Abstract
The La1−xBaxCrO3 (x = 0–0.2) negative temperature coefficient (NTC) ceramics have been prepared by the traditional solid-state reaction method at 1600 °C. Scanning electron microscope images show that the doping of Ba2+ contributes to the increase in the density. X-ray diffraction analysis has revealed that the sintered ceramics crystallize in a single perovskite structure. X-ray photoelectron spectroscopy analysis confirm the existence of Cr3+ and Cr6+ ions on lattice sites, which result in hopping conduction. The presence of the Cr3+ and Cr6+ ions is one of the significant factors that affect the electrical conductivity of La1−xBaxCrO3 ceramics. The resistance of NTC thermistors decreases with the increase of Ba content as a result of the enhancement of Cr6+ Concentration. The obtained values of ρ −50, B −50/−25 and E a are in the range of 49.278–1.9839 × 105 Ω cm, 1767.4–3496.9 K, 0.1523–0.3013 eV, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The LaCrO3 is a refractory oxide with a high melting point (>2400 °C) and a distorted perovskite structure with orthorhombic symmetry (Pnma, space group 62) at room temperature [1]. It exhibits excellent chemical stability in both oxidizing and reducing atmospheres, and a good conductivity [2]. The lanthanum chromite-based ceramics gain interest for a variety of high-temperature electrochemical applications including interconnector in solid oxide fuel cell [3], thin film electric heaters [4], energy-saving material [5], composite thermistor ceramics [6] and so on. There are some mechanism of lanthanum chromite solid solutions that has been widely investigated for stabilizing phase transformation [7], optimizing sintering behavior [8], and modifying electrical properties [9]. Most frequently, the divalent alkaline earth cations dope LaCrO3, such as Mg2+ [10, 11], Ca2+ [5, 12], Sr2+ [13, 14] and so on. What’s more, LaCrO3 doped with appropriate impurity ions can be applied as the NTC thermistor materials [15].
However, it is well known that it is very difficult to sinter LaCrO3 ceramics in air atmosphere [16]. In order to enhance the density of LaCrO3, the A-site and B-site were doped, such as La1−xSrxCrO3 [17], LaCr1−xCoxO3 [1] and so on. This article will introduce that the A-site is doped by Ba2+ for adjusting the electrical properties and density of LaCrO3.
2 Experimental procedures
The La1−xBaxCrO3 powders were synthesized by conventional solid-state method at 1200 °C for 5 h. High-pure lanthanum oxide (La2O3, purity >99.99%, Sinopharm Chemical Reagent co., Ltd, China), Chromium oxide (Cr2O3, purity >99%, Sinopharm Chemical Reagent co., Ltd, China), and Barium carbonate (BaCO3, purity >98%, Sinopharm Chemical Reagent co., Ltd, China) were used as the initial raw material. According to the stoichiometry of La1−xBaxCrO3, the La2O3, Cr2O3 and BaCO3 were well weighed, mixed, ground, and calcined at 1200 °C for 5 h. The calcined powders were then ground in mortar for 8 h. Subsequently, the powders were pressed into green pellets under a uniaxial pressure of 20 MPa to form the disks of 10 mm in a diameter, and then cold isostatic pressing at 200 MPa was used to enhance densities. The pressed disks were sintered in the temperature of 1600 °C for 5 h.
X-ray diffraction (XRD; BRUKERD8-ADVANCE, Cu K α radiation) analysis was used to identify the crystalline phases of the sintered ceramic samples. Scanning Electron Microscope (SEM; Zeiss SUPRA 55 VP, Germany) was used to observe the microstructure of the sintered ceramic samples. X-ray photoelectron spectroscopy (XPS; Thermo, ESCALAB 250XI) was used to analyze the chemical states of the sintered ceramics. In order to obtain the electrical conductivity, the sintered ceramic samples were coated with some silver paste, and then annealed at 800 °C for 30 min. The resistance was measured in the temperature of −75 to 50 °C by Agilent 34970A multimeter in an oil bath. The density and open porosity of samples were measured by using Archimedes method.
3 Result and discussion
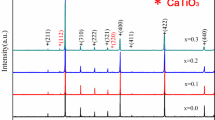
The XRD patterns and lattice constants of the sintered ceramics are shown in Fig. 1. Clearly, the samples of ceramics were consisted of a single phase isomorphic to the orthorhombic perovskite LaCrO3 (JCPDS 24-1016). Typically no another phase occurred with the increase of Ba concentration in the ceramic samples. It indicates that the Ba2+ has all entered the perovskite lattice to form the solid solution in the process of high temperature sintering. From Fig. 1b, we can see that the lattice constants of sintered ceramics gradually increase with the increase of Ba2+ content. What’s more, the diffraction peaks slightly shift toward lower angles with the increase of Ba2+ content. It indicates that the cell volume of the ceramic samples decrease with the increase of Ba2+ content according to the Bragg’s Law:
The main reason is that the ionic radius of substitution Ba2+ (0.135 nm) is greater than that of La3+ (0.1032 nm).
The SEM micrographs of the ceramics are shown in Fig. 2. As can be seen, there are many pores on the surface of sintered LaCrO3 ceramics and the pores gradually decrease with the increase of Ba2+ content. Furthermore, the morphology of the grain gradually changes from a polyhedron into a cuboid. And the density of the sintered ceramics has been markedly improved with the increase of Ba2+. The relative density of the sintered samples can be calculated by the following equation [1]:
The ρ geom was obtained by Archimedes method. The ρ theor was calculated as follows [1]:
where n is the number of elements per unit cell (n = 4 for perovskite type La1−xBaxCrO3), N A is the Avogadro constant, M i is the atomic weight of element i (La, Cr, Ba, O). The a, b, c are the lattice constants which are calculated by XRD patterns. The ρ theor, ρ geom, relative density and open porosity for the La1−xBaxCrO3 ceramics are listed in Table 1. The relative density and open porosity of La0.8Ba0.2CrO3 ceramics are 90.6 and 8%, respectively. Comparing with La0.7Ba0.3CrO3 [9], the density of La0.7Ba0.3CrO3 ceramic is 92% but there is the impure phase BaCr2O4. It may be that the doping content of Ba2+ exceeds the solid solution limit.
The XPS spectra of La 3d which is obtained from the sintered La1−xBaxCrO3 ceramic samples is shown in Fig. 3. It can be seen that the spectra peaks are associated with the presence of lanthanum in the form of La3+ [18]. Figure 4 shows the XPS spectra of Ba 3d obtained from the sintered ceramic samples. The spectra can be fitted using one component, which is characteristic of Ba2+ [19].
Figure 5 shows the XPS spectra and the curve-fitting example of ceramics in the regions of Cr 2p core-level peaks. As can be see, the peaks in the Cr 2p 3/2 and Cr 2p 1/2 spectra can be split into two peaks through Gaussian–Lorentzian curve fitting. The lower binding energy peak should be owing to Cr3+, and the higher binding energy peak is the result of Cr6+. The XPS results show that there are two different Cr ions in these samples, mainly Cr3+ and Cr6+. The result is in good agreement with other authors [20]. What’s more, the content of Cr6+ increases with the increase of Ba2+. The reason is that the introduction of Ba2+ into the lattice could be compensated by the oxidation of Chromium ion from Cr3+ to Cr6+ just like the influence of doping Sr2+ [17].
Figure 6 shows the relationship of natural logarithm of the resistivity (ρ) and the reciprocal of absolute temperature (1000/T) for the NTC thermistors. It can be seen that the resistivity of sintered ceramics decreases with the increase of Ba2+ content. And the relationship of lnρ and 1000/T displays an approximate line in the measured temperature range. The linear dependence depends on the small-polaron hopping transport mechanism [21], which can be described by the Arrhenius relationship [22]:
where ρ 0 is the resistivity at infinite temperature, E a is the activation energy for electrical conduction, k is the Boltzmann constant, T is the absolute temperature. The thermal constant B which describes the temperature sensitivity of material can be calculated as follows [22]:
where R 1 is the resistance at temperature T 1, and R 2 is the resistance at temperature T 2. The value of ρ −50, B −50/−25 and E a are shown in Table 2. The value of ρ −50, B −50/−25 and E a are in the range of 49.278–1.9839 × 105 Ω cm, 1767.4–3496.9 K, 0.1523–0.3013 eV, respectively. The electrical properties can be adjusted by adjusting the Ba2+ concentration.
4 Conclusions
The preparation, structure and electrical properties of La1−xBaxCrO3 ceramics have been investigated. The sintered ceramic samples are a single orthorhombic perovskite phase. The density of the sintered ceramics has been greatly improved by the doping of Ba2+. The electrical conductivity of these sintered ceramics is that the polarons jumps between different oxidation states of chromium ions. The resistivity decreases with the increase of Ba2+ content. The values of ρ−50, B−25/−50 constant and activation energy Ea of the thermistors are in the range of 49.278–1.9839 × 105 Ω cm, 1767.4–3496.9 K, 0.1523–0.3013 eV, respectively. The electrical properties can be adjusted by adjusting the Ba2+ concentration. These materials could be used as potential candidates for NTC thermistors.
References
A. Bonet, M. Baben, N. Travitzky, P. Greil, J. Am. Ceram. Soc. 99, 917 (2016)
H. Yokokawa, N. Sakai, T. Kawada, M. Dokiya, J. Electrochem. Soc. 138, 1018 (1991)
J. Sfeir, J. Power Sources 118, 276 (2003)
Y. Ito, K. Wakisaka, H. Kado, S. Yoshikado, Key Eng. Mater. 301, 171 (2006)
Z. Han, J. Liu, X. Li, Y. Chen, G. Liu, J. Li, J. Am. Ceram. Soc. 97, 2705 (2014)
B. Zhang, Q. Zhao, A. Chang, Y. Wu, H. Li, J. Alloys Compd. 675, 381 (2016)
M. Mori, Y. Hiei, N.M. Sammes, Solid State Ionics 135, 743 (2000)
M. Iwasaki, H. Takizawa, K. Uheda, T. Endo, M. Shimada, J. Mater. Chem. 8, 2765 (1998)
S.P. Jiang, L. Liu, K.P. Ong, P. Wu, J. Li, J. Pu, J. Power Sources 176, 82 (2008)
B.K. Flandermeyer, M.M. Nasrallah, A.K. Agarwal, H.U. Anderson, J. Am. Ceram. Soc. 67, 195 (1983)
K. Azegami, M. Yoshinaka, K. Hirota, O. Yamaguchi, Solid State Commun. 112, 281 (1999)
Y. Fu, H. Wang, C. Weng, S. Hu, Y. Liu, J. Am. Ceram. Soc. 98, 2561 (2015)
T. Takeuchi, Y. Takeda, R. Funahashi, T. Aihara, M. Tabuchi, H. Kageyama, J. Electrochem. Soc. 147, 3979 (2000)
H. Qi, Y. Luan, S. Che, L. Zuo, X. Zhao, C. Hou, Inorg. Chem. Commun. 66, 33 (2016)
K. Azegami, M. Yoshinaka, K. Hirota, O. Yamaguchi, J. Electrochem. Soc. 147, 2830 (2000)
L. Groupy, H.U. Anderson, J. Am. Ceram. Soc. 59, 449 (1976)
X. Liu, W. Su, Z. Lu, J. Liu, L. Pei, W. Liu, L. He, J. Alloys Compd. 305, 21 (2000)
H. Berthou, C.K. Jørgensen, C. Bonnelle, Chem. Phys. Lett. 38, 199 (1976)
H. Van Doveren, J.A.T.H. Verhoeven, J. Electron. Spectrosc. Relat. Phenom. 21, 265 (1980)
L. Li, Q. Wei, Z. Kang, M. Rui, W. Su, J. Alloys Compd. 249, 264 (1997)
A.N. Kamlo, J. Bernard, C. Lelievre, D. Houivet, J. Eur. Ceram. Soc. 31, 1457 (2011)
A. Feteira, J. Am. Ceram. Soc. 92, 967 (2009)
Acknowledgements
This study was supported by the Autonomous Region Youth Science and Technology Innovation Personnel Training Project (No. QN2015JQ010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, M., Zhang, H., Liu, T. et al. Preparation, structure and electrical properties of La1−xBaxCrO3 NTC ceramics. J Mater Sci: Mater Electron 28, 18873–18878 (2017). https://doi.org/10.1007/s10854-017-7839-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7839-9