Abstract
Conventional polymethylmethacrylate (PMMA) cements and more recently Bisphenol-a-glycidyl dimethacrylate (BIS-GMA) composite cements are employed in procedures such as vertebroplasty. Unfortunately, such materials have inherent drawbacks including, a high curing exotherm, the incorporation of toxic components in their formulations, and critically, exhibit a modulus mismatch between cement and bone. The literature suggests that aluminium free, zinc based glass polyalkenoate cements (Zn-GPC) may be suitable alternative materials for consideration in such applications as vertebroplasty. This paper, examines one formulation of Zn-GPC and compares its strengths, modulus, and biocompatibility with three commercially available bone cements, Spineplex®, Simplex® P and Cortoss®. The setting times indicate that the current formulation of Zn-GPC sets in a time unsuitable for clinical deployment. However during setting, the peak exotherm was recorded to be 33°C, the lowest of all cements examined, and well below the threshold level for tissue necrosis to occur. The data obtained from mechanical testing shows the Zn-GPC has strengths of 63 MPa in compression and 30 MPa in biaxial flexure. Importantly these strengths remain stable with maturation; similar long term stability was exhibited by both Spineplex® and Simplex® P. Conversely, the strengths of Cortoss® were observed to rapidly diminish with time, a cause for clinical concern. In addition to strengths, the modulus of each material was determined. Only the Zn-GPC exhibited a modulus similar to vertebral trabecular bone, with all commercial materials exhibiting excessively high moduli. Such data indicates that the use of Zn-GPC may reduce adjacent fractures. The final investigation used the well established simulated body fluid (SBF) method to examine the ability of each material to bond with bone. The results indicate that the Zn-GPC is capable of producing a bone like apatite layer at its surface within 24 h which increased in coverage and density up to 7 days. Conversely, Spineplex®, and Simplex® P exhibit no apatite layer formation, while Cortoss® exhibits only minimal formation of an apatite layer after 7 days incubation in SBF. This paper shows that Zn-GPC, with optimised setting times, are suitable candidate materials for further development as bone cements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polymethlymethacrylate (PMMA) has been employed as a bone cement since the 1960s [1]. Originally used for the fixation of prostheses these cements have more recently been employed to stabilise compression fractures of the vertebrae [2–4], and to treat both vertebral tumors [5] and haemangiomas [6] by percutaneous vertebroplasty. Despite these successes, PMMA is beset with inherent drawbacks, including the risk of thermal and chemical necrosis to healthy bone tissue [7], impaired functioning of the immune system [8, 9] and a variety of systemic and cardiovascular reactions [10]. Given these deficiencies, research literature relating to the investigation of alternative materials for skeletal applications is abundant [11–19]. In addition to the much discussed calcium phosphates, an additional group of materials; Bisphenol-a-glycidyl dimethacrylate (BIS-GMA) resins are of significant interest.
BIS-GMA resins have been investigated for potential skeletal applications since the 1970s [20]. One commercially available brand; Cortoss® (Orthovita, Malvern, USA) is currently being marketed as bone void filler, for the stabilisation of compression fractures in vertebrae. In contrast to PMMA cements, Cortoss® benefits from a high degree of monomer conversion [21], decreasing the amount of leachable toxic monomer from the cement mantle. Additionally, this cement is reinforced with ceramic particles to stimulate bone apposition at the interface, and has improved interfacial bond strengths between implant and bone [22]. Cortoss® is currently undergoing clinical trials in the USA [23] for use as a bone void filler with particular emphasis on the augmentation of vertebral bodies. Notwithstanding the improvements Cortoss® offers, there remains a number of drawbacks associated with its use in skeletal applications including an excessively high exothermic reaction (63°C) relative to the threshold associated with thermal necrosis of healthy bone tissue (56°C) [24, 25]. Many studies have also documented a cytotoxic potency of BIS-GMA and triethyleneglycol-dimethacrylate (TEG-DMA) [26–30]. These components have also been associated with decreased recruitment of leukocytes to sites of inflammation as well as inducing deletions in DNA sequences resulting in increased mutation frequency [31].
Potential alternatives to PMMA and BIS-GMA bone cements are glass polyalkenoate cements (GPCs); formed by the reaction of an acid degradable alumino-silicate glass with an aqueous solution of polyalkenoic acid, usually polyacrylic acid (PAA) [32]. GPCs are bioactive materials [33] with mechanical properties similar to bone, and have an established record of success in dental applications. However, numerous cases of aluminium induced encephalopathy have been reported [34–37], due to the release of the neurotoxic Al3+ ion from the mantle of set GPCs in vivo. Subsequently, aluminium based GPCs were contraindicated for use in skeletal applications, particularly for procedures where the cement could come in contact with cerebrospinal fluid (CSF). The authors have previously reported the development of aluminium free GPCs for consideration as skeletal materials [14–19]. These materials are based on predicate dental materials and exhibit similar properties to their predecessors but are formed from a calcium–strontium–zinc–silicate glass, thus eliminating the threat of aluminium induced neurotoxicity. The novel zinc based GPCs (Zn-GPCs) have strengths suitable for load bearing applications [19], demonstratable bioactivity in vivo [14], and are inherently antibacterial [16] due to the release of bacteriocidal ions from the cement mantle.
It is the aim of this paper to compare a selection of the physical and mechanical properties, and the biocompatibility of Zn-GPCs with commercially available bone cements Simplex® P, Spineplex® (both PMMA) and Cortoss® (BIS-GMA), with the objective of offering a critical review of which materials are most suitable to clinical applications.
2 Materials and methods
2.1 Materials
2.1.1 Glass synthesis
One CaO–SrO–ZnO–SiO2 glass composition, 0.04SrO/0.12CaO/0.36ZnO/0.48SiO2 (mol. fraction), was synthesized. Appropriate amounts of analytical grade calcium carbonate, strontium carbonate, zinc oxide and silicon dioxide (Sigma Aldrich, Dublin, Ireland), were weighed out in a plastic tub and mixed in a ball mill for 1 h, then dried (100°C, 1 h). The pre-fired glass batch was then transferred to a platinum crucible for firing (1,480°C, 1 h). The glass melt was subsequently quenched into water and the resulting frit was dried, ground and sieved to retrieve a <45 μm glass powder. The glass was then annealed (645°C, 3 h) to relieve internal stresses within the glass network such that Zn-GPC specimen preparation was possible.
2.1.2 Commercial bone cements
The following commercial bone cements were reviewed in this study:
-
1.
Surgical Simplex® P (Stryker International, Limerick, Ireland) powder lot # 0700-2-136, liquid lot # 865EK.
-
2.
Spineplex® (Stryker International, Limerick, Ireland) powder lot # V03062006-09, liquid lot # 893GN060764.
-
3.
Cortoss® (Orthovita, Malvern, USA) lot number A603014, mixing tips lot # A612012 and delivery gun lot # A603018.
2.1.3 Cement preparation
Zn-GPCs (termed BT101) were prepared by mixing the glass with an aqueous solution of PAA; M w = 80,800 (Advanced Healthcare, UK) on a clean glass slab with a dental spatula at a powder liquid ratio of 2:1.5. Mixing of the Zn-GPCs was completed within 20 s. All commercial materials were produced in strict compliance with manufacturer’s instructions.
2.1.4 Determination of setting times and exotherm
The setting time of cements was measured in accordance with ISO 9917 [38]. An empty mould 10 mm × 8 mm × 5 mm was placed on aluminium foil and filled to a level surface with mixed cement. Sixty seconds after mixing commenced, the entire assembly was placed on a metal block (8 mm × 75 mm × 100 mm) in an oven maintained at 37°C. Ninety seconds after mixing, a Vicat needle indenter (mass, 400 g) was lowered onto the surface of the cement. The needle was allowed to remain on the surface for 5 s, the indent was then observed and the process repeated every 30 s until the needle failed to make a complete circular indent when viewed at 2× magnification. The net setting time of three tests was recorded. Plastic moulds (12.6 mm height × 12.5 mm ϕ) were packed with cement (n = 3) in order to determine the peak exotherm during setting. A thermocouple attached to an Accumet® portable AP6 multimeter (Reagecon, Shannon, Ireland) was placed into the cement 30 s after mixing commenced and the peak exotherm was recorded directly from the meter.
2.1.5 Determination of compressive strength
The compressive strength of each cement was assessed using the protocols outlined in ISO9917. Spilt ring moulds (4 mm Ø, 6 mm height), were filled to excess with freshly mixed cement then covered with acetate. The moulds were then sandwiched between 2 stainless steel plates, clamped, and incubated (37°C, 1 h). The moulds were then removed from the clamps. Flash around the moulds was removed using a grinding wheel (100 rpm) and 1200 grit silicon carbide paper. This ensured that all compression specimens had flat ends, which were parallel to one another. Specimens were incubated (37°C) for 1, 7, 30 and 90 days. After each incubation period, the specimens (n = 5) were loaded on an Instron 4082 Universal Testing Machine (Instron Ltd., High Wycombe, Bucks, UK) using a load cell of 5 kN at a crosshead speed of 1 mm min−1.
2.1.6 Determination of biaxial flexural strength and biaxial flexural modulus
The biaxial flexural strength (BFS) of cements was determined in a similar fashion to that used by Williams et al; using three support bearings on the test jig. After mixing, rubber moulds (12 mm Ø, 2 mm thick) were filled to excess with cement then sandwiched between 2 acetate covered stainless steel plates and incubated (37°C, 1 h). Following incubation the samples were de-moulded and flash was removed from the edges of each disc using 1200 grit silicon-carbide paper. Specimens were then placed in distilled water and incubated for 1, 7, 30 and 90 days. Specimen thickness was measured using Vernier callipers. After each incubation period, the specimens (n = 5) were loaded on an Instron 4082 Universal Testing Machine (Instron Ltd., High Wycombe, UK) using a load cell of 5 kN at a crosshead speed of 1 mm min−1. Biaxial flexural strength (BFS) was calculated according to Eq. 1.
where ρ is the fracture load (N), t the sample thickness, and r is the radius of the support diameter (mm).
Biaxial flexural modulus was determined using the method of Higgs et al. [39] where poisson’s ratio was assumed to be 0.3.
2.1.7 Preparation of simulated body fluid (SBF)
SBF was produced in accordance with the literature [40]. The composition is illustrated in Table 2. Reagents were dissolved in sequential order (as per Table 1) into 500 ml of purified water (Reagecon, Shannon, Ireland) using a magnetic stirrer. The solution was maintained at 36.5°C (±1.5°C) using a water bath. One mole-HCl was titrated to adjust the pH of the SBF to 7.40. Purified water was then added to adjust the total volume of liquid to 1 l. Once prepared, the SBF was stored for 24 h (5°C) to ensure that no precipitation occurred.
2.1.8 Evaluation of biocompatibility using SBF
Each bone cement (n = 3 per incubation period) was prepared as described previously. Each specimen of cement was subsequently immersed in a volume of SBF such that the following equation was satisfied:
where V s is the volume of SBF (ml) and S a is the surface area of the specimen (mm2).
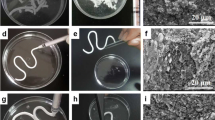
Specimens were stored in plastic containers for 24 and 168 h. After the specified incubation times, the cements were removed from the SBF, gently rinsed, and stored in a desiccator prior to analysis. A JOEL JSM-840 scanning electron microscope was used to obtain secondary electron images of the surface of cement discs.
3 Results and discussion
3.1 Setting times and exotherm
The setting times and peak exotherm of each cement are listed in Table 2. Cortoss® and Simplex® P have average setting times of 5 m 45 s and 6 m 18 s, respectively. Spineplex® has a setting time of 7 m 15 s, whilst the experimental Zn-GPC set after 55 s.
The desirable setting times of a bone-cement is dependant on the surgical procedure being undertaken. For example, the optimum setting time of cements for use in applications such as the stabilisation of compression fractures of the vertebrae is in the range of 5–8 min [41]. In this regard, the commercial materials are well tailored in respect of their applications. In contrast, BT101 exhibits a setting time too rapid for surgical deployment in any application. However, the authors have recently published data which proves that controlled additions of tri-sodium citrate to the formulation of Zn-GPCs like BT101 can significantly improve working and setting times without adversely effecting strengths [42].
A additional aspect of the setting reaction of many commercial bone cements is the evolution of heat during curing, resulting in the thermal necrosis of healthy bone stock in vivo [25, 43, 44]. The peak exotherm for 1 cm3 (approx.) of each cement was examined in this work (results illustrated in Table 2). The data shows that both Simplex® P and Spineplex® exhibit temperatures well above the threshold for tissue necrosis to occur. Whilst Cortoss® exhibits a lower peak exotherm than Simplex® P and Spineplex®, it generated more heat than BT101. However, the maximum exotherm reached for both Cortoss® and BT101 are below the threshold for tissue necrosis. In this work, exothermic evaluation was undertaken on small amounts of each material (approx. 1.1 cm3), and it is likely that the heat generated from a larger volume of material would be greater in magnitude and may exceed the threshold for exothermic necrosis. Thus, whilst it is evident that BT101 exhibits the lowest peak exotherm, an expanded, volume dependent, study will be required in the future for completeness.
3.2 Compressive and biaxial flexural strength
From Fig. 1, it is evident that Cortoss® exhibits the highest compressive strength (CS) of all cements investigated. However, the compressive strength of Cortoss® decreases significantly with maturation (179–91 MPa, from 1 to 30 days). No degradation in strength was associated with the maturation of the PMMA materials. Rather, slight increases in the mean compressive strengths were observed (99–113 MPa, from 1 and 7 days for Simplex® P and 98–116 MPa from 7 to 30 days for Spineplex®). BT101 performed in a similar fashion to Simplex® P and Spineplex®, with no deterioration in strength observed with maturation.
In relation to the biaxial flexural strength (Fig. 2), Simplex® P exhibits the highest biaxial flexural strength of all cements investigated, with Spineplex® and Cortoss® exhibiting similar BFS after 1-day maturation. However, as with CS there is a significant decrease in the BFS of Cortoss® with respect to time (96–59 MPa for 1 and 30 days, respectively). No deterioration in BFS, with respect to time, was evident for Spineplex®, Simplex® P or BT101.
The deterioration in strengths recorded for Cortoss® over both test modalities is related to the chemistry of the cement. The hydroxyl groups of BIS-GMA cements contribute to water absorption, which leads to plasticization of the cement matrix with time and a concomitant decrease in strength [45, 46]. In addition to plasticization of the matrix Abe et al. [47] have indicated that water absorption into reinforced BIS-GMA materials like Cortoss® results in filler failures and filler-matrix de-bonding resulting in reduced strengths. Given that clinicians have previously stated that ‘reinforcement should not deteriorate over time’ [41] such time-dependant deterioration in strength as observed for Cortoss® in this paper is cause for clinical concern.
In contrast to Cortoss®, conventional GPCs exhibit increasing strengths with maturation time in aqueous environments; owing to the continuous setting process which takes place within the matrix of conventional GPCs [32]. The authors have previously shown that Zn-GPCs, similar in composition to BT101 can be made to exhibit increased strengths with time [14]. However, in this work, no variations in strength were evident with maturation time (Figs. 1 and 2), a feature attributable to the unique cement composition used in this study which ensures complete evolution of strength within 24 h, thus providing clinicians with a cement implant which achieves full strength rapidly, and does not lose integrity with age.
Such stability was also a feature of the PMMA based bone cements (Figs. 1 and 2). However, whilst both Simplex® P and Spineplex® have similar strengths at all maturation times, in biaxial flexure, Simplex® P is stronger than Spineplex® at all times. There are two contributing factors to this. Firstly, the biaxial flexural test is deemed a far more sensitive test than compression testing, mainly because it eliminates the influence of intersecting planes of shear and edge defects from the result [48]. Thus, differences in the integrity of materials are more readily detected by the biaxial flexural test. However, the underlying reason for decreased strengths of Spineplex® as compared with Simplex® P is due to compositional differences between both cements. Firstly, the levels of radio-pacifying agent Barium sulphate (BaSO4) incorporated in each cement formulation are influential. In the case of Simplex® P, 10% w/w of the formulation is BaSO4 [12], however for improved visualization under fluoroscopy, the BaSO4 content of Spineplex® is 30% w/w [49]. Importantly, the literature indicates that BaSO4 can reduce the mechanical strengths of PMMA bone cements [50]. Furthermore, and as a consequence of an increased BaSO4 loading in Spineplex®, there is a concomitant decrease in the percentage of methylmethacrylate (MMA)-styrene-copolymer and prepolymerized PMMA beads in the cement. Previous data in the literature shows that decreasing the MMA-styrene-copolymer content of PMMA bone cements results in significant reductions in the compressive strength of the final cements. Therefore, the reduction in strength of Spineplex® as compared with Simplex® P is as a result of increased BaSO4 content and decreased MMA-styrene-copolymer content of Spineplex®.
In relation to the clinical potential of Zn-GPCs in applications such as vertebroplasty, the stiffness of the material, rather than its compressive and biaxial flexural strengths, is very important. Fractures adjacent to vertebroplasty sites are being increasingly reported [51–53]. While the etiology for such fractures is complex, excessive stiffness of bone cements relative to vertebral trabecular bone has been implicated as an area of considerable concern [54, 55].
The modulus of PMMA is far greater than trabecular vertebral bone, therefore after augmentation of a vertebra with PMMA cement, the stiffness of the trabecular bone increase 12-fold. This change in vertebral body stiffness results in re-distribution loads through the treated vertebrae and onto adjacent vertebrae, resulting in fracture [52, 54, 55]. In order to overcome this, a material with a modulus matched to that of trabecular vertebral bone would be a significant advantage in the field of vertebroplasty. As can be seen in Fig. 3, both Spineplex®, Simplex® P and Cortoss® exhibit excessively high moduli when compared to the Zn-GPC, which exhibits a modulus matched closely to that of vertebral trabecular bone [56]. As such this will eliminate modulus mismatch difficulties associated with these stiffer materials and thus will help to reduce adjacent fractures caused by increased stiffness of vertebrae after vertebroplasty.
3.3 Evaluation of biocompatibility using SBF
In 1991 it was proposed that the ability of an artificial material to bond with bone tissue lay in that material’s ability to form an apatite layer at its surface in vivo. Furthermore, this in vivo apatite formation could be reproduced using SBF with ion concentrations similar to those of blood plasma (Table 3) [57].
Since then, the use of SBF as a preliminary screen for biocompatibility has become widespread, with the literature clearly demonstrating a well established correlation between a materials ability to induce apatite formation in SBF and its ability to bond directly to living bone tissue [40].
In this paper, three commercial bone cements and one experimental bone cement were evaluated in SBF. In relation to Simplex® P and Spineplex®, the SEM images (Fig. 4) do not provide any evidence for the formation of an apatite layer in SBF. The precipitation of an apatite layer in SBF is governed by complex thermodynamics, consisting of 17 association/disassociation reactions [58]. Tanihara et al. [59] have identified the factors, which govern the nucleation of apatite on a substrate and primary amongst these factors is the ability of a material to increase the ionic activity product of apatite [58]; with such an increase being facilitated by release of Ca2+, PO3− 4, or OH−, from the cement (Ca2+ being most effective). Therefore, the lack of apatite at the surface of Simplex® P and Spineplex® is likely due to the compositional deficiencies of the cements.
The last commercial cement examined was Cortoss®; a BIS-GMA cement containing silane treated glass ceramic particles (Na2O–CaO–P2O5–SiO2), silane treated baria-boroalumino-silicate glass and silane treated amorphous silicon dioxide [12]. The SEM results (Fig. 5) indicate no apatite layer after 1-day incubation in SBF. However, after 7 days, evidence of formation of an apatite like surface precipitate was present; a feature that has been attributed to the release of ions from the silane treated glass ceramic particles, with the slow evolution of apatite being related to minimal ion release from the glass-ceramic particles [23].
Conversely, the experimental Zn-GPC gave the best results in the SBF trail where the formation of an apatite layer was observed after 1 day. The coverage and density also appeared to increase from 1 to 7 days. These results correlate well with previously published data which, using EDX has chemically analysed such surface precipitates and has identified amorphous calcium phosphate layers at the surface of similar Zn-GPCs after 24 h [14, 23]. Such results indicate that the experimental Zn-GPCs may have potential to bond directly to living bone tissue upon implantation.
4 Conclusions
The aim of this study was to compare a selection of the physical and mechanical properties, and biocompatibility of Zn-GPCs with three commercial bone cements, Simplex® P, Spineplex® and Cortoss®. The results indicated that the experimental cement sets far too quickly for clinical deployment, as compared with commercial materials. However recent developments have shown that the setting time of such cements can be modified without adversely affecting strengths. As part of the investigation, the peak exotherm was also determined, and it was found that the experimental bone cement exhibited the lowest exotherm (34°C) of all materials examined and consequently could eliminate clinical concerns relating to the thermal necrosis. In relation to mechanical properties, the commercial cements exhibited the higher strengths than the experimental cement. More importantly, the modulus of the commercial materials was found to be excessively high as compared with the modulus of vertebral trabecular bone. However, the modulus of the experimental cement matched that of vertebral trabecular bone, indicating that its use in such procedures as vertebroplasty could reduce the instances of adjacent fractures in the spine. A final point to note from the analysis of the mechanical properties of the materials is that, while the strengths of BT101, Simplex® P and Spineplex® remain static with time, the strength of Cortoss® decreases with time in an aqueous environment and this is cause for clinical concern. Finally, as expected, neither Simplex® P nor Spineplex® induced the formation of an apatite layer in SBF. Cortoss® was shown to have an apatite layer after 7 days; however, the experimental bone cement facilitates the formation an apatite layer after 24 h indicating that in vivo it may form a direct bond with bone quicker than its commercial counterparts.
The study has limitations, primarily the quantity of material used to evaluate setting times and peak exotherm is not equivalent to the volume of material that would be deployed in a procedure like vertebroplasty (up 8 cm3). Notwithstanding this limitation, the study has highlighted some issues relating to commercial materials and has shown that an alternative material based on aluminium free glass polyalkenoate chemistry may be suitable for surgical procedures such as vertebroplasty.
References
J. Charnley, Clin. Orthop. Relat. Res. 72, 7 (1970)
K. Hochmuth, D. Proschek, W. Schwarz, M. Mack, A. Kurth, T. Vogl, Eur. Radiol. 16, 998 (2006)
T.H. Diamond, B. Champion, W.A. Clark, Am. J. Med. 114, 257 (2003)
H. Deramond, C. Depriester, P. Galibert, D. Le Gars, Radiol. Clin. North Am. 36, 533 (1998)
E. van der Linden, L.J.M. Kroft, P.D.S. Dijkstra, J. Vasc. Intervent. Radiol. 18, 741 (2007)
P. Galibert, H. Deramond, P. Rosat, D. Le Gars, Neurochirurgie 33, 166 (1987)
R.S. Laskin, Controversies in Total Knee Arthroplasty. (University press, Oxford, New York, 2001)
W. Petty, J. Bone Joint Surg. 60a, 492 (1978)
W. Petty, J. Bone Joint Surg. 60a, 752 (1978)
A.T. Berman, J.L. Parmet, S.P. Harding, C.L. Israelite, K. Chandrasekaren, J.C. Horrow, M.D. Singer, H. Rosenberg, J. Bone Joint Surg. 80a, 389 (1998)
T.M. Turner, R.M. Urban, K. Singh, D.J. Hall, S.M. Renner, T.-H. Lim, M.J. Tomlinson, H.S. An, Spine J. (in press)
I.H. Lieberman, D. Togawa, M.M. Kayanja, Spine J. 5, S305 (2005)
T. Ishida, T. Hashimoto, K. Shigenobu, M. Kanayama, F. Oha, S. Yamane, Spine J. 4, S84 (2004)
D. Boyd, M.R. Towler, J. Mater. Sci. -Mater. Med. 16, 843 (2005)
D. Boyd, M.R. Towler, R.V. Law, R.G. Hill, J. Mater. Sci. -Mater. Med. 17, 397 (2006)
D. Boyd, H. Li, D.A. Tanner, M.R. Towler, J.G. Wall, J. Mater. Sci. -Mater. Med. 17, 489 (2006)
M.R. Towler, S. Kenny, D. Boyd, T. Pembroke, M. Buggy, R.G. Hill, Bio-Med. Mater. Eng. 14, 565 (2004)
M.R. Towler, S. Kenny, D. Boyd, T. Pembroke, M. Buggy, A. Guida, R.G. Hill, J. Mater. Sci. -Mater. Med. 17, 835 (2006)
D. Boyd, O.M. Clarkin, A.W. Wren, M.R. Towler, Acta Biomaterialia (in press)
J. Vainio, Arch. Orthop. Trauma Surg. 92, 169 (1978)
G.J. Pomrink, M.P. Dicicco, T.D. Clineff, E.M. Erbe, Biomaterials 24, 1023 (2003)
E.M. Erbe, T.D. Clineff, G. Gualtieri, Eur. Spine J. 10, S147 (2001)
O. Johnell, Eur. Spine J. 12(Suppl. 2), S168 (2003)
L. Eschbach, Injury Int. J. Care Injured 31, S (2000)
F.L.W. Geurtsen, W. Spahl, G. Leyhausen, J. Biomed. Mater. Res. 41, 474 (1998)
K. Kehe, F.X. Reichl, J. Durner, U. Walther, R. Hickel, W. Forth, Biomaterials 22, 317 (2001)
E. Yoshii, J. Biomed. Mater. Res. 37, 517 (1997)
Y.T.C. Theilig, G. Leyhausen, W. Geurtsen, J. Biomed. Mater. Res. 53, 632 (2000)
M.L. Lena Stanislawski, K. Bourd, E. Soheili-Majd, M. Goldberg, A. Périanin, J. Biomed. Mater. Res. Part A 66, 476 (2003)
H. Darmani, A.S. Al-Hiyasat, Dent. Mater. 22, 353 (2006)
J.W. Nicholson, Biomaterials 19, 485 (1998)
P.V. Hatton, K. Hurrell-Gillingham, I.M. Brook, J. Dent. Proc. 2nd Eur. Glass Ionomer Conf., May 2004 34, 598 (2006)
K. Hoang-Xuan, P. Perrotte, F. Dubas, J. Philippon, F.M. Poisson, Lancet 347, 910 (1996)
E. Reusche, J. Rohwer, W. Forth, J. Helms, G. Geyer, Lancet 345, 1633 (1995)
P. Hantson, P. Mahieu, M. Gersdorff, C.J.M. Sindic, R. Lauwerys, Lancet 344, 1647 (1994)
E. Reusche, P. Pilz, G. Oberascher, B. Lindner, R. Egensperger, K. Gloeckner, E. Trinka, B. Iglseder, Human Pathol. 32, 1136 (2001)
International Standard 9917-1:2003, Dental Water Based Cements. International Organization for Standardization, Case Postale 56, CH1211, Geneve, Switzerland
W.A.J. Higgs, P. Lucksanasombool, R.J.E.D. Higgs, J. Biomed. Mater. Res. (Appl. Biomater.) 58, 188 (2001)
T. Kokubo, H. Takadama, Biomaterials 27, 2907 (2006)
P.F. Heini, U. Berlemann, Eur. Spine J. 10, S205 (2001)
D. Boyd, O.M. Clarkin, A.W. Wren, M.R. Towler, Acta Biomaterialia (in press)
I. Rehman, E.J. Harper, W. Bonfield, Biomaterials 17, 1615 (1996)
N.J. Dunne, J.F. Orr, J. Mater. Sci. Mater. Med. 13, 17 (2002)
C.A. Khatri, J.W. Stansbury, C.R. Schultheisz, J.M. Antonucci, Dent. Mater. 19, 584 (2003)
K.J. Salderholm, P.D. Calvert, J. Mater. Sci. 18, 2957 (1983)
Y. Abe, M.J.A. Braem, P. Lambrechts, S. Inoue, M. Takeuchi, B. Van Meerbeek, Biomaterials 26, 3405 (2005)
Stryker Intruments, Spineplex: Instructions for use. Stryker Instruments, Raheen, Limerick, Ireland
M. Baleani, L. Cristofolini, C. Minari, A. Toni, J. Eng. Med. 217, 9 (2002)
H. Deramond, C. Depriester, P. Galibert, D. Le Gars, Radiol. Clin. North Am. 36, 533 (1998)
A. Polikeit, L.P. Nolte, S.J. Ferguson, Spine 28, 991 (2003)
G.H. Zoarski, P. Snow, W.J. Olan, M.J.B. Stallmeyer, B.W. Dick, J.R. Hebel, M. De Deyne, J. Vasc. Intervent. Radiol. 13, 139 (2002)
G. Baroud, J. Nemes, P. Heini, T. Steffen, Eur. Spine J. 12, 421 (2003)
G. Baroud, M. Bohner, Joint Bone Spine 73, 144 (2006)
J.C.M. Teo, K.M. Si-Hoe, J.E.L. Keh, S.H. Teoh, J. Mater. Sci. Eng. 27, 333 (2007)
T. Kokubo, Biomaterials 12, 155 (1991)
X. Lu, Y. Leng, Biomaterials 26, 1097 (2005)
H. Nalwa, Handbook of Organic–Inorganic Hybrid Materials and Nanocomposites. (American scientific publishers, California, 2003)
A.W. Wren, D. Boyd, M.R. Towler, J. Mater. Sci. Mater. Med. (in press)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boyd, D., Towler, M.R., Wren, A. et al. Comparison of an experimental bone cement with surgical Simplex® P, Spineplex® and Cortoss® . J Mater Sci: Mater Med 19, 1745–1752 (2008). https://doi.org/10.1007/s10856-007-3363-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3363-4