Abstract
NiFe2−xSmxO4 (x = 0.0, 0.01, 0.025, 0.05, 0.075, 0.1) nanoferrites samples were prepared by sol–gel method. The structural properties of the samples were studied by X-ray powder diffraction, where the formation of a cubic spinel phase was confirmed. The lattice parameter and the particle size were determined by applying Rietveld refinement. A slight change in the lattice parameter was observed for Sm doped nanosamples. Whereas, the particle sizes were found to decrease with increasing Sm concentration (x) and have the highest value (42 nm) for pure NiFe2O4 nanoparticles. The magnetic properties were characterized using Mössbauer effect (ME) spectroscopy and vibrating sample magnetometer (VSM). ME spectroscopy was used to study the distribution of cations in tetrahedral (A) and octahedral [B] sites of the nanoparticles and the degrees of inversion values were obtained. The saturation magnetization, coercivity and remanent magnetization were determined using VSM measurements. These magnetic parameters vary with Sm substitution and found to be mainly affected by the change in the cations distribution and particle sizes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rare earth (R) doped nano-ferrites have been investigated because of their novel physics and chemical properties. These (R) doped nano-ferrites are technologically important materials that are used to produce permanent magnets, electronic and microwave devices, magnetic fluids, spintronics, data storage, magnetic filters, transformers and magnetic switches [1,2,3,4]. The magnetic properties of these spinel ferrites are governed by the chemical composition and the method of preparation [5,6,7]. In the case of nickel ferrites, altering the chemical composition can be achieved by doping with different transition metals and rare earth (R) ions [5, 8].
Not only is the type of the dopant but also its amount is critical in changing the cation distribution and accordingly the physical properties of the doped Ni-ferrite. Using different synthesizing methods could yield different particle sizes, particle size distribution, morphology and cation distribution [8, 9]. There are many preparation methods of nickel ferrite nanocrystals; mechanical milling, sol–gel methods, ceramic method, microwave processing approaches and co-precipitation, microemulsions. Among these methods, sol–gel process stands as an important method used to obtain ferrite nanoparticles with enhanced homogeneity and controlled particle size in a relatively short processing time and low reaction temperatures.
Single phase NiFe1.99R0.01O4 (R = Sm, Gd, Eu, La) samples were prepared using the standard ceramic method [8]. Mössbauer effect (ME) measurements suggested that R ions substitute Fe3+ ions in the octahedral B site. An increase in the coercivity and saturation magnetization was observed for all doped samples, where the highest saturation magnetization was obtained for Sm-doped Ni-ferrite sample. Then, Zn and Ga doped Ni1−xZnxFe1.49Ga0.5Sm0.01O4 samples were prepared using the standard ceramic method [10]. Measurements confirmed that Sm3+ ions favor the B sites and support magnetism in the studied system, whereas Zn substitution clearly destabilizes the magnetic order and suppresses magnetism in the studied systems. Later, ultrasmall Zn-substituted Ni–Sm–Ga (NiFe1.99Sm0.01O4 and Ni1−xZnxFe1.49Ga0.5Sm0.01O4) ferrites nanoparticles were synthesized using sol–gel method [9]. The particle size ranged from D = 4–8 nm, and hence a pronounced superparamagnetism was observed for all the prepared nanoparticles.
In this work, Sm-substituted Ni ferrites NiFe2−xSmxO4 (x = 0.0, 0.01, 0.025, 0.05, 0.075, 0.1) were prepared by the sol–gel technique. The influence of Sm3+ doping on the structural and magnetic properties of Ni ferrite were systematically studied. The crystal structure was investigated using X-ray powder diffraction and Fourier transform-infrared (FTIR), whereas the cation distribution and the magnetic properties were studied using ME spectrometer and vibrating sample magnetometer (VSM).
2 Experimental
NiFe2−xSmxO4 (x = 0.0, 0.01, 0.025, 0.05, 0.075, 0.1) nanoparticles were synthesized using Fe(NO3)3·9 H2O [99% Merck, Germany], Ni(NO3)2·6H2O [99.9% Strem Chemicals, USA], Sm(NO3)3·6H2O [99.9% Strem Chemicals, USA], citric acid (C6H8O7·H2O) [99.7% Winlab, UK] and distilled water. The structure of the prepared samples was studied at room temperature using X-ray diffraction (XRD) (Philips X’pert multipurpose diffractometer) with CuKα radiation (with λ = 1.5418 Å). The crystal structure was refined applying Rietveld profile method using the Rex software [11]. The crystallographic parameters: lattice constant (a), ionic radius per molecule of the tetrahedral and octahedral sites (rA, rB), bond length in the two crystallographic sites (RA and RB) and oxygen position parameter (u) have been calculated. Jasco FT-IR 310 spectrophotometer were used to carry out FTIR measurements in the frequency range of 2000–250 cm−1. Austin Science Mössbauer Effect Spectrometer with constant acceleration laser-interferometer-controlled drive and data acquisition system is used in a standard transmission setup with a personal Computer Analyzer (PCA II-card with 1024 channel). The radioactive source is 57Co embedded in Rh matrix with initial activity of 50 mCi. Metallic iron spectrum is used for the calibration of both observed velocities and hyperfine magnetic fields. The cation distributions and all (ME) parameters are calculated from the recorded Mössbauer spectra. Magnetic measurements on the synthesized nanoferrites were carried out using VSM (9600-1 LDJ, USA) with a maximum applied field of nearly 20 kG at room temperature and the saturation magnetization MS and the coercivity HC were determined.
3 Results and discussion
3.1 XRD measurements
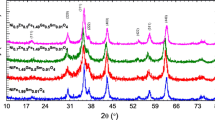
The XRD patterns of NiFe2−xSmxO4 (x = 0.0, 0.01, 0.025, 0.05, 0.075, 0.1) nanoparticles are shown in Fig. 1. For all samples, the observed diffraction peaks can be indexed to NiFe2O4 with Fd-3m space group. The diffraction patterns exhibit additional impurity peaks of Ni. Using Rex software (Fig. 2), the percentage of Ni phase was calculated and ranged from 3.4% for pure Ni ferrite to < 1% for all Sm doped samples. The lattice parameter aexp obtained from the Rietveld refinement of XRD measurements is summarized in Fig. 3b. On the other hand, the lattice parameter ath can be theoretically calculated using the cation distribution obtained from ME spectroscopy (Sect. 3.2). The Mean ionic radius per molecule of the A-site (rA) and B-site (rB) are given by [12]:
where r(Fe3+), r(Ni2+) and r(Sm3+) are the ionic radii of Fe3+, Ni2+ and Sm3+ ions given in Table 1. The concentration of Fe3+, Ni2+ and Sm3+ ions in A- and B- sites are CFe, CNi and CSm, respectively and were taken from (ME) results. The average (A–O) and (B–O) bond lengths are RA and RB respectively and can be expressed as:
where rO= 1.32 Å is the radius of oxygen ion. The lattice parameter ath can be obtained from:
a Variation of particle size with the substitution of Sm content. b Variation of the lattice parameter (a) with Sm content x. aexp is the lattice parameter obtained from the XRD measurements and ath is the lattice parameter calculated according to the cation distribution obtained from the ME measurements
The calculated values of ath, rA, rB, RA, RB and u are illustrated in Table 2. The comparison between the calculated lattice parameter ath and the experimentally obtained aexp are shown in Fig. 3b. According to the ME measurements (Sect. 3.3), nano NiFe2O4 is a partial inverse spinel, where most of the Ni2+ ions set on the B-site, while a small fraction (~ 9%) enters the A-site. By increasing x, Sm3+ ions have a clear preference to inter the B-site and gradually substitute Fe3+ ions. This process is accompanied by the migration of the Ni2+ from the A-site to the B-site. The ionic radius r(Sm3+) is bigger than both r(Fe3+) and r(Ni2+). This why by increasing x, both rA and RA slightly decrease, while rB and RB increase monotonically. The resultant effect is an increase in the lattice parameter. The values of the lattice parameter are very close to the values observed for the bulk Ni ferrite sample [4]. For the A-site, center of symmetry is at \(\left( {\frac{3}{8}\frac{3}{8}\frac{3}{8}} \right)\) and the ideal value of the oxygen positional parameter (u43m) is 0.375. While, the center of symmetry for the B-site is at \(\left( {\frac{1}{4}\frac{1}{4}\frac{1}{4}} \right)\) and the ideal value of u3m is 0.25. In this case, the arrangement of O2− ions correspond exactly to a cubic closed packing. However, in the actual spinel lattice a small deformation from this ideal pattern is observed. As shown in Table 2, the values of u3m and u43m are higher than the ideal values, which indicate that O2− ions move away from the tetrahedrally coordinated A-site cation along the \(\langle 111\rangle\) directions due to the contraction of the tetrahedral interstices, correspondingly the octahedral B-sites become smaller [9]. Such changes in the oxygen positional parameter will affect the A–A interactions and B–B magnetic interactions.
The particle size of NiFe2−xSmxO4 nanoparticles can be obtained from the Rietveld refinement of XRD measurements. The broad diffraction peaks observed for all the samples reflect the reduced particle size. The particle size D are shown in Fig. 3, where it varies from ~ 42 nm for pure Ni-ferrite to ~ 30 nm for NiFe1.9Sm0.1O4. Generally, by increasing the Sm content x, the particle size decreases. A similar behavior was observed for Nd3+ substituted nano Mg–Cd ferrites [14, 15].
3.2 FTIR analysis
FTIR spectra of NiFe2−xSmxO4 (x = 0.0, 0.01, 0.025, 0.05, 0.075, 0.1) nanoferrites are shown in Fig. 4 and the vibration frequencies of all the samples are listed in Table 3. Spinel ferrites generally are branded with two vibrational frequency bands, ʋ1 around 600–500 cm−1 which is related to the Fe3+–O2− and Ni2+–O2− complexes at the tetrahedral site (A) and vibrational frequency ʋ2 around 450–350 cm−1 which is related to the Fe3+–O2−, Sm3+–O2− and Ni2+–O2−complexes at octahedral (B) site [16]. The differences in vibration frequencies between the two crystallographic A and B-sites are attributed to the differences in bond length between the two sites, where the ionic radius of A-site is smaller than B-site. In this case, the change in values of ʋ1 and ʋ2 with increasing Sm content reflects the change in bond lengths of Fe3+, Sm3+ and Ni2+ metal ions complexes present in this ferrite. As discussed before in Sect. 3.1, XRD measurements suggest a slight change in the average bond length RA, RB and the lattice parameter a. Accordingly, a minor change in the vibrational frequency ʋ1 and ʋ2 was observed. The bands at 1613 cm−1, 1359 cm−1 and 1115 cm−1 are attributed to; (a) the stretching vibration in the residual H–O–H modes of water molecules, (b) the symmetric stretching of the carboxyl group (COOH) and (c) the asymmetric stretching vibration of the adsorbed NO3 produced as a residue from the nitrate group after the combustion of the samples [17].
3.3 ME measurements
Figure 5 shows the room temperature transmission ME spectra of NiFe2−xSmxO4 (x = 0.0, 0.01, 0.025, 0.05, 0.075, 0.1). ME spectra spot the hyperfine interactions between the nucleus and its neighboring electrons. There are three main hyperfine interactions, one originates from the electron density at the nucleus (Isomer shift δ), the other from the gradient of the electric field at the nucleus (Quadrupole splitting ΔEQ), and finally from the unpaired electron density at the nucleus (Hyperfine field Hf). The spectra of all samples were fitted with two magnetic sextets and a paramagnetic doublet. All the fitting parameters are summarized in Table 4. The magnetic sextets are a direct proof of the magnetic order in the studied samples. The existence of two sextets is typical for inverse and partially inverse spinel ferrites, where they refer to Fe3+ in both tetrahedral and octahedral environments. For this reason, the area of these two sextets are used for the determination of Fe amount in the A- and B-sites. The sextets attributed to the Fe3+ ions at the octahedral (B) have a larger isomer shift and hyperfine field [18]. As presented in Fig. 6, the Fe3+ amount in the B-site is slightly higher than that in the A-site, which straight forwardly refers that a portion Ni2+ ions set on A-site (~ 9%). This unusual partial inverse Ni ferrite is due to the reduced particle size [4]. For x = 0.01, Sm3+ ions replace Fe3+ in the B-site and in the same time some of the Ni2+ ions transfer from the A- to the B-site. By increasing the Sm content x, the behavior continues and the amount of Fe3+ ions in the A-site exceed that in the B-site for x > 0.05.
In addition to the two sextets components, the spectra of all samples have doublets due to the presence of nano-sized ferrite crystallites below the single domain size (Dsp) with superparamagnetic behavior [4, 19]. The area of the doublets is ranging from (3.58–16.24%). δ values are typical for Fe3+ ions in octahedral and tetrahedral environment [4, 5]. Since, the s-electrons charge density of the Fe3+ ions is not influenced by crystalline size, the observed values of the isomer shift show a weak change with decreasing D. The quadrupole splitting (ΔEQ) has values close to zero for both (A)- and [B]-sites.
Figure 7 presents the hyperfine field (HfA and HfB) for both A- and B-sites. Generally, the hyperfine field reflects the internal magnetic field produced by the magnetic ordering on Fe3+ ions on both sites. This field is strongly affected by intersite A–A and B–B interactions and the interasites A–B interactions. As the cation distribution changes due to the Sm3+ doping, these magnetic interactions change and consequently affect both hyperfine fields. Also, the hyperfine field is sensitive to the decreased particle size, where decreasing the particle size tends to decrease the hyperfine field [20, 21]. Figure 7 suggests that increasing Sm3+ concentration slightly changes HfA and HfB. Generally, for x ≤ 0.1, Sm3+ ions has a minor effect on the magnetic interactions and magnetic order. However, the changes in the cation distribution due to Sm doping is reflected in the difference between HfB and HfA. The difference between HfB and HfA increases gradually by introducing Sm3+ ions and it peaks at x = 0.05 and then decreases again for higher concentration (x > 0.05) where the Fe3+ in the A-site exceeds that in the B-site. The change in the cation distribution can also be spotted in the doublets parameters. As indicated in Fig. 8, pronounced differences are observed in the behavior of δd, ΔEQd and doublet linewidth ωd for x < 0.05 and x > 0.05. Such behavior can be attributed to the changes in the A–B interactions due to partial transfer of the Fe3+ ions from the B-site to the A-site by increasing Sm3+ concentration.
3.4 Magnetic properties
Figure 9 shows the variation of magnetization as a function of the applied magnetic field for various NiFe2−xSmxO4 (x = 0.0, 0.01, 0.025, 0.05, 0.075, 0.1) nanoparticles at 300 K. The M–H curve provides information regarding the magnetic parameters such as saturation magnetization (MS), coercivity (Hc) and remanent magnetization (Mr), which are summarized in Table 5. Saturation magnetization (MS) and Coercivity (Hc) are shown in Fig. 10. Generally, the magnetic properties should mainly depend on Sm3+ content and the cation distribution of different ions between A and B sites. However, the magnetic properties of nanoferrites also strongly depend on the particle size and the particle size distribution. The saturation magnetization (MS) is expected to decrease with decreasing the particle size [4]. The magnetic moment per formula unit in Bohr magneton (µB) can be calculated using the relation [8]:
The value of Mobs is presented in Table 4 and Fig. 10a. The magnetic properties of ferrites are mainly governed by super exchange interactions and therefore changing the cation distribution and introducing rare earth Sm would affect A–A, A–B and B–B magnetic interactions. According to Néel’s theory, the magnetic moment Mcal is expressed as:
The concentration of different ions in the A- and B- sites are obtained from the ME measurements and the magnetic moment of Ni2+, Fe3+ and Sm3+ ions are 2 µB, 5 µB and 5 µB, respectively. The dashed line is the expected value for complete inverse NiFe2O4, which is exact 2 µB. For all the samples, Mcal values are significantly higher than the observed values. MS value depends on the ions magnetic moments, and the distance and angle between two adjacent magnetic ions. The larger ionic radii of Sm3+ ions compared to Fe3+ ions causes changes in the lattice parameter, bond lengths, bond angles and oxygen positional parameter, which would lead to the variation of the magnetic interactions. Moreover, the change in the cation distribution will also affect the magnetic interactions. Finally, the reduced particle size of the prepared nanoparticles decreases MS values [3, 22, 23]. The coercivity of the ferrites nanoparticles depends on internal micro stress due to distortion of lattice, different electronic configuration of the ions and particle size. Sm3+ ions concentration would logically affect HC since Sm3+ 4f electrons have a strong spin–orbit coupling [22]. The change in coercivity with Sm3+ ions concentration can be attributed to the decrease in particle size and the changes in the cation distribution. The squareness R2 = MR/MS for all the studied samples is ranging from 0.235–0.314. Since the squareness is smaller than 0.5, NiFe2−xSmxO4 nanoparticles would have uniaxial anisotropy which arises due to surface effect [23, 24].
4 Conclusion
NiFe2−xSmxO4 (x = 0.0, 0.01, 0.025, 0.05, 0.075, 0.1) nanocrystalline ferrites were synthesized by sol–gel technique. The structural parameters; lattice parameter (a), bond lengths and oxygen positional parameter (u) were calculated from the X-ray powder diffraction measurements. These structure parameters vary systemically with the substitution of Sm3+ ions. ME spectroscopy was utilized to study the magnetic properties and determine the cation distribution. For all the samples, ME spectra contain two magnetic sextets and a paramagnetic doublet, which suggest the coexistence of the magnetic order for large particle sizes and superparamagnetic behavior for small particle sizes below Dsp. The saturation magnitization (MS), coercivity (HC) and remanent magnetization (Mr) were determined from the VSM measurements. The squareness ratio suggests that all the studied nanoparticles have uniaxial anisotropy. The observed magnetic moment values (Mobs) for all samples are significantly lower than the calculated magnetic moments according to Néel’s theory (Mcal). These reduced values of Mobs can be attributed to the reduced particle sizes of the prepared nanoferrites.
References
K.V. Zipare, S.S. Bandgar, G.S. Shahane, J. Rare Earths 36, 86–94 (2018)
S. Ikram, M.I. Arshad, K. Mahmood, A. Ali, N. Amin, N. Ali, J. Alloys Compd. 769, 1019–1025 (2018)
M.N. Akhtar, A.B. Sulong, M.N. Akhtar, M.A. Khan, J. Rare Earths 36, 156–164 (2018)
M. Yehia, Sh Labib, S.M. Ismail, Phys. B 446, 49–54 (2014)
A. Hashhash, M. Yehia, S.M. Ismail, S.S. Ata-Allah, J. Supercond. Nov. Magn. 27, 2305–2310 (2014)
M. Rahimi-Nasrabadi, M. Behpour, A. Sobhani-Nasab, M.R. Jeddy, J. Mater. Sci.: Mater. Electron. 27, 11691–11697 (2016)
M. Rahimi-Nasrabadi, M. Behpour, A. Sobhani-Nasab, S.M. Hosseinpour-Mashkani, J. Mater. Sci.: Mater. Electron. 26, 9776–9781 (2015)
M. Yehia, S.M. Ismail, A. Hashhash, J. Supercond. Nov. Magn. 27, 771–774 (2014)
M. Yehia, S.M. Ismail, M.B. Mohamed, J. Supercond. Nov. Magn. 28, 3335–3342 (2015)
S.M. Ismail, M. Yehia, S.S. Ata-Allah, J. Supercond. Nov. Magn. 28, 2875 (2004)
M. Bortolotti, I. Lonardelli, J. Appl. Cryst. 46, 259–261 (2013)
V.K. Lakhani, T.K. Pathak, N.H. Vasoya, K.B. Modi, Solid State Sci. 13, 539–547 (2011)
A. Dean John, Lange’s Handbook of Chemistry (McGraw-Hill, New York, 1999)
S.R. Bhongale, H.R. Ingawale, T.J. Shinde, P.N. Vasambekar, J. Rare Earths 36, 390–397 (2018)
M. Tsvetkov, M. Milanova, I. Ivanova, D. Neov, Z. Cherkezova-Zheleva, J. Zaharieva, M. Abrashev, J. Mol. Struct. (2019). https://doi.org/10.1016/j.molstruc.2018.07.083
M. Deepty, Ch Srinivas, E.R. Kumar, N.K. Mohan, C.L. Prajapat, T.C. Rao, S.S. Meena, A.K. Verma, D.L. Sastry, Ceram. Int. (2019). https://doi.org/10.1016/j.ceramint.2019.01.029
A. Hashhash, M. Kaiser, J. Electron. Mater. 45, 462–472 (2016)
K.M. Srinivasamurthy, V.J. Angadi, S.P. Kubrin, S. Matteppanavar, P.M. Kumar, B. Rudraswamy, Ceram. Int. 44, 18878–18885 (2018)
S. Joshi, M. Kumar, H. Pandey, M. Singh, P. Pal, J. Alloys Compd. 768, 287–297 (2018)
S. Mørup, M.F. Hansen, C. Frandsen, Beilstein J. Nanotechnol. 1, 182–190 (2010)
S. Mørup, M.F. Hansen, C. Frandsen, Beilstein J. Nanotechnol. 1, 48–54 (2010)
S.I. Ahmad, S.A. Ansari, D.R Kumar, Mater. Chem. Phys. 208, 248–257 (2018)
A. Pathania, S. Bhardwaj, S.S. Thakur, J.L. Mattei, P. Queffelec, L.V. Panina, P. Thakur, A. Thakur, Phys. B 531, 45–50 (2018)
T. Vigneswari, P. Raji, J. Mol. Struct. 1127, 515–521 (2017)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yehia, M., Hashhash, A. Structural and magnetic study of Sm doped NiFe2O4 nanoparticles. J Mater Sci: Mater Electron 30, 6768–6775 (2019). https://doi.org/10.1007/s10854-019-00988-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-00988-9