Abstract
Single-phase spinel ferrite Ni 1−x Zn x Ga y Fe 2−y O 4 with (0.0≤x≤0.5) and y=0.5 samples were synthesized using solid-state reaction technique. These ferrites were investigated using X-ray diffraction (XRD), vibrating sample magnetometer (VSM) and Mössbauer effect (ME) spectroscopy. XRD patterns confirmed the formation of single-phase cubic spinel ferrites for all samples. Lattice parameter was found to decrease with the introduction of Ga 3+ in NiFe 2 O 4, and then to increase with the increasing of Zn 2+ content x. ME measurements illustrated a strong dependence on the zinc concentration. The cation distribution calculated from the ME spectra at room temperature indicated that the Ga 3+ ion substituted iron in both octahedral B and tetrahedral A- sites. Zn 2+ ions firstly introduced in A- site, and for higher x, they distribute in both B- and A- sites. VSM measurements indicated that the change in the value of saturation magnetization can be explained using the cation distribution obtained from ME measurements. The coercivity values can be interpreted on the basis of magneto-crystalline anisotropy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ferrimagnetic spinel ferrites are important magnetic materials, which are widely used in high-frequency devices due to their high permeability in the radio frequency region, high electrical resistance, mechanical hardness, and chemical stability [1]. Spinel ferrite chemical formula is MFe 2 O 4, where M represents a divalent transition metal ion. In this structure, oxygen ions form face centered cubic lattice and the metal ions are surrounded by either 4 (tetrahedral) or 6 (octahedral) oxygen ions. The properties of these materials depend upon the distribution of cation (metal ions) between tetrahedral (A)- and octahedral [B]- sites. Cation distribution in these systems is very much sensitive to the method of preparation, particle size, and doping. Hence, the electrical, magnetic and optical properties of ferrites can be tuned by the type and amount of substituting elements [2–4]. Substitutions of nonmagnetic ions in spinel ferrites have received a great interest because they alter the magnetic and electric properties. This isomorphous substitutions are particularly apparent in the Mössbauer effect spectra, where the magnetic interactions drastically reduced, resulting in the lower magnetic ordering and decreased the hyperfine fields [5, 6]. The present work reports the preparation procedures and the results of X-ray diffraction (XRD), vibrating sample magnetometer (VSM), and Mössbauer effect (ME) spectroscopy measurements of the spinel ferrites Ni 1−x Zn x Ga y Fe 2−y O 4 with (0.0≤x≤0.5) and y=0.5, aiming to shed more lights on the microscopic picture of the structure parameters and magnetic properties in this diluted ferrimagnets.
2 Experimental Details
Polycrystalline samples of the studied compounds were prepared using the solid-state reaction method. The powders of NiO, ZnO, Ga 2 O 3, and Fe 2 O 3 oxides (with purity 99.99 %) in the required stoichiometric proportions were thoroughly mixed and milled. The mixture is pre-sintered at (1,000–1,150 ∘C), depending on the ZnO content, for 60 h, then slowly cooled to room temperature. The product is reground and fired again under the same conditions to improve homogeneity. X-ray diffraction patterns of the prepared samples were recorded at room temperature using monochromatic CuK α radiation (with λ=1.5418 Å). The crystallographic parameters: lattice constant (a), ionic radius per molecule of the tetrahedral and octahedral sites (r A,r B), bond length in the two crystallographic sites (R A and R B) and oxygen position parameter (u) have been calculated. Austin Science Mössbauer Spectrometer with constant acceleration and data acquisition system is used in a standard transmission setup with a Personal Computer Analyzer (PCA II card with 1,024 channels). The radioactive source is 57Co imbedded in Rh matrix with initial activity of 50 mCi. Metallic iron spectrum is used for the calibration of both observed velocities and hyperfine magnetic fields. The absorber thickness is approximately 10 mg cm −2 of natural iron. The cation distributions and all ME parameters are calculated from the recorded Mössbauer spectra [9]. Magnetic measurements on the prepared ferrites were carried out using vibrating sample magnetometer (VSM; 9600-1 LDJ, USA) with a maximum applied field of nearly 20 kG at room temperature and the saturation magnetization M s and the coercivity H c were determined.
3 Results and Discussion
3.1 X-ray Measurements
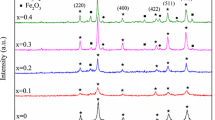
The X-ray diffraction patterns of Ni 1−x Zn x Ga y Fe 2−y O 4 with (y=0.5 and 0.0≤x≤0.5) samples are shown in Fig. 1. XRD patterns confirm the formation of single-phase face center cubic (FCC) spinel structures with \(Fd\bar {3}m\) space group, and no diffraction lines attributed to the presence of impurities or metal oxides were observed. As illustrated in Fig. 2, the partial replacement of Fe 3+ by Ga 3+ in NiFe 2 O 4 causes a decrease in the lattice constant (a). However, by replacing Ni 2+ by Zn 2+, a gradual increase in the lattice constant is observed. The lattice parameter a can be calculated theoretically based on the cation distribution obtained from Mössbauer effect spectroscopy (Section 3.3). The values of mean ionic radius per molecule of the A- site (r A) and B- site (r B) are given by the following relationships [8]:
where r(Fe3+), r(Ni2+), r(Ga3+), and r(Zn2+) are the ionic radii of Fe 3+, Ni 2+, Ga 3+, and Zn 2+ ions given in Table 1. While C Fe,C Ni,C Ga, and C Zn are the concentration of the mentioned ions in A- and B- sites, respectively. The concentration of each ion in both A and B- sites are taken from ME results. The average (A-O) and (B-O) bond lengths are R A and R B, respectively, and are given by:
where r o=1.32 Å is the radius of oxygen ion. The lattice parameter a th can be obtained from the following:
Variation of the lattice parameters (a) for NiFe 2 O 4 and Ni 1−x Zn x Ga 0.5Fe 1.5 O 4 spinel ferrites. a exp is the lattice parameter obtained from the XRD measurements and a th is the lattice parameter calculated according to the cation distribution obtained from the Mössbauer effect measurements
Table 2 summarizes the calculated values of a th, r A, r B, R A, R B, and u. The experimentally obtained values of the lattice parameter a exp are compared to a th. As shown in Fig. 2, the theoretically calculated values of (a th) are in good agreement with (a exp) and they both have the same trend. For x=0, the cation distribution suggests that Ga 3+ substitutes Fe 3+ in both A- and B- sites, and since r(Ga3+) < r(Fe3+), r A, r B, and the average bond lengths (R A and R B) decrease and consequently the lattice parameter will decrease. By introducing Zn to the system, and since r(Zn2+) > r(Ni2+), a gradual increase in the lattice parameter with increasing Zn content (x) would be expected. However, the cation distribution suggests a more complicated picture. For x < 3, Zn 2+ goes to A- site and transfers mainly Ga 3+ to the B- site. The r(Zn2+) is bigger than both r(Ga3+) and r(Fe3+), which leads to an increase in r A and R A accompanied with a decrease in r B and R B. The resultant effect is a gradual increase in the lattice parameter. For x=3, the ME measurements suggest that Zn 2+ distributes between A- and B- sites and a considerable portion of Zn 2+ ions substitute Ni 2+ in the B- sites. This causes an inverse behavior, where r A and R A decrease, while both r B and R B increase and the resultant effect is a slight decrease in the lattice parameter. For x>3, Zn continues to distribute between A- and B- sites. The ionic radius of Zn 2+ ions in the octahedral coordinates is bigger than that in the tetrahedral coordinates. As a resultant, the lattice parameter increases again for higher Zn content.
The oxygen positional parameter or anion parameter (u) for each composition was calculated using the following [8]:
The oxygen positional parameter (u) depends on the chemical composition, preparation conditions, and sintering procedure. Taking the center of symmetry at (\(\frac {1}{4}\frac {1}{4}\frac {1}{4}\)) (origin at B- site), the ideal value of parameter u is 0.25, while assuming center of symmetry at (\(\frac {3}{8}\frac {3}{8}\frac {3}{8}\)) (origin at A- site), u ideal is 0.375. For these ideal values the arrangement of O 2− ions are equals exactly a cubic closed packing, but in actual spinel lattice, this ideal pattern is slightly deformed. As illustrated in the table, both u 3m and u 43m are higher than the ideal values, which means that O 2− ions move away from the tetrahedrally coordinated A- site cation along the 〈111〉 directions due to the contraction of the tetrahedral interstices, correspondingly the octahedral B- sites become smaller.
3.2 Mössbauer Effect Measurements
Figure 3 shows room temperature Mössbauer effect measurements of NiFe 2 O 4 and Ni 1−x Zn x Ga 0.5Fe 1.5 O 4 samples. For NiFe 2 O 4, spectrum consists of two clearly split Zeeman sextets due to the almost equally distributed Fe 3+ ions between A and B- sites. For 0.0≤x≤0.3, Mössbauer effect spectra are broadening gradually and each spectra still consists of two Zeeman sextets. By increasing Zn content to x=0.4, in addition to the two sextets, a central quadruple doublet is observed. However, for x=0.5 sample, sextets disappears and two quadruple doublets completely dominate the Mössbauer effect spectrum.
The broadening of Mössbauer effect spectra is expected to develop gradually because of the introduction of two successive nonmagnetic ions (Ga 3+ and Zn 2+) with closed 3 d 10 shell. Both Ga 3+ and Zn 2+ substitute Fe 3+ and Ni 2+ and accordingly will weaken the A-A, B-B, and A-B interactions, which is expected to decrease the transition temperature (T N). The disappearance of the sextets and the appearance of the quadruple doublets in the x=5 spectrum are a straight forward signature of the paramagnetic nature of this sample.
The hyperfine parameters, isomer shift (δ), quadrupole splitting (ΔE Q), and hyperfine field (H f), of all the studied samples are summarized in Table 3. The isomer shifts (δ) of the sextets lie between 0.13 and 0.25 mm/s, which is typical values of Fe 3+ ion octahedral and tetrahedral environments. Typically for ferrites, the bond distance Fe 3+-O 2− is larger for the [B] sites than (A) sites. Therefore, the overlapping of Fe ion orbital is small at the B- sites and δ B >δ A is expected [9, 10]. The developing of isomer shift for different samples tends to have reverse behavior in A and B- sites as illustrated in Fig. 4. By introducing Ga and increasing Zn content x, δ A increases gradually in the same time δ B decreases. Such behavior may be connected to the expansion of the A-site suggested by the increase of R A and the contraction of the B- site illustrated in the decrease of R B. Generally, the quadrupole splitting (ΔE Q) has values close to zero for both (A)- and [B]- sites. In the NiFe 2 O 4 and Ni 1−x Zn x Ga 0.5Fe 1.5 O 4 system, ΔE Q has small values close to zero, but it increases for higher Zn content. Such increase can be explained that by introducing Ga 3+ and Zn 2+ ions in both A- and B- sites, it is going to increase chemical disorder in spinel structure, which generates electrical field gradient of varying magnitude, direction, sign, and symmetry [11]. Figure 4 shows that the H fB of the octahedral [B]-site is larger than the H fA of the tetrahedral (A)- site [9, 11]. The hyperfine fields of both A- site and B- sites are decreasing with introducing Ga and Zn. Ga 3+ and Zn 2+ ions (3d 10) have no unpaired d electrons and no magnetic moment, and so Ga 3+ and Zn 2+ ions do not participate in the A-A, B-B, and A-B interactions and will destructively affect magnetism and decrease H f and the transition temperatures.
3.3 Cation Distribution
The cation inversion parameter varies based on a complex set of factors, such as, preparation conditions, the electrostatic contribution to the lattice energy, cationic radii, cationic charge, and crystal-field effects [8]. Although Mössbauer effect spectroscopy is only sensitive for the Fe ions in the samples, we used the Fe 3+ content in the A- and B- sites (Fig. 4) as a building block to the whole cation distribution picture. For Ni ferrite, it is straightforward; only Fe and Ni should be distributed between the A and B sites. It is well established that Ni ferrite is an inverse spinel ferrite, where Ni 2+ ions occupy the B- site and Fe 3+ ions distribute equally between the A- and B- sites. Depending on Fe content in both sites, the rest available places will be filled with Ga or Zn ions. The suggested cation distribution is summarized in Table 4. For x=0 sample, we considered Ni ions in the B- site and Ga ions are distributed between both sites. However, introducing Zn makes the situation more complicated. Zn ferrite is known to be normal spinel; Zn 2+ ions have strong preference to occupy A-site. For some preparation methods, Zn ion substitutes Fe in the B- site and cause a degree of inversion in Zn ferrite [12–14]. In our case, for small x concentration (x < 0.3), Zn is situated as expected in the A- site and transfer Ga from A- to B- site. However, for x>0.3 and since Mössbauer effect measurements suggest an almost equal distribution of Fe between A- and B- sites, therefore Zn ions would substitute Ni in B-site.
3.4 VSM Measurements
Vibrating sample magnetometer (VSM) measurements of NiFe 2 O 4 and Ni 1−x Zn x Ga 0.5Fe 1.5 O 4 samples are shown in Fig. 5. The magnetic parameters obtained from the M-H curves such as saturation magnetization (M s) and coercivity (H c) are summarized in Table 5. The saturation magnetization M s and coercivity H c are plotted in Fig. 6. M s is found to increase by the initial replacement of Fe 3+ by Ga 3+ and then it decreases slightly for x≤0.2. However, for x≥0.3, M s decreases rapidly. From the measured saturation magnetization, the observed magnetic moment (μ obs) per formula unit in Bohr magneton (μ B ) is given by the following [9]:
where MW is the molar weight of the samples. On the other side, the magnetization can be calculated using Néel’s model and depending on the cation distribution obtained from ME measurements. According to Néel’s model, the μ cal is the vector sum of the magnetic moments of the individual (A) and [B] sublattices:
Variation of saturation magnetization M s and coercivity H c for the NiFe 2 O 4 and Ni 1−x Zn x Ga 0.5Fe 1.5 O 4 samples. In the upper panel, a comparison between the observed moment μ obs obtained from the saturation magnetization M s and the calculated moment μ cal obtained from the Néel model. We used the cation distribution deduced from the Mössbauer effect measurements
The only magnetic ions in Ni 1−x Zn x Ga y Fe 2−y O 4 system are Ni 2+ and Fe 3+ and have magnetic moments 2 μ B and 5 μ B, respectively. For complete inverse Ni ferrite, the cation distribution is (Fe 3+)[Ni 2+Fe 3+]O\(^{2-}_{4}\) and μ cal will be exact 2 μ B. The μ cal calculated using the cation distribution obtained from ME results is shown in Table 5. Figure 6 represents the comparison between μ obs and μ cal. The values and the behavior of the calculated moments are in good agreement with the observed moments, which strongly support the cation distribution suggested by the Mössbauer effect measurements. As indicated in Fig. 6, the coercivity H c initially increases by introducing Ga 3+ ions and then decreases with increasing Zn content x. H c depends on anisotropy constant, K. By increasing Zn content, which has low anisotropy constant value compared to Ni, H c is expected to decrease [15].
4 Conclusion
Ni-Ga-Zn ferrite samples are synthesized using solid-state reaction technique and found to have a single-phase cubic spinel. The structural and magnetic properties have been studied with different techniques. These properties were found to be influenced by the Ga and Zn substitution. Mössbauer effect results showed that Ga 3+ ion substitutes Fe 3+ in both A and B- sites. For low Zn content, Zn 2+ goes to A- site but for x≥0.3, unusually, it distributes between both A- and B- sites. Depending on this cation distribution, the calculated lattice parameter a th and the calculated moment μ cal are in very good agreement with the observed values from XRD and VSM measurements.
References
Igarash, H., Okazaki, K., Am, J.: Ceram. Soc. 60, 51 (1977)
Ata-Allah, S.S., Fayek, M.K., Sayed, H.A., Yehia, M.: Mater. Chem. Phys. 92, 278 (2005)
Birajdar, D.S., Mane, D.R., More, S.S., Kawade, V.B., Jadhav, K.M.: J. Mater. Lett. 59, 2981 (2005)
Dixit, G., Pal Singh, J., Srivastava, R.C., Agrawal, H. M.: J. Magn. Magn. Mater. 324, 479 (2012)
Ata-Allah, S.S.: J. Solid State Chem. 177, 4443 (2004)
Kanno, R., Kawamoto, N., Takeda, Y., Masegawa, M., Yamamoto, O., Kinomura, N.: J. Solid State Chem. 96, 397 (1992)
Lange’s Handbook of chemistry, John A. Dean, McGRAW-HILL, INC (1999)
Lakhani, V.K., Pathak, T.K., Vasoya, N.H., Modi, K.B.: Solid State Sci. 13, 539 (2011)
Yehia, M., Ismail, S. M., Hashhash, A., Supercond, J.: Nov. Magn. 27, 771 (2014)
Yehia, M., Labib, Sh., Ismail, S. M.: Physica B 446, 49 (2014)
Ata-Allah, S.S., Fayek, M.K., Yehia, M., Magn, J.: Magn. Mater. 279, 411 (2004)
Tanaka, K., Makita, M., Shimizugawa, Y., Hirao, K., Soga, N., Phys, J.: Chem Solids 59 (9), 1611 (1998)
Šepelák, V., Steinike, U., Uecker, D. Chr. , Wißmann, S., Beckerà, K. D.: J. Solid State Chem. 135, 52 (1998)
Anantharaman, M.R., Jagatheesan, S., Malini, K.A., Sindhu, S., Narayanasamy, A., Chinnasamy, C.N., Jacobs, J.P., Reijne, S., Seshan, K., Smits, R.H.H., Brongersma, H.H.: J. Magn. Magn. Mater 189, 83 (1998)
Najmoddin, N, Beitollahi, A, Kavas, HS, Mohseni, SM, Rezaie, H, Åkerman, J, Toprak, MS: J. Ceram. Inter. 40, 3619 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hashhash, A., Yehia, M., Ismail, S.M. et al. Structural and Magnetic Study of Zn-Substituted NiGa y Fe 2−y O 4 Ferrite. J Supercond Nov Magn 27, 2305–2310 (2014). https://doi.org/10.1007/s10948-014-2594-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-014-2594-0