agents with branches of methacrylate usually does not influence the micro-tensile bond strength or the degree of conversion [12, 18, 19]. Additionally, according to the reports, samples containing quaternary ammonium methacrylate displayed the lowest decreases in bond strength compared to those without an antibacterial agent, after 6 and 12 months of storage in distilled water [12]. The QAC are active against a series of bacteria and fungi, even at low amounts. The antibacterial activity of quaternary ammonium salts arises from the mutual electrostatic interaction between the positive charge of a nitrogen atom and the negatively charged bacterial membrane cell, and in this synthesized monomer, the quaternary ammonium unit and methyl acrylic moiety act as the polar head and polymerizable part, respectively. These structural features are responsible for the antimicrobial effect of the gemini quaternary ammonium monomer [20, 21]. This antimicrobial impact mainly arises from their long alkyl chains as hydrophobic fragments and therefore the ability to penetrate the hydrophobic bacterial membrane [22]. On the other hand, the presence of the double bond relating to the methacrylate in this structure provides the possibility of chemical binding to the backbone of dental resin. The C=C bonds of QAC have analogous reactivity to the comonomers of bis-GMA and TEGDMA [24]. Based on previous investigations, usually mono-QAC with a branch of methacrylate has been employed as an antibacterial agent [23]. Additionally, the application of bis-QAC has been remarkably increased over the past years [12, 24, 25]. Gemini or bis-quaternary ammonium salts are a class of surfactants made of two symmetric quaternary ammonium groups linked via different spacers; therefore, they have a greater surface activity and more antibacterial potency than conventional traditional mono-QAC. Accordingly, in the present study, a series of novel bis-quaternary ammonium salts based on dimethacrylates bearing different spacer lengths were synthesized as expedient antimicrobial dental monomers and were incorporated into the dental materials to obtain dental antibacterial adhesives.
Experimental
Materials
The dimethylaminoethyl methacrylate (DMAEMA), 1,4-dibromobutane and 1,6-dibromohexane, isopropyl alcohol and diethyl ether were purchased from the Merck Company. All materials were used without any further purification.
Experimental method of characterization
The NMR spectra were recorded with a Bruker DRX-500 AVANCE instrument (500.1 MHz for 1H, 125.0 MHz for 13C). The spectra were measured in DMSO-d6 as a solvent. The IR spectra of synthesized materials were carried out in a wave number range of 400–4000 cm−1 at a resolution of 4 cm−1 on a (Nicolet IS10.USA) spectrophotometer using KBr disks. The TG/DTG technique was performed in a nitrogen atmosphere in the temperature range from 25° to 600 °C in thermal analysis apparatus (Perkin Elmer, Pyris 1). A heating rate of 10 °C/min was chosen for the measurements.
General method for the synthesis of new bis-quaternary ammonium dimethacrylates
Some dimethylaminoethyl methacrylate (DMAEMA, 0.2 mol, 33.44 g) was reacted with 1,4-dibromobutane (21.59 g) or 1,6-dibromohexane (dibromoalkane, 0.1 mol) combined with 100 ml of isopropyl alcohol in a round-bottomed flask under a nitrogen atmosphere and refluxed for 2 h at 80 °C. After completion of the reaction, the mixture was cooled to room temperature, then filtered and washed with 50 ml of diethyl ether several times and then dried in a vacuum oven at 40 °C for 4 h to attain the product. The yields of the reactions for compound 1 (DMBB) and compound 2 (DMBH) were 95.6 and 97.3%, respectively. The structures of the obtained bis-quaternary ammonium dimethacrylate (bis-QAMS) are shown in Scheme 1.
Preparation of QAMS-containing dental bonding (1:99)
In this study, a commercially available adhesive resin (Tetric N-Bond) without any effective antibacterial component was used as the control group and a mixture of 1 wt% QAMS and 99 wt% Tetric N-Bond was prepared as experimental groups. In order to achieve a homogeneous adhesive, the mixture was placed into an oven at 50 °C and was stirred several times manually. The samples were kept in the dark before experimentation.
Measuring the degree of conversion (DC) by FTIR spectroscopy
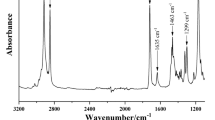
The purpose of this test is to measure the degree of conversion of the monomers to polymers. Inadequate polymerization bonding lowers the quality, and when a monomer is not polymerized properly, it exhibits toxic effects. A FTIR device was used to assess the degree of carbon–carbon double bond conversion, before and after polymerization (curing process). The time of light curing was 20 s. The FTIR spectra of Tetric N-Bond as a control group and additionally two newly synthesized antibacterial monomers were monitored to determine DC percentage (QAMS) (NICOLET IS10, USA). The FTIR spectra of materials were obtained at room temperature in KBr pellets over the range of 400–4000 cm−1 with 16 scans. In this work, there are two peak important absorbance values: (1) aliphatic peak of carbon–carbon double bonds (C=C) at the frequency of 1635 cm−1 that will change after curing; (2) aromatic peak attributed to the polymerized and unpolymerized monomer at 1608 cm−1 (C=C) which is almost constant and therefore acts as an internal standard (IS). Comparing the ratios of absorbance peak intensities ascribed to (C=C) before and after polymerization represents the unreacted carbon double bonds, and accordingly, the degree of conversion can be explained by the following equation [19, 26].
Mechanical properties test (teeth preparation and micro-tensile bond strength evaluation)
Thirty-five extracted human third molars were collected after obtaining the donors’ information. The caries-free teeth were stored in 0.5% chloramine to fumigate them, and then, they were transferred to distilled water. After cutting the roots, wet grinding of the occlusal enamel with 180-grit SiC paper was carried out to form a flat dentin surface without enamel on each tooth and the exposed dentin surface was polished with 600-grit wet silicon carbide paper to create a standardized smear layer. Table 1 shows the dental restorative materials used in the present study. The bonding agent processing was performed according to the manufacturer’s instructions. At the first step, the dentin surface was etched with 37% phosphoric acid gel for 15 s, rinsed with water for 15 s and then dried until it was still slightly wet. Two bonding coats were applied and the adhesive was light-cured by LED light curing for 20 s (10 s for each coat). This method was repeated with three bonding antibacterial agent groups (two experimental groups) incorporated into the dental adhesive and control adhesive, as indicated in Table 2. Resin composite buildups were made with 2-mm-thick increments of resin (Z250). Each layer of composite also was light-cured for 20 s and afterward stored in deionized water at 37 °C for 24 h. After storage, samples were cut vertically to the bonding surface by means of a CNC device where consequently several beam-shaped sticks with a cross surface of about 1.0 mm2 were created. The next step involved an aging process through thermocycling [500 cycles (20 20 20), 500 cycles (5–55 °C)]. Finally, the bond strength values were measured using a universal testing machine (SANTAM-STM-20). The beams were attached to the micro-tensile tester with a cyanoacrylate adhesive. They were then stressed to failure at a crosshead speed of (0.5) mm/min. The bond strength values were calculated with the divided force at failure by the bonded cross-sectional surface area and were registered in MPa. Data were analyzed using one-way ANOVA and the Games–Howell’s post hoc test (p = 0.05).
Antibacterial test
Minimum inhibitory concentration (MIC)
In this test, we used Streptococcus mutans bacteria. A starting solution was prepared in this method, using 15 ml of stock S. mutans cultured in 15 ml of brain heart infusion (BHI), together with the addition of sucrose (0.2%), and incubated at 37 °C and 5% CO2, and they were kept for 1 day. The obtained QAMS was sterilized by gamma ray. After sterilization, each group of uncured monomer was dissolved in BHI at a concentration of 200 mg/ml, and from this suspension, serial dilutions were prepared into 1 ml volumes of BHI broth (the ratio of 1/2, 1/4, 1/8, 1/16, 1/32, etc.). Then, 50 µl of suspension with specified serial dilution was transferred into each well in a 96-well plate filled with 2 × 106 CFU/ml (volume of 13 µl) of cultured bacteria, coupled with the addition of 50 µl of inoculum into the wells. After 48-h incubation at + 37 °C, the results were checked and the absorbance was measured (A550). The MIC value was the lowest concentration of monomer where no turbidity could be observed in the wells compared to the control group.
Agar disk diffusion test (ADT)
Briefly, to evaluate antimicrobial activity, disks involving QMAS and bonding were molded and then light-cured for 20 s for all groups. The disks had a diameter of 6 mm with a thickness of 1 mm, and every disk was sterilized by gamma ray. The Streptococcus mutans bacteria suspension was prepared according to 0.5 McFarland standards (1.5 × 108). A volume of 10 μl of bacterial suspension was spread on mitis salivarius agar. After that, the disks were placed on the surface. The plates were incubated for 48 h at 37 °C in order to measure the inhibition zone diameters around each disk with a caliper.
Cytotoxicity assay (MTT assay)
Assessment of the cell viability/cytotoxicity is one of the essential experiments for each novel material or agent like polymers which are to be used in humans and animals. The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay is a colorimetric assay that measures the enzymatic reduction of MTT a yellow tetrazole, to purple crystals of formazan. This method was used to estimate cell metabolic activity of antibacterial monomers. Human foreskin fibroblast (HFF2) cells (Department of Cell Bank, Pasteur Institute of Iran) were cultured at a density of 1× 104 cells/well in 96-well plates by the use of fibroblast medium (RPMI 1640) with growth supplement including 10% fetal bovine serum (FBS) and 1% Pen strep. Disks were made according to the instructions explained in “Agar disk diffusion test” section. Every disk was sterilized by gamma ray. For preparation extracts of specimens, disks were transferred to the cell culture medium and were incubated at 37 °C in pH 7 for 24 h. The extracts were filtered through 0.22-mm cellulose acetate filters (Millipore) and used for the cytotoxicity test; RPMI 1640 medium was removed, replaced with the extracts, added to each tested well of the 96-well plates and incubated for 24 and 72 h. Subsequently, 100 μl of medium in 10 μl of MTT solution (5 mg of MTT/ml of PBS) was added to the wells. After 4 h of incubation at 37 °C in darkness, the purple formazan precipitate was formed and then 100 μl per well of dimethyl sulfoxide (DMSO) was added. The absorbance values of the solutions were measured by an ELISA test at 570 nm.
Results and discussion
Characterization of the product: Fourier transform infrared (FTIR) analysis and NMR spectroscopy
FTIR
Figure 1 shows the FTIR spectra of the prepared compounds. In the FTIR spectra of DMBH, the band at 3005 cm−1 corresponds to C=C–H stretching vibration of methacrylate units; 2800–2970 cm−1, aliphatic C–H stretching vibration; 1712 cm−1, C=O stretching vibration of methacrylate units; 1632 cm−1, C=C stretching vibration of methacrylate units; 1460 cm−1, bending vibration of CH2 units; 1322 cm−1, bending vibration of CH3 units; 1298 cm−1, C–N stretching vibration of quaternary ammonium; 1170 cm−1, C–O stretching vibration.
In the FTIR spectra of DMBB, the band at 3020 cm−1 corresponds to C=C–H stretching vibration of methacrylate units; 2900–2970 cm−1, aliphatic C–H stretching vibration; 1716 cm−1, C=O stretching vibration of methacrylate units; 1635 cm−1, C=C stretching vibration of methacrylate units; 1456 cm−1, bending vibration of CH2 units; 1322 cm−1, bending vibration of CH3 units; 1298 cm−1, C–N stretching vibration of quaternary ammonium; 1170 cm−1, C–O stretching vibration.
NMR
Figure 2 shows the NMR spectra of the prepared compounds. The bis-quaternary ammonium dimethacrylate monomers were analyzed through 1H NMR and 13C NMR spectroscopy.
NMR of DMBB
The chemical structure of DMBB was identified with 1H and 13C NMR spectral data. Observing 1H NMR spectrum of DMBB, 1H chemical shifts of vinyl protons in trans and cis position obviously indicated that the trans isomer emerged more downfield (δ 6.09 ppm) than that for the cis isomer (δ 5.77 ppm). The protons of methylene attached to the ester group appeared at δ 4.54 ppm. Moreover, 1H chemical shifts related to methyl and methylene groups appended to the quaternary ammonium were assigned at δ 3.13 and 3.74 ppm, respectively. The singlet peak at δ 1.91 ppm can be ascribed to the protons of methyl substituent of vinyl carbon. Protons of the middle methylene groups (between two ammonium groups) appeared at δ 1.72 and 3.47 ppm. The 13C-NMR spectrum of DMBB showed a signal at δ 166.33 ppm which is relevant to carbonyl of ester (methacrylate unit). The chemical shifts of vinyl carbons were assigned at δ 135.78 and 127.20 ppm. In addition, the methylene carbon attached to the ammonium group appeared at δ 63.41 ppm. The carbon of methylene attached to the ester group appeared at δ 58.56 ppm. Carbons of the middle methylene groups (between two ammonium groups) appeared at δ 62.23 and 19.42 ppm. Methyl groups of the ammonium unit were observed at δ 51.04 ppm. The lowest amount of chemical shift (δ 18.42 ppm) corresponded to methyl substituent bonded to vinyl carbon.
NMR of DMBH
The chemical structure of DMBH was identified with 1H and 13C NMR spectral data. Observing 1H NMR spectrum of DMBH, 1H chemical shifts of vinyl protons in trans and cis position obviously indicated that the trans isomer emerged more downfield (δ 6.08 ppm) than that for the cis isomer (δ 5.76 ppm). The protons of methylene attached to the ester group appeared at δ 4.50 ppm. Moreover, 1H chemical shifts related to methyl and methylene groups appended to the quaternary ammonium were assigned at δ 3.75 and 3.42 ppm, respectively. The singlet peak at δ 1.91 ppm can be ascribed to the protons of methyl substituent of vinyl carbon. Protons of the middle methylene groups (between two ammonium groups) appeared at δ 1.75 and 1.3 and 3.44 ppm. The 13C NMR spectrum of DMBH showed a signal at δ 166.76 ppm which is relevant to carbonyl of ester (methacrylate unit). The chemical shifts of vinyl carbons were assigned at δ 136.23 and 127.48 ppm. Furthermore, the methylene carbon attached to the ammonium group appeared at δ 64.52 ppm. The carbon of methylene attached to the ester group appeared at δ 59.01 ppm. Carbons of the middle methylene groups (between two ammonium groups) appeared at δ 62.53 and 22.38 and 25.95 ppm. Methyl groups of the ammonium unit were observed at δ 51.42 ppm. The lowest amount of chemical shift (δ 18.79 ppm) corresponded to methyl substituent bonded to vinyl carbon.
The results of FTIR, 1H NMR and 13C NMR analyses confirmed that bis-quaternary ammonium dimethacrylate monomers were successfully synthesized.
Thermogravimetry (TG)/derivative thermogravimetry (DTG)
The TG/DTG technique discusses the analysis of the thermal properties of synthesized compounds. The study of thermal behavior is one test for the characterization of polymers. Although in this study these materials are not exposed to high temperatures, these synthesized materials may be used in other cases where thermal stability is important; thus, this investigation was carried out. The heat resistance of these compounds is of great importance because the resulting thermal stability reduces volatility and consequently an antibacterial agent added to dental bonding will have a longer-lasting antibacterial effect (e.g., when hot drinks and food are consumed, or when mixing an antibacterial agent with bonding, which is accompanied by a temperature rise).
Figure 4 shows the TG/DTG curve for commercial bonding (control group) and the antibacterial agent added commercial bonding. The findings showed that the addition of the antimicrobial agent to the bonding agent resulted in a highly cross-linked three-dimensional (3D) network, and therefore, these lead to thermal stability compared to that of the control group. The TG/DTG curve of the synthesized monomers (Fig. 3) shows the DMBH monomer has more thermal stability than the DMBB due to its higher molecular weight. However, after incorporating these monomers to the bonding (Fig. 4) and performing polymerization, the DMBH-containing bonding showed a lower thermal stability than the DMBB-containing bonding. This is explained by the fact that the DMBH has lower cross-link density and will create a 3D network with more hollow space. As a result, it exhibited a lower softening point and more degradation to heat.
Degree of conversion
Figure 5 displays comparative FTIR spectra of the adhesive including bis-QAC and a control group under cured and uncured conditions. The average values of data points obtained from the DC (%) of the samples are reported in Fig. 5.
The conversion of monomers to polymers is very important, and a low amount of conversion will lead to an increase in toxicity and a decrease in strength [31, 32]. Incompletely polymerized dental material contains partially unbounded monomers released directly in the oral environment [33]. The results obtained from this test indicate that the addition of QACs (at a concentration of 1%) to the commercial adhesive (as a control group) did not significantly affect the degree of conversion of monomer to polymer; therefore, there was suitable curing.
Bond strength
The results of the bond strength are presented in Fig. 6. The QACs in combination with the adhesive showed a slightly higher bond strength compared to the control adhesive, although the data obtained showed no statistically significant difference among groups which contained antibacterial agents and commercial adhesives (p > 0.05), indicating that the incorporation of the QACs’ monomers into the dental adhesive did not adversely influence the bonding properties of the carrier material. In previous studies, antibacterial compounds exhibit reduced bond strength [29], but our results indicate that the addition of ammonium quarts not only did not have any destructive effect on bond strength, but also enhanced mechanical properties (bond strength). This can be due to the presence of two groups of methacrylates on both sides of the molecule.
Antibacterial
Evaluation of the antibacterial activity in this study was performed using MIC (minimum inhibitory concentration) and the inhibition zone diameter measurement against the Streptococcus mutans bacteria, which are bacteria in dental plaque. The assessment of minimal inhibitory concentrations provides a method for measuring the amount of microbial activity of bis-quaternary ammonium salts with different spacers ((CH2)4 or (CH2)6). The results of the antibacterial activity are summarized in Table 3.
Gemini quaternary ammonium salts (QAS) have an unbeatable structure and generally exhibit stronger antibacterial potency and antifungal activity than the mono-QAS [25, 27]. Mono-quaternary ammonium monomer has antibacterial activity [30], but our bis-quaternary ammonium monomer has higher antibacterial activity than it, even at very low concentrations (only 1%), while in other research it has been shown to amount to 10% or more [18]. Its increased antibacterial effect is related to the structure of bis-quaternary ammonium, which enhances the contact surface with the bacteria due to the enlargement of the molecule. This novel monomer comprises of two methacrylate and two ammonium parts that increase antimicrobial properties in comparison with the mono-structure. This structural design is more effective in connecting with the cell surface because it has two relatively long chains on both sides, which makes the hydrophobic section larger and also leads to the more efficient attack on the wall of the bacterial membrane. As displayed in “Results,” according to the influence of the monomer size on the antimicrobial features, the DMBH group demonstrates higher antibacterial activity on account of the larger spacer. Previous studies have evaluated antibacterial activity of gemini QAC with different alkyl chain and spacer lengths [24]. Research studies have also elucidated that gemini QAS with longer spacers displayed more antibacterial activity in comparison with samples with short spacers [24, 28]. The results demonstrated that the antibacterial effect of compounds containing six methylene groups as spacers (DMBH) was stronger than gemini QAC with four methylene groups as spacers (DMBB).
MTT
Material with biomedical applications must have the lowest cytotoxicity to host the tissues. Figure 7 shows the result of the cytotoxicity (MTT assay) of the samples, and Figs. 8 and 9 show microscopy images of viable cells after 24 and 72 h.
Figures 8 and 9 show microscopic photographs of the cell viability in contact with the commercial substance (control group) and the synthesized antibacterial compounds with the bonding group. A large percentage of cells have been destroyed by the commercial material, although the antibacterial compound has also added a little to this destruction. However, after the cure process, toxicity is reduced, which is presented by calculating the cell viability in percent as shown in Fig. 7.
Moreover, results showed that cured resins including 1 wt% DMBB have considerable biocompatibility and a high percentage of cell viability compared with the adhesive as the control sample (without antibacterial agent). However, under the same conditions, in the presence of 1 wt% gemini DMBH reduced cell viability was observed. Samples containing antibacterial agents disclosed cytotoxicity against mammalian cells. Thus, it is important to establish a balance between antibacterial effects and cytotoxicity for some applications. Accordingly, this study reports that bis-QAC, a novel antibacterial additive for bonding systems, did not show any adverse influence on micro-tensile bond strength or on the degree of conversion of a widely used commercial adhesive. However, mixing gemini QAC with adhesive demonstrated slight increases in bond strength value compared to the control adhesive (commercial bonding). Presumably, this increase corresponds to the presence of the methacrylate groups.
Conclusions
A new bis-quaternary ammonium dimethacrylate monomer was synthesized, characterized and utilized as an antibacterial agent in an adhesive system for dental restoration. Samples including DMBH indicated a higher antibacterial characteristic than adhesives containing DMBB, but exhibited reduced cell viability. A mixture of synthesized antibacterial compound with adhesive did not exhibit any adverse effect on the degree of conversion and bond strength of the experimental adhesive. Therefore, this mixture has promising applications in a wide range of bonding agents.
References
Pal C, Bengtsson-Palme J, Kristiansson E, Larsson DJ (2015) Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genom 16:964
Pupo YM, Farago PV, Nadal JM, Esmerino LA, Maluf DF, Zawadzki SF, Michél MD, Santos FA, Gomes OM, Gomes JC (2013) An innovative quaternary ammonium methacrylate polymer can provide improved antimicrobial properties for a dental adhesive system. J Biomater Sci Polym Ed 24:1443–1458
Qiu T, Zhang L, Xing XD (2014) Synthesis and antibacterial activities of novel polymerizable gemini quaternary ammonium monomers. Des Monomers Polym 17:726–735
Imazato S, J-h Chen, Ma S, Izutani N, Li F (2012) Antibacterial resin monomers based on quaternary ammonium and their benefits in restorative dentistry. Jpn Dent Sci Rev 48:115–125
Ribeiro J, Ericson D (1991) In vitro antibacterial effect of chlorhexidine added to glass-ionomer cements. Scand J Dent Res 99:533–540
Jaymand M, Lotfi M, Lotfi R (2016) Functional dendritic compounds: potential prospective candidates for dental restorative materials and in situ re-mineralization of human tooth enamel. RSC Adv 6:43127–43146
Moszner N, Salz U (2007) Recent developments of new components for dental adhesives and composites. Macromol Mater Eng 292:245–271
Ge Y, Wang S, Zhou X, Wang H, Xu HH, Cheng L (2015) The use of quaternary ammonium to combat dental caries. Materials 8:3532–3549
Liang X, Huang Q, Liu F, He J, Lin Z (2013) Synthesis of novel antibacterial monomers (UDMQA) and their potential application in dental resin. J Appl Polym Sci 129:3373–3381
Gottenbos B, van der Mei HC, Klatter F, Nieuwenhuis P, Busscher HJ (2002) In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials 23:1417–1423
Cheng L, Zhang K, Melo MA, Weir M, Zhou X, Xu H (2012) Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J Dent Res 91:598–604
Pupo YM, Farago PV, Nadal JM, Simão LC, Esmerino LA, Gomes OM et al (2014) Effect of a novel quaternary ammonium methacrylate polymer (QAMP) on adhesion and antibacterial properties of dental adhesives. Int J Mol Sci 15:8998–9015
Chai Z, Li F, Fang M, Wang Y, Ma S, Xiao Y et al (2011) The bonding property and cytotoxicity of a dental adhesive incorporating a new antibacterial monomer. J Oral Rehabil 38:849–856
Song Y, Gao Y, Wan X, Luo F, Li J, Tan H, Fu Q (2016) Dual-functional anticoagulant and antibacterial blend coatings based on gemini quaternary ammonium salt waterborne polyurethane and heparin. RSC Adv 6:17336–17344
Tezel U, Pavlostathis SG (2015) Quaternary ammonium disinfectants: microbial adaptation, degradation and ecology. Curr Opin Biotechnol 33:296–304
Cheng L, Weir MD, Zhang K, Arola DD, Zhou X, Xu HH (2013) Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J Dent 41:345–355
Imazato S (2009) Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dent Mater J 28:11–19
Li F, Weir MD, Xu HK (2013) Effects of quaternary ammonium chain length on antibacterial bonding agents. J Dent Res 92:932–938
Makvandi P, Ghaemy M, Mohseni M (2016) Synthesis and characterization of photo-curable bis-quaternary ammonium dimethacrylate with antimicrobial activity for dental restoration materials. Eur Polym J 74:81–90
Mathias LJ, Shemper BS, Alirol M, Morizur JF (2004) Synthesis of new hydroxylated monomers based on methacrylate, dimethacrylate, and tetramethacrylate michael adducts and photopolymerization kinetics of bulk cross-linkers. Macromolecules 37:3231–3238
Destais N, Adès D, Sauvet G (2000) Synthesis, characterization and biocidal properties of epoxy resins containing quaternary ammonium salts. Polym Bull 44:401–408
Tiller JC, Liao CJ, Lewis K, Klibanov AM (2001) Designing surfaces that kill bacteria on contact. Proc Natl Acad Sci USA 98(11):5981–5985
He J, Söderling E, Österblad M, Vallittu PK, Lassila LV (2011) Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules 16:9755–9763
Obłąk E, Piecuch A, Krasowska A, Łuczyński J (2013) Antifungal activity of gemini quaternary ammonium salts. Microbiol Res 168:630–638
Zhou F, Maeda T, Nagamune H, Kourai H (2004) Synthesis and antimicrobial characteristics of novel biocides, 1, 1′-(Decanedioyl) bis (4-methy1-4-alkylpiperazinium iodide) s with a gemini structure. Biocontrol Sci 9:61–67
Hoshika T, Nishitani Y, Yoshiyama M, Key WO, Brantley W, Agee KA et al (2014) Effects of quaternary ammonium-methacrylates on the mechanical properties of unfilled resins. Dent Mater 30:1213–1223
Shirai A, Sumitomo T, Yoshida M, Kaimura T, Nagamune H, Maeda T et al (2006) Synthesis and biological properties of gemini quaternary ammonium compounds, 5, 5′-[2, 2′-(α, ω-polymethylnedicarbonyldioxy) diethyl] bis-(3-alkyl-4-methylthiazolium iodide) and 5, 5′-[2, 2′-(p-phenylenedicarbonyldioxy) diethyl] bis (3-alkyl-4-methylthiazolium bromide). Chem Pharm Bull 54:639–645
Piecuch A, Obłąk E, Guz-Regner K (2016) Antibacterial activity of alanine-derived gemini quaternary ammonium compounds. J Surfactants Deterg 19:275–282
Gürgan S, Bolay S, Kiremitçi A (1999) Effect of disinfectant application methods on the bond strength of composite to dentin. J Oral Rehabil 26:836–840
Lu G, Wu D, Fu R (2007) Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethy-laminoethyl methacrylate. React Funct Polym 67:355–366
Pithon MM, Santos RL, Martins FO, Romanos MTV, Araújo MTS (2010) Evaluation of cytotoxicity and degree of conversion of orthodontic adhesives over different time periods. Mater Res 13:165–169
Lovell LG, Lu H, Elliott JE, Stansbury JW, Bowman CN (2001) The effect of cure rate on the mechanical properties of dental resins. Dent Mater 17:504–511
Goldberg M (2008) In vitro and in vivo studies on the toxicity of dental resin components: a review. Clin Oral Investig 12:1–8
Acknowledgements
The Research Council of the Islamic Azad University of Yazd is gratefully acknowledged for their financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manouchehri, F., Sadeghi, B., Najafi, F. et al. Synthesis and characterization of novel polymerizable bis-quaternary ammonium dimethacrylate monomers with antibacterial activity as an efficient adhesive system for dental restoration. Polym. Bull. 76, 1295–1315 (2019). https://doi.org/10.1007/s00289-018-2414-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2414-y