Abstract
Ultrasmall nanocrystalline NiSm0.01Fe1.99O4 and Ni1−x Zn x Sm0.01Ga0.5Fe1.49O4 (x = 0.0, 0.3, 0.5) ferrites were synthesized by sol-gel method. The effect of Ga and Zn substitution on the particle size and structural and magnetic properties was investigated using X-ray diffraction patterns (XRD), transmission electron microscope (TEM), Mössbauer effect spectroscopy (ME), and vibrating sample magnetometer (VSM) techniques. XRD measurements suggest the formation of single-phase spinel ferrites with ultrasmall particles size ranging from 4 to 8 nm. Mössbauer effect measurements show a clear signature of superparamagnetism in the studied samples, where the reduced particle size causes the broadening of the Mössbauer spectra and the presence of a pronounced central doublet in all the measured samples. VSM measurements show a strong reduction of saturation magnetization due to size effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nickel ferrites are soft magnetic materials which have been studied for several decades because of their wide range of technological applications. The magnetic properties of these inverse spinel ferrites are governed by the chemical composition and the method of preparation. Altering the chemical composition is achieved by doping nickel ferrites with different transition metals and rare earth (R) ions [1–3]. Both the type of the dopant and its amount are critical in changing the cation distribution and consequently the properties of the doped Ni-ferrite [1, 2]. By using different methods of preparation, different size, shapes, and size distribution are obtained [4]. Several chemical methods have been developed to produce nanocrystalline ferrites, e.g., sol-gel [5, 6], citrate precursor [7], co-precipitation [8], and microwave-assisted hydrothermal [9]. Among different dopants, nickel-zinc ferrites happen to be the most researched materials due to their remarkable magnetic properties such as large permeability at high frequency, remarkably high electrical resistivity, and low power loss [10–13].
On the frame of a research project, we aimed to systematically study the effect of both doping (Ga, Zn, and R) and preparation method on the structural and magnetic properties of nickel ferrites. For the doping part, a single-phase-R (Sm, Gd, Eu, La)-doped NiFe 1.99 R 0.01 O 4 samples were prepared using the standard ceramic method [1]. Mössbauer effect measurements suggested that R 3+ ions substitute Fe 3+ ions in the octahedral B site. An increase in the coercivity and saturation magnetization was observed for all doped samples, where the highest saturation magnetization was obtained for Sm-doped Ni-ferrite sample. Later, Ga- and Zn-doped Ni 1−x Zn x Fe 1.5Ga 0.5 O 4 (0≤x≤0.5) were prepared by the same standard ceramic method [2]. Ga 3+ ions substituted Fe 3+ ions in both A and B sites with slight preference to the A site. By introducing Zn to the unit cell, Zn 2+ ions initially set on the A site and transfer Ga 3+ ions gradually to the B site. For x≥0.3, Zn 2+ ions unusually distributed in both A to B sites. Recently, the standard ceramic method was used to prepare Ga-, Zn-, and Sm-doped Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 (0.0≤x≤0.5) samples [3]. Our measurements confirmed that Sm 3+ ions favor the B sites and support magnetism in the studied system. On the other hand, Ga 3+ and Zn 2+ ions tend to distribute similar to their distribution in Ni 1−x Zn x Fe 1.5Ga 0.5 O 4 system. For Sm-doped and Sm-free Ni 1−x Zn x Fe 1.5Ga 0.5 O 4 samples, Zn substitution in the octahedral B site (x≥0.3) clearly destabilizes the magnetic order and suppresses magnetism in the studied systems.
On the other hand, Ni-ferrite samples were synthesized using different preparation methods [4], which yields samples with different particle sizes. The effect of decreasing the particle size (D) on the magnetic properties was carefully studied. The analysis of Mössbauer effect measurements suggested that NiFe 2 O 4 nanoparticles deviate from the perfect inverse spinel structure to a partial inverse spinel structure with decreasing D. The Mössbauer hyperfine field (H f) and M s shows the same particle size dependence, where they decrease gradually by decreasing D. The coercivity (H c) showed a remarkable D dependence, where it reaches its maximum around D≈48 nm and then decreases gradually for lower D. The appearance of strong broad doublets in the Mössbauer effect spectra for D<20 nm samples and the reduced values of H f and M s illustrated the superparamagnetic behavior of nano-sized ferrite crystallites. From the coercivity size dependence, the superparamagnetic region was estimated below D sp=14 nm.
In the present paper, ultrasmall NiSm 0.01Fe 1.99 O 4 and Ni 1−x Zn x Sm 0.01Ga 0.5Fe 1.49 O 4 (x = 0.0, 0.3, 0.5) nanoparticles were prepared using sol-gel method. The structure and particle size were studied using X-ray diffraction and transmission electron microscope. Mössbauer effect spectroscopy and VSM were utilized to investigate the magnetic properties of the studied samples. Our measurement confirms the critical effect of the reduced particle size on the magnetic properties, and a clear signature of superparamagnetism was observed in the studied samples. The results obtained for NiSm 0.01Fe 1.99 O 4 and Ni 1−x Zn x Sm 0.01Ga 0.5Fe 1.49 O 4 nanoparticles are compared to our results obtained before for the bulk NiSm 0.01Fe 1.99 O 4 and Ni 1−x Zn x Sm 0.01Ga 0.5Fe 1.49 O 4 samples prepared by the standard ceramic method and published in Ref. [3].
2 Experimental Details
NiFe 1.99Sm 0.01 O 4 and Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 (x = 0, 0.3, 0.5) nanocrystalline samples were synthesized using stoichiometric amounts of iron, nickel, gallium, zinc, samarium nitrates, and citric acid using deionized water as a solvent. The solution was heated under stirring to evaporate the solvent water. The gel was dried at 180 ∘C for 1 h, then heated at 400 ∘C for an hour and then cooled to room temperature [14]. X-ray powder diffraction patterns of the samples were collected on a Philips diffractometer (X’pert MPD) with a goniometer using Cu-K α radiation. The diffracted intensities were collected in step-scan mode (step size 2𝜃 = 0.02 ∘; counting time 2 s) in the angular range 2𝜃 = 10–80 ∘. To correct instrumental broadening, LaB 6 standard was used. The crystal structure and microstructure were refined applying Rietveld profile method [15], using MAUD program [16] and applying Popa anisotropic model [17]. Transmission electron microscope (TEM) images were performed using a JEOL 4010 transmission electron microscope to confirm the formation of NiFe 1.99Sm 0.01 O 4 and Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 (x = 0, 0.3, 0.5) nanoparticles.
Austin Science Mössbauer Spectrometer with constant acceleration and data acquisition system is used in a standard transmission setup with a Personal Computer Analyzer (PCA II-card with 1024 channels). The radioactive source is 57Co imbedded in Rh matrix with initial activity of 50 mCi. Metallic iron spectrum is used for the calibration of both observed velocities and hyperfine magnetic fields. The absorber thickness is approximately 10 mg cm −2 of natural iron. The cation distributions and all (ME) parameters are calculated from the recorded Mössbauer spectra [1]. Magnetic measurements on the prepared ferrites were carried out using vibrating sample magnetometer VSM (9600-1 LDJ, USA) with a maximum applied field of nearly 20 kG at room temperature, and the saturation magnetization M s and the coercivity H c were determined.
3 Results and Discussion
3.1 Structural and Microstructure Analysis
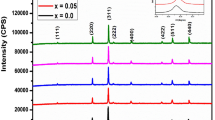
Figure 1 shows the XRD patterns of NiFe 1.99Sm 0.01 O 4 and Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 (x = 0, 0.3, 0.5) samples. The phase identification indicated that all patterns refer to a single phase cubic spinel structure (space group \(Fd\bar {3}m\)). The phase analysis was carried out by matching the obtained diffractograms with the standard ICDD card number 22-1086. The first clue of the reduced particle size is clear from the broad peaks in all diffraction patterns. The structural and microstructural parameters obtained from the Rietveld analysis carried out applying the MAUD program are given in Fig. 2 and Table 1. The lattice parameter (a) values are very close to the values observed for the bulk samples [3]. From Table 1, a initially increases up to x=0.3 and then decreases for higher Zn content. The increase and decrease of the lattice parameters is connected to spinel ferrite structural parameters such as (i) the different percentage of oxygen vacancies and pores present in each Zn composition and (ii) the type and amount of the metal ions in the tetrahedral A site compared with those in the octahedral B site [18, 19]. Although the nanoparticles and bulk samples have the same composition (Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4), the cation distribution and percentage of oxygen can be different due to the reduced particle size and the change of the preparation method. This may cause small differences in the lattice parameters and its behavior.
For bulk Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4, a increased almost linearly for 0≤x≤0.4 and then decreased. This results were explained in the frame of the cation distribution, where for x≤0.4 Zn 2+, ions go to the A site and transfer Ga 3+ ions to the B site. Only for x>0.3, Zn 2+ distributes between A and B sites. Depending on these changes in the cation distribution and the differences in the ionic radii of Fe 3+, Ga 3+, Zn 2+, and Ni 2+, the behavior of the lattice parameter was explained [3]. For the present nanoparticles, it is difficult to determine the cation distribution for Zn doped samples from the ME measurements since the ME spectra are relaxed and contains only a doublet and a singlet for x=0.3 and x=0.5 samples as discussed in the next section. However, the similarity in the lattice parameter behavior for the bulk and nanoparticles would refer that they both have similar cation distributions.
As illustrated in Table 1, the substitution of Ga 3+ ions reduces the grain size from 8 to 4 nm for NiFe 1.99Sm 0.01 O 4 and NiFe 1.99Ga 0.5Sm 0.01 O 4 samples, respectively, and similar result was obtained for NiFe 2−x Ga x O 4 nanoparticles [20]. The effect of Zn in Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 matrix is increasing the grain size, and similar result was obtained for Zn-doped Ni 1−x Zn x Fe 2 O 4 nanoparticles [12]. The oxygen positional parameter or anion parameter (u) depends on the chemical composition, preparation conditions, and sintering procedure and can be obtained from Rietveld refinement of XRD data. As seen in Table 1, the oxygen position parameter first decreases for NiFe 1.99Ga 0.5Sm 0.01 O 4, then increases for Zn 2+-doped samples. Taking the center of symmetry at (\(\frac {1}{4}\frac {1}{4}\frac {1}{4}\)) (origin at B site), the ideal value of u is 0.25, while assuming center of symmetry at (\(\frac {3}{8}\frac {3}{8}\frac {3}{8}\)) (origin at A-site), u ideal is 0.375. For these ideal values, the arrangement of O 2− ions correspond exactly to a cubic closed packing, but in actual spinel lattice, this ideal pattern is slightly deformed. When u>0.375, O 2− ions move away from the cations in tetrahedral A site along 〈111〉 direction due to the expansion of the tetrahedral interstices; accordingly, the octahedral B sites become smaller. This leads to a decrease in the A-A interactions and an increase in the B-B interactions. When u<0.375, the effect is reversed. The bond lengths between cations (Me-Me) (b, c, d, e, and f) and cations-anion (Me-O) (p, q, r, and s) and the bond angles (𝜃 1, 𝜃 2, 𝜃 3, 𝜃 4, and 𝜃 5) between the cations and cation-anion, Fig. 3, are given by the following relations [21]:
The values of the interatomic distances between cation-anion (Me-O) and cation-cation (Me-Me) are listed in Table 2. The interatomic distances between the cation-anion (p, q, r, and s) and those between the cations (b, c, d, e, and f) change by increasing the Zn concentration. This can lead to the change in the super-exchange strength (wave-function overlapping) and change the magnetic properties. Table 3 shows the bond angles for all samples. For NiFe 1.99Ga 0.5Sm 0.01 O 4, the angles 𝜃 1, 𝜃 2, and 𝜃 5 increase, while 𝜃 3 and 𝜃 4 decrease. The angles 𝜃 1, 𝜃 2, and 𝜃 5 suggest the strengthening of the A-B and A-A interactions, while the decrease in 𝜃 3 and 𝜃 4 indicates the weakening of the B-B interaction in the system. Generally in ferrimagnetic materials, the increase in A-B (𝜃 1 and 𝜃 2 angles) enhances the super-exchange strength [21]. As Zn 2+ ions substitute nickel, the situation is reversed; A-B (𝜃 1 and 𝜃 2 angles) decreases; hence, super-exchange strength decreases.
Transmission electron microscopy provides information about morphological characteristics such as the particle size, shape, and size distribution. TEM micrographs for different NiFe 1.99Sm 0.01 O 4 and Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 samples are presented in Fig. 4. Most of the particles appear to be spherical in shape. The average grain size values obtained from TEM pictures are from 10 to 15 nm, which are slightly greater than the corresponding particle sizes obtained from XRD patterns. This might indicate that every particle is formed by the agglomeration of a small number of crystallites or grains [22].
3.2 Mössbauer Effect Measurements
Figure 5 shows Mössbauer effect spectra for NiFe 1.99 Sm 0.01 O 4 and Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 samples at room temperature. The values of the Mössbauer effect parameters, isomer shift (δ), quadrupole splitting (ΔE Q) and Hyperfine field (H f), are summarized in Table 4. The spectra of NiFe 1.99Sm 0.01 O 4 and NiFe 1.49Ga 0.5Sm 0.01 O 4 consist of two sextets and a doublet. The presence of the two sextets is a clear indication of the ferrimagnetic nature of the samples and confirms the existence of two Fe sites (A site and B site) in this spinel structure. δ values are within the expected values for Fe 3+ ions in the tetrahedral (A) and octahedral (B) environments [1, 4], and ΔE Q has values close to zero for both sites.
The area ratio between the two sextets reflects the ratio between Fe 3+ contents in A and B sites and allows the determination of the cation distribution. Only NiFe 1.99 Sm 0.01 O 4 and NiFe 1.49Ga 0.5Sm 0.01 O 4 spectra contain magnetic sextets. The suggested cation distributions for these samples are (Fe 0.98Ni 0.02) A [Fe 1.01Ni 0.98Sm 0.01] B O 4 and (Fe 0.81Ga 0.19) A [Fe 0.68NiGa 0.31Sm 0.01] B O 4, respectively. The cation distribution of NiFe 1.99Sm 0.01 O 4 nanoparticles is the same as the bulk sample; however, the values of H f are significantly smaller for the nanoparticles [1]. For NiFe 1.49Ga 0.5Sm 0.01 O 4 nanoparticles, smaller portion of Ga 3+ ions sets on the A site in comparison to the bulk sample; however, the value of H f is slightly higher for the nanoparticles [3]. In nanoparticles, the magnetic anisotropy energy is proportional to the volume, and for very small particles, it may therefore be comparable to the thermal energy. This results in superparamagnetic relaxation, i.e., thermally induced reversals of the magnetization direction [4, 23]. Such magnetic fluctuations yield a particle size D-dependent magnetization and hyperfine field, where \(H_{\text {f}}\propto \frac {1}{D^{3}}\). The doublet seen in Mössbauer effect spectra is due to superparamagnetic nature of ferrite nanoparticles. The coexistence of the two sextets and the doublet is attributed to the particle size distribution in the studied samples, where particles with D>D sp are responsible for the magnetic sextets and particles with D<D sp would contribute to the superparamagnetic doublet. The effect of the particle size is combined with the effect of doping with nonmagnetic Ga 3+ ions for NiFe 1.49Ga 0.5Sm 0.01 O 4 sample, which leads to a stronger doublet contribution as indicated in Fig. 5.
The situation is different for Ni 0.7Zn 0.3Fe 1.49 Ga 0.5 Sm 0.01 O 4 and Ni 0.5Zn 0.5Fe 1.49Ga 0.5Sm 0.01 O 4 nanoparticles. In this case, in addition to the doublet, each Mössbauer effect spectrum contains a singlet. Figure 6 presents the comparison between the ME spectrum for Ni 0.7Zn 0.3 Fe 1.49Ga 0.5Sm 0.01 O 4 nanoparticles and Ni 0.7Zn 0.3Fe 1.49 Ga 0.5Sm 0.01 O 4 bulk sample. In Ni 1−x Zn x Fe 1.49Ga 0.5 Sm 0.01 O 4 bulk samples, increasing Zn content to x=0.3 and above is associated with destabilizing the magnetic order and a gradual broadening in ME spectra was observed. Along with the broadening of the spectra, a central quadruple doublet is emerged for x>0.3, which indicates the weakening of the A-A, B-B, and A-B magnetic interactions and the decrease of the transition temperature (T N) for these samples. In Ni 0.7Zn 0.3Fe 1.49Ga 0.5Sm 0.01 O 4 and Ni 0.5Zn 0.5Fe 1.49Ga 0.5Sm 0.01 O 4 nanoparticles, the reduced particle size coexists with a fragile magnetic order and smears magnetism in the two samples, so consequently the magnetic sextets disappear.
A comparison between Mössbauer effect spectrum for Ni 0.7Zn 0.3Fe 1.49Ga 0.5Sm 0.01 O 4 nanoparticles and Ni 0.7Zn 0.3Fe 1.49Ga 0.5Sm 0.01 O 4 bulk sample from Ref. [3]
3.3 VSM Measurements
Figure 7 shows the variation of the magnetization as a function of the applied magnetic field for NiFe 1.99Sm 0.01 O 4 and Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 at room temperature. The saturation magnetization (M s) and coercivity (H c) obtained from the M–H curves are shown in Table 1.
Magnetic properties of Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 are strongly dependent on Zn content and the cation distribution between A and B sites. For nanoparticles, the magnetic properties depend on the particle size and the particle size distribution. M s decreases for smaller size because the surface of the nanoparticles is composed of some distorted spins that repel the core spins to align the field direction and they act as a dead layer with inconsiderable magnetization [25]. M s values obtained for NiFe 1.99Sm 0.01 O 4 and Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 nanoparticles are significantly smaller than the values obtained for bulk samples [1, 3]. Similar to the bulk samples, the saturation magnetization values for Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 (x = 0, 0.3, 0.5) nanoparticles are smaller than M s for NiFe 1.99Sm 0.01 O 4 nanoparticles. However, M s is almost constant for all x = 0, 0.3 and 0.5 samples. The strong reduced values of M s and its almost constant values for different x content would suggest that the effect of the reduced particle size is stronger than the effect of doping with Zn 2+ and Ga 3+ nonmagnetic ions in this system.
The coercivity is influenced by factors such as particle size, particle morphology, and size distribution. Large particle sizes energetically prefer the formation of domain walls, and the magnetization reversal happens mainly through nucleation and motion of these walls. In this case, coercivity decreases with increasing D. As the particle size decreases to the single domain value D c, the formation of domain walls becomes unfavorable [4, 24] and the magnetization process happens through a coherent relation of magnetic moment, resulting in large coercivity. As the particle size decreases below D c, H c decreases with decreasing the particle size. Below D sp, the magnetic ordering state is easily collapsed due to thermal fluctuation and the material exhibits a superparamagnetic feature.
For NiFe 1.99Sm 0.01 O 4 and Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 nanoparticles, the coercivity values are very small, indicating that D<D c. These results are supported by Mössbauer effect measurement where a pronounced central doublet is observed in ME spectra. The contribution of the doublet to the whole spectra is increasing for Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 samples, which is reflected in lower values of the H c.
4 Conclusion
Ultrasmall nanocrystalline Zn-substituted Ni-Sm-Ga ferrites were synthesized using sol-gel method. X-ray diffraction and transmission electron microscope measurements suggest the formation of single-phase spinel NiFe 1.99Sm 0.01 O 4 and Ni 1−x Zn x Fe 1.49Ga 0.5Sm 0.01 O 4 nano particles with D = 4 ∼8 nm. The pronounced central doublet appeared in the ME spectra for all samples is a strong indication of superparamagnetism in the studied samples. VSM measurements show a strong reduction of saturation magnetization and coercivity due to particle size effects. Our measurements clearly confirm the critical effect of the reduced particle size on the magnetic properties of this system.
References
Yehia, M., Ismail, S.M., Hashhash, A.: J. Supercond. Nov. Magn. 27, 771 (2014)
Hashhash, A., Yehia, M., Ismail, S.M., Ata-Allah, S.S.: J. Supercond. Nov. Magn. 27, 2305 (2014)
Yehia, M., Ismail, S.M., Ata-Allah, S.S.: J. Supercond. Nov. Magn. (2015). doi:10.1007/s10948-015-3132-4
Yehia, M., Labib, S.h., Ismail, S.M.: Physica B 446, 49 (2014)
George, M., Nair, S.S., John, A.M., Joy, P.A., Anantharaman, M.R.: J. Phys. D: Appl. Phys. 39, 900 (2006)
Mohamed, B.M., Yehia, M.: J. Alloy. Comp. 615, 181 (2014)
Singh, A.K., Verma, A., Thakur, O.P., Prakash, C., Goel, T.C., Mendiratta, R.G.: Mater. Lett. 57, 1040 (2003)
Mohamed, B.M., Wahbab, A.M., Yehia, M.: Mater. Sci. Eng. B 190, 52 (2014)
Kothawale, M.M., Tangsali, R.B., Naik, G.K., Budkuley, J.S.: J. Supercond. Nov. Magn. 25, 1907 (2012)
Huili, H., Grindi, B., Viau, G., Tahar, L.B.: Ceram. Int. 40, 16235 (2014)
Kurmude, D.V., Kale, C.M., Aghav, P.S., Shengule, D.R., Jadhav, K.M.: J. Supercond. Nov. Magn. 27, 1889 (2014)
Kurmude, D.V., Barkule, R.S., Raut, A.V., Shengule, D. R., Jadhav, K.M.: J. Supercond. Nov. Magn. 27, 547 (2014)
Venkatesh, D., Himavathi, G., Ramesh, K.V.: J. Supercond. Nov. Magn. (2015). doi:10.1007/s10948-015-3098-2
Mohamed, B.M., El-Sayed, K.: Mater. Res. Bull. 48, 1778 (2013)
Rodriguez-Carvajal, J.: Physica B 192, 55 (1993)
Lutterotti, L.: Maud 2.33, http://www.ing.unitn.it/maud/
Popa, N.C.: J. Appl. Cryst. 31, 176 (1988)
Pradhan, S.K., Bidb, S., Gateshki, M., Petkov, V.: Mater. Chem. Phys. 93, 224 (2005)
Carta, D., Casula, M.F., Falqui, A., Loche, D., Mountjoy, G., Sangregorio, C., Corrias, A.: J. Phys. Chem. C 113, 8606 (2009)
Heiba, Z.K., Mohamed, B.M., Hamdeh, H.H., Ahmed, M.A.: J. Alloy.Comp. 618, 755 (2015)
Karimi, Z., Mohammadifar, Y., Shokrollahi, H., Khameneh Asl, Sh., Yousefi, Gh., Karimi, L.: J. Magn. Magn. Mater. 361, 150 (2014)
Pradeep, A., Priyadharsini, P., Chandrasekaran, G.: J. Alloys Compd. 509, 3917 (2011)
Mørup, S., Frandsen, C., Fougt Hansen, M.: Beilstein J. Nanotechnol. 1, 48 (2010)
Tapan, K.S., Andrey, L.R.: Complex-shaped metal nanoparticles. Wiley (2012)
Mastai, Yitzhak: Crystalization in spinel ferrite nanoparticles, advances in crystallization processes, ISBN 978-953-51-0581-7, InTech (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yehia, M., Ismail, S.M. & Mohamed, M.B. Synthesis and Characterization of Ultrasmall Nanocrystalline Zn-substituted Ni-Sm-Ga Ferrites. J Supercond Nov Magn 28, 3335–3342 (2015). https://doi.org/10.1007/s10948-015-3162-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-015-3162-y