Abstract
The La1−xSrxCrO3 (x = 0–0.1) negative temperature coefficient (NTC) ceramics have been prepared by the traditional solid state reaction method. X-ray diffraction (XRD) analysis has revealed that the as-sintered ceramics crystallize in a single perovskite structure. Scanning Electron Microscope (SEM) images show that the doped Sr2+ contributes to in the decrease in porosity. X-ray photoelectron spectroscopy (XPS) analysis indicates the existence of Cr3+ and Cr6+ ions on lattice sites, which result in hopping conduction. The presence of the Cr6+ is one of the key factors that affect the electrical conductivity of La1−xSrxCrO3. Resistance–temperature characteristics were studied in the range of −80 to 10 °C for the ceramic samples, the electrical characterizations show that the electrical resistivity and material constant B decrease with the increase of the strontium content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
LaCrO3 material is stable perovskite rare earth oxide having attracted extensive attention of researchers because of their variety of properties, such as good conductivity, excellent catalytic performance, outstanding ferroelectric and etc. [1–5]. LaCrO3 material by doping other metallic elements in A or B-sites get rapid development owing to their excellent properties, such as high melting points, high mechanical, chemical stability and good electrical conductivity [6–8]. Furthermore, LaCrO3 doped with appropriate impurity ions can be applied to the NTC thermistor materials [9, 10]. NTC thermistors are thermally sensitive resistors whose resistance decreases with increasing temperature [11, 12]. However, LaCrO3 material has too high electrical resistivity at room temperature because of the weak sintering properties in the air. In order to improve the electrical properties of LaCrO3, the A-site was doped by alkaline-earth metals [13, 14]. Considering the ions radius of Sr2+ and La3+ is similar, and the introduction of Sr2+ into the lattice could be compensated by the oxidation from Cr3+ to Cr6+ [15, 16], the substitution of Sr2+ for La3+ in LaCrO3 will promote the hopping conductivity due to the presence of Cr3+ and Cr6+ ions and thereby decrease resistivity [15, 17]. Therefore, it is possible that NTC thermistor material could be obtained through the substitution of Sr2+ for La3+ in LaCrO3. In this paper, the new NTC ceramic material La1−xSrxCrO3 (x = 0–0.1) were studied. The structure and electrical properties of La1−xSrxCrO3 (x = 0–0.1) ceramics were investigated.
2 Experimental procedures
Powders of La1−xSrxCrO3 (x = 0–0.1) were prepared by solid-state reaction. Stoichiometric amounts of La2O3 (purity >99.99%, Sinopharm Chemical Reagent co., Ltd, China), Cr2O3 (purity >99%, Sinopharm Chemical Reagent co., Ltd, China), and SrCO3 (purity >99%, Sinopharm Chemical Reagent co., Ltd, China) were well mixed, ground, and calcined at 1100 °C for 5 h. The calcined powders were then milled in mortar for 8 h. Subsequently, the powders were pressed at 10 MPa to form a disk of 10 mm in diameters and 3 mm in thickness, and then cold isostatic pressing at 150 MPa was used to enhance densities. The pressed disks were sintered in the temperature of 1600 °C for 5 h.
X-ray diffraction (XRD; BRUKERD8-ADVANCE, Cu K α radiation) analysis was used to identify the crystalline phases of the sintered ceramic samples. Scanning Electron Microscope (SEM; Zeiss SUPRA 55 VP, Germany) was used to observe the microstructure of the sintered ceramic samples. X-ray photoelectron spectroscopy (XPS; Thermo, ESCALAB 250XI) was used to analyze the chemical states of the sintered ceramics.
In order to obtain the electrical conductivity, the disks were coated with a platinum paste, and then annealed at 1200 °C for 2 h. The resistivity was measured in the temperature of −80 to 10 °C by Agilent34970A multimeter in an oil bath.
3 Results and discussion
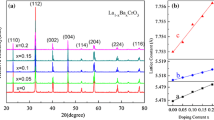
Figure 1 shows the XRD patterns of the as-sintered La1−xSrxCrO3 ceramics. Analysis of these diffractograms has revealed that the ceramics are indexed to be orthorhombic perovskite phase isomorphic to LaCrO3 (32-1240 card) described by the space group is P bmn. There is no other impurity phase, which shows that Sr2+ has all entered the perovskite lattice to form the solid solution. It is noted that the peaks corresponding to the perovskite phase in the ceramic samples slightly shift toward higher angles with the increase of Sr2+, which means that the unit cell volume decreases. This result from many factors such as the ionic radius of the acceptor dopant (Sr2+), the mixed valence state of the chromium ion, and the oxygen deficiency. The substitution of size larger Sr2+ (0.144 nm) for La3+ (0.136 nm) should cause an incremental increase in the until cell volume. However, the crystal structure cell volume decreased with the increase of Sr2+. Comparing the sizes of the relevant ions, it is clear that the amount of the relative increment in the unit cell volume due to the substitution of larger Sr is less than that of the relative decrement caused by the formation of chrome ions, which leads to the lattice contraction. La1−xSrxCrO3 ceramics introduction of Sr2+ contributes to the Cr3+ was oxidized to Cr6+, which results in the decrease in the cell volume as a result of Cr6+ (0.044 nm) radius less than Cr3+ (0.055 nm). The results will be discussed with XPS analysis.
Figure 2 shows the SEM micrographs of the as-sintered La1−xSrxCrO3 ceramics. As can be seen, there was high porosity on the surface of ceramics, and the size of agglomerate Sr2+ doped LaCrO3 samples slightly decreases compared to pure LaCrO3. This may be due to the chromium volatilization at the high temperature in air atmosphere [15, 18]. Therefore, the chromium vaporization will be results in a poor sinterability of these ceramics and thereby producing little pore.
Figure 3 shows the XPS spectra of La 3d obtained from the ceramic samples. It can be seen that the spectra peaks are associated with the presence of lanthanum in the form of La3+ [19]. Figure 4 shows XPS spectra of Sr 3d obtained from the ceramic samples. It can be seen that the spectra only contained one single peak associated with the presence of strontium in the form of Sr2+ [20].
Figure 5 shows the XPS spectra and the curve-fitting example of La1−xSrxCrO3 ceramics in the regions of Cr 2p core-level peaks. As can be seen, the peaks in the Cr 2p3/2 and Cr 2p1/2 spectra can be split into two peaks. The peak at 576.0 eV is assigned to Cr3+ and 580.0 eV to Cr6+ [21]. Similar result has been reported on other doped lanthanum chromite systems [13, 21]. From Fig. 5, it can be seen that Cr6+ concentration increases with increasing Sr concentration, which lead to decreasing the cell volume. This is agreement with the previous XRD results.
Figure 6 shows the relationship between the natural logarithm of the resistivity (In ρ) and the reciprocal of the absolute temperature (1000/T) for the as-sintered La1−xSrxCrO3 NTC thermistors. It can be seen that the samples exhibited typical NTC characteristic whose resistivity decreases with increasing temperature over the measured temperature range. The resistance of NTC thermistors can be expressed by the equation [22, 23]:
where \(\uprho\) is the resistance at infinite temperature, Ea is the activation energy, K is the Boltzmann constant and T is the absolute temperature. Ea can be calculated from the slope of the In ρ versus 1/T plots. It is also noted that the relationship between ln ρ and 1/T of all the samples turned out to be linear from Fig. 6. This linear dependence indicates an electrical conduction phenomena explained by polarons jumps [24]. The chromium volatilization at the high temperature in air atmosphere lead to the formation of Cr6+ ions. Due to the presence of these higher valent cations, holes are generated as per the following defect reaction [25]:
The holes, generated from the above equation, along with the lattice interaction form small polarons. In the present study, it not create any additional defect in LaCrO3 ceramics, and therefore the conductivity is due to the polarons generated from Eq. (2). When 0.01 ≤ x ≤ 0.1, for the substitution of La3+ by Sr2+, the valence state of Cr3+ ions partially oxidized to Cr6+ to maintain the electrical neutrality. The ratio of Cr6+ to Cr3+ increases with x, this lead to the decrease in resistivity of La1−xSrxCrO3 system [17, 26] as the concentration of small polarons increases. This is agreement with the previous XPS results. As shown in Table 1, the values of ρ−70, ρ−10, B−70/−10 constant and activation energy Eα of the thermistors were in the range of 16.51–84100, 4.77–2170 Ωcm, 1106–3268 K, and 0.095–0.283 eV, respectively. The B value can be calculated by the following equation [12]:
where R1 and R2 are the resistance value that measured by the temperature at T1 and T2, respectively. From Table 1 and Fig. 6 the variation trend of activation energy for different compositions of La1−xSrxCrO3 indicates that activation energy continuously decreases with Sr2+ ions increases. These results indicate that the electrical properties can be adjusted by the Sr2+ ions content.
In order to reveal the relation between ρ−70, B−70/−10 and Sr ions content clearly, Fig. 7 is presented. As shown in Fig. 7, the two lines have similar shapes, these results indicate that ρ−70 and B−70/−10 of La1−xSrxCrO3 ceramics have the same tendency to increase of Sr2+ ions content.
4 Conclusions
The structure and electrical properties of La1−xSrxCrO3 ceramics has been investigated. The sintered ceramic samples are a single orthorhombic perovskite phase. The electrical conductivity in these ceramics is due to the polarons jumps between different oxidation states of chromium ions. The resistivity decreases with the increase of Sr2+ content, which is attributed to the increase of Cr6+. The values of ρ−70, ρ−10, B−70/−10 constant and activation energy Ea of the thermistors are in the range of 16.51–84100, 4.77–2170 Ωcm, 1106–3268 K, and 0.095–0.283 eV, respectively. The electrical properties can be adjusted by adjusting the Sr2+ concentration. These compounds could be used as potential candidates for NTC thermistors.
References
R. Koc, H.U. Anderson, J. Mater. Sci. 27, 5477 (1992)
V.V. Kharton, A.P. Viskup, E.N. Naumovich, N.M. Lapchuk, Solid State Ion. 104, 67 (1997)
Y. Jiang, J. Gao, M. Liu, Y. Wang, G. Meng, Mater. Lett. 61, 1908 (2007)
M. Panneerselvam, K.J. Rao, J. Mater. Chem. 13, 596 (2003)
D.H. Peck, M. Miller, D. Kobertz, H. Nickel, K. Hilpert, J. Am. Ceram. Soc. 79, 3266 (1996)
N. Sakai, T. Kawada, H. Yokokawa, M. Dokiya, T. Iwata, J. Mater. Sci. 25, 4531 (1990)
I. Yasuda, T. Hikita, J. Electron. Soc. 140, 1699 (1993)
S.P. Jiang, L. Liu, K.P. Ong, P. Wu, J. Li, J. Pu, J. Power. Sour. 176, 82 (2008)
S. Hayashi, K. Fukaya, H. Saito, J. Mater Sci. Lett. 7, 457 (1988)
W.J. Weber, H.L. Tuller, T.O. Mason, A.N. Cormack, Mater. Sci. Eng. B 18, 52 (1993)
A. Feteira, K. Reichmann, Adv. Sci. Technol. 67, 124 (2010)
A. Feteira, J. Am. Ceram. Soc. 92, 967 (2009)
X. Liu, W. Su, Z. Lu, J. Phys. Chem. Solids 62, 1919 (2001)
H. Qi, Y. Luan, S. Che, L. Zuo, X. Zhao, C. Hou, Inorg. Chem. Commun. 66, 33 (2016)
S.R. Nair, R.D. Purohit, A.K. Tyagi, P.K. Sinha, B.P. Sharma, J. Am. Ceram. Soc. 91, 88 (2008)
H. Jiao, J. Wang, J. Ge, L. Zhang, H. Zhu, S. Jiao, Solid State Commun. 231, 53 (2016)
X.N. Liu, W.H. Su, Z. Lu, J. Liu, L. Pei, W. Liu, L.Y. He, J. Alloy. Compd. 305, 21 (2000)
H. Yokokawa, N. Sakai, T. Kawada, M. Kokiya, J. Electron. Soc. 138, 1018 (1991)
H. Berthou, C.K. Jørgensen, C. Bonnelle, Chem. Phys. Lett. 38, 199 (1976)
H. Van Doveren, J.A.T.H. Verhoeven, J. Electron. Spectrosc. Relat. Phenom. 21, 265 (1980)
L. Li, Q. Wei, Z. Kang, M. Rui, W. Su, J. Alloy. Compd. 249, 264 (1997)
A.N. Kamlo, J. Bernard, C. Lelievre, D. Houivet, J. Eur. Ceram. Soc. 31, 1457 (2011)
M.A.L. Nobre, S. Lanfredi, Appl. Phys. Lett. 81, 451 (2002)
B. Zhang, Q. Zhao, A. Chang, H. Yan, Y. Wu, J. Mater. Sci. Mater. Electron. 24, 4452 (2013)
A.K. Tripathi, H.B. Lal, J. Mater. Sci. 17, 1595 (1982)
W.J. Weber, C.W. Griffin, J.L. Bates, J. Am. Ceram. Soc. 70, 265 (1987)
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant Nos. 61671447, 51502335), the Innovation Fund of Chinese Academy of Sciences (Grant No. CXJJ-15M047), and the West Light Foundation of the Chinese Academy of Sciences (Grant No. 2015-XBQN-B-13).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Luo, P., Zhang, B., Zhao, Q. et al. Characterization and electrical conductivity of La1−xSrxCrO3 NTC ceramics. J Mater Sci: Mater Electron 28, 9265–9271 (2017). https://doi.org/10.1007/s10854-017-6662-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6662-7