Abstract
Glass–ceramic materials of the CaO–B2O3–SiO2 (CBS) system added with various amounts of Al2O3 have been prepared by conventional melting and heat treated crystallization method. The effects of Al2O3 on the sintering characterization and dielectric properties have been investigated. With increasing the Al2O3 content, the bending and stretching vibrations in [SiO4]2− became weak. XRD patterns of samples with ≥3 wt% Al2O3 introduce cyclowollastonite (α-CaSiO3) as single crystallized phase of sintered glass–ceramics. It is found that the optimal sintering temperature for this glass ceramics is 800 °C for 30 min, and the sinterability was obviously improved by Al2O3 additive. CBS glass ceramics sample with 5 wt% Al2O3 achieved the best sintering characterization and dielectric properties (εr ≈ 7, tan δ = 1.9 × 10−3 at 1 MHz), which is proposed to be suitable for LTCC application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Low temperature co-fired ceramics (LTCC) technology, because of its excellent electrical, mechanical and thermal properties, is nowadays being increasingly used to make substrates for the production of multilayer ceramic circuits, mainly for telecommunication, automotive and medical applications [1]. The most important parameter which is expected for LTCC component is the low sintering temperature because of the use of low melting point metals e.g. Cu, Ag and Au. In addition to these basic requirements, the LTCC substrate materials should exhibit a low permittivity (εr < 10) to reduce the signal propagation delay, and low dielectric losses [2]. The low temperature sintered for LTCC materials can be achieved with use of three main types of materials: glass–ceramics, glass/ceramic and single-phase ceramics. Of these, the glass–ceramic composite system is the most frequently used.
As an attractive glass–ceramic system, CaO–SiO2–B2O3 (CBS) has been investigated as the candidate substrate materials in the electronic packaging filed, because of its low sintering temperature, relatively low dielectric constant (~6.5) and low material cost [3–16]. Wang and Zhou [11] studied the effects of the glasses of different compositions, holding time and heating speed on CaO–B2O3–SiO2 system. Zhu et al. [15] reported the effect of temperature, frequency and composition for CBS on the dielectric properties. Zhou et al. [16]found that the La2O3–B2O3 addition promoted the crystallization of the CaSiO3 and enhanced flexural strength. However, the large number of pores appeared in CBS system during the crystallization process, which led to low sintering density. Thus, it is difficult to control the proper shrinkage for LTCC application. Many studies indicated that Al2O3 is effective material filler for the glass + ceramic composites]. [17, 18]
In this study, a small amounts of Al2O3 as additives were mixed with CBS glasses and the sinterability of glass–ceramic increased dramatically. The effects of Al2O3 for CBS glasses on the crystalline phases, microstructures and dielectric properties are investigated systematically and discussed in detail.
2 Experimental procedure
The composition of the glass is 40 wt% CaO, 40 wt% SiO2 and 20 wt% B2O3 and starting materials are analytical reagent-grade CaCO3, SiO2 and H3BO3 with purities higher than 99 wt%. The raw materials were dry-mixed for 4 h in polyethylene jars. After mixed uniformly, the raw materials were put into an alumina crucible and melted at 1450 °C for 2 h. The molten glass was quenched into distilled water to form cullet. The crushed glass powders were sieved through a 20-mesh screen. Subsequently, the glasses were re-milled in ethyl alcohol for 4 h in a planetary ball mill and then oven-dried at 80 °C. As a next step, Al2O3 powders (99.9 %; AR) in a range from 0 to 10 wt% were mixed with the CBS glass frit by ball milling in ethanol for 4 h. The mixtures were added with about 8 wt% of 5 %-PVA solution, and uniaxially pressed under a pressure 80 MPa to obtain green compacts with diameter of 13 mm and thickness of 6 mm. The compacts were then heat treated at 550 °C for 2 h with a heating rate of 2 °C/min to eliminate the PVA, followed by sintering at temperatures between 800 and 900 °C for 15 min–1 h with a heating rate of 5 °C/min.
Density measurements on the CBS glass with Al2O3 were done using the Archimedes method. X-ray diffraction with CuKα radiation was used to determine the phase composition of these samples. FTIR spectra of the powdered samples were recorded at room temperature in the range 400–4000 cm−1 using Nicolet 5700. Glass transition and crystallization temperature of samples were determined by differential scanning calorimeter (DSC) at N2 atmosphere with a heating rate of 10 °C/min.
(NETZSCH-STA 449F3, α-alumina reference material). Scanning electron microscopy (SEM) was used to reveal the microstructures of sintered samples. The dielectric properties were measured at 1 MHz by Agilent4278A impedance analyzer at 20 °C.
3 Results and discussions
3.1 Crystallization and structure of glass
Figure 1 illustrates XRD patterns of all samples sintered at 750–850 °C for 30 min. No crystallization occurred at 750 °C because sintering temperature was below crystallization temperature (illustrated in Fig. 4). At 800 °C, pure CBS sample is composed of main crystallization phase of cyclowollastonite (α-CaSiO3) and minor crystallization phase of wollastonite (β-CaSiO3), phase transition from β-CaSiO3 to α-CaSiO3 happened for sample with <3 wt% Al2O3, resulting in the decrease of β-CaSiO3 phase; α-CaSiO3 as single crystallized phase was observed for sample with ≥3 wt% Al2O3. When the sintering temperature is increased to 850 °C, the diffraction peaks intensities of samples increased, indicating that the crystallization is more complete; as 7 wt% Al2O3 was introduced, the β-CaSiO3 phase disappeared. In addition, no Al2O3 or containing Al2O3 crystallization phase is detected in all samples. It indicates that Al2O3 has already melted, becoming glass network former in CBS glasses. Normally, the phase transformation from β-CaSiO3 to α-CaSiO3 is 1125 °C. In this study, more Al2O3 are favorable to α-CaSiO3 being crystallized. The reason may due to high field strength of Al3+ ions resulting in the reduction of activation energy of crystallization.
Figure 2 depicts the FT-IR spectra of CBS glass–ceramic in the presence of variable amounts of Al2O3 additives. All spectra exhibit four broad transmittance bands in the region of 400–2000 cm−1. The IR features located in the first region that range between 1300 and 1600 cm−1 are attributed to the asymmetric stretching relaxation of B–O bonds of from the [BO3] trigonal units (Fig. 2a). The second region range between 800 and 1200 cm−1 and its spectral features are related to the presence of the B–O bonds stretching vibrations of [BO4] tetrahedral units and the Si–O–Si bonds stretching vibrations [9] (Fig. 2b). The bands in the 600–800 cm−1 region are due to the stretching vibrations of Al–O bond in [AlO2]− tetrahedral and the bending vibration of the B–O–B bonds in the borate network [8, 14] (Fig. 2c). The band in 400–600 cm−1 can be attributed to O–Si–O bending vibrations and Al–O stretching vibrations in [AlO3]3− octahedral [19](Fig. 2d). The reason may due to Al replacing Si as a glass network former, resulting in the weaker bending and stretching intensity in [SiO4]. The band located at about 430 cm−1 results from Al–O stretching vibrations in [AlO3]3− octahedral. As the amount of Al2O3 increased, the band at about 430 cm−1 became stronger because more [AlO3]3− octahedral formed. However, the absorbance of the sample with 10 wt% Al2O3 is higher than that of the sample with 5 wt% Al2O3, which is caused by more [AlO3]3− octahedral forming and less [SiO4] being destroyed.
3.2 Sinterability and microstructure
Figure 3 displays the bulk densities of CBS glass ceramic with various Al2O3 content sintered at 800–900 °C for 30 min and sintered at 800 °C for 15 min–1 h. It was obvious that the densification strongly depends on the sintering temperature, time and Al2O3 content. Among these factors, the Al2O3 content was the most important factor in the densification process of the composites. As the Al2O3 content from 0 to 3 wt%, the bulk density increased markedly; and further increasing the Al2O3 content, the bulk density change little. Moreover, it is found that the appropriate sintering temperature and holding time are 800 °C and 30 min, respectively. According to the previous work [1], densification mechanisms for the glass/ceramic system can be divided into two categories: owing to the coexistence of filler and glass in LTCC, liquid-phase sintering can be considered; due to the large amount of glass, viscous sintering can be assumed [1]. For the present study, it is considered to be the latter mechanism that dominates the densification process, because small amount of Al2O3 was added in CBS glass and melted in it. Generally, the crystallizing in the compacts will increase the relative amount of B2O3 in CBS glass, leading to the decrease of the viscosity of CBS glass which contributes to densification [20]. In this study, the higher temperatures (≥800 °C) and longer holding time (≥30 min) can promote the crystallization and densification of CBS glass; however, the samples possessed lower densities. This cause is probably attributed to the boron-rich CBS glass volatilization. Besides, the shorter holding time (<30 min) also achieved the low densities, which is due to high viscosity of CBS glass restraining further densification. This above results indicated that the addition of Al2O3 dramatically improved the sinterability of CBS, which acted as the fluxes and increased the liquid fluidity during viscous flow [21].
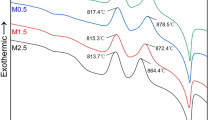
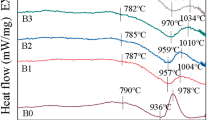
Figure 4 shows the DSC curves for CBS glass with different Al2O3 contents. All of these samples have exothermic peaks corresponding to crystallization (as indicated in Fig. 1) at about 790 °C. The crystallization peaks are believed as correlated to the crystallization of α-CaSiO3 and β-CaSiO3 as identified in the results of the XRD patterns in Fig. 1. The exothermic peak at about 580 °C is the softening temperature of pure CBS glass. The samples with Al2O3 found no peaks at about 580 °C. The possible reason is that the temperature of Al2O3 melting in CBS glass was at about 580 °C.
Figure 5 shows the microstructures of the cross section of sintered CBS glass ceramic with different Al2O3 contents at 800 °C for 30 min. The CBS specimen with no Al2O3 exhibited large number of open pores as shown in Fig. 5a. Both the amount and the size of pores decreased with increase of Al2O3. Dense structure with a few pores with small size were observed for the samples with 3–10 wt%. However, their densities didn’t absolutely agree with the change of pores. It can be explained by the fusion of Al2O3. When Al2O3 melted in CBS glass, it formed [AlO2]− tetrahedral or [AlO3]3− octahedral. The [AlO3]3− octahedral as glass network intermediate can reduce the density of CBS glass. With Al2O3 adding, there are more [AlO3]3− octahedral formed in CBS glass.
3.3 Dielectric properties
Dielectric properties of CBS samples with various Al2O3 content sintered at 800–850 °C for 30 min are shown in Fig. 6. The changed trends of dielectric constants of specimens agree with the trends of their densities in Fig. 3. The dielectric constant of the sample sintered at 800 °C first increased, reached the maximum value at 3 wt%, and then decreased with increasing Al2O3 content. However, the dielectric constant of the sample sintered at 850 °C shows a little different changing tendency. The sample with 5 wt% Al2O3 has a lower dielectric constant than the one with 7 wt% Al2O3, which is caused by its lower density. It is concluded that the porosities play decisive role in the variations of dielectric constants in specimens with relative low densities. With the addition of Al2O3 increasing, the dielectric loss of samples sintered at 850 °C increases firstly and then decreases. However, the dielectric loss of samples sintered at 800 °C increases with the rising of addition, except the one with 5 wt% Al2O3. Pure CBS sample sintered at 800 °C for 30 min have lower dielectric loss. Overall, the addition of Al2O3 would cause the dielectric loss to increase. Many factors are believed to affect the dielectric loss and these factors can be divided into two categories: the intrinsic loss and the extrinsic loss. Intrinsic losses are mainly caused by lattice vibration modes while extrinsic losses are dominated by second phases, oxygen vacancies, grain sizes and densification or porosity [14]. In this study, it can be concluded that the dielectric properties of the samples in this study are mainly affected by [AlO3]3− stretching vibrations.
4 Conclusions
In this study, the crystallization, sintering and dielectric properties of CBS glass ceramic containing Al2O3 were investigated. With increasing the Al2O3 content, the bending and stretching vibrations in [SiO4]2− became weak. XRD patterns of samples with ≥3 wt% Al2O3 introduce cyclowollastonite (α-CaSiO3) as single crystallized phase of sintered glass–ceramics. It is found that the optimal sintering temperature for this glass ceramics is 800 °C for 30 min, and the sinterability was obviously improved by Al2O3 additive. CBS glass ceramics sample with 5 wt% Al2O3 achieved the best sintering characterization and dielectric properties (εr ≈ 7, tan δ = 1.9 × 10−3 at 1 MHz), which is proposed to be suitable for LTCC application.
References
M. Liu, H.Q. Zhou, X.Y. Xu, Z.X. Yue, M. Liu, H.K. Zhou, J. Mater. Sci. Mater. Electron. 24, 3985–3994 (2013)
M.K. Zitani, M. Rezvani, R.A. Tabrizi, Electron. Mater. Lett. 10, 113–117 (2014)
S.F. Wang, Y.R. Wang, Y.F. Hsu, C.C. Chiang, J. Alloys Compd. 498, 211–216 (2010)
X.Y. Chen, W.J. Zhang, S.X. Bai, Y.G. Du, Ceram. Int. 39, 6355–6361 (2013)
N. Santha, S. Shamsudeen, N.T. Karunakaran, J.I. Naseemabeevi, Int. J. Appl. Ceram. Technol. 8, 1042–1049 (2011)
C.C. Chiang, S.F. Wang, Y.R. Wang, Y.F. Hsu, J. Alloys Compd. 461, 612–616 (2008)
H.K. Zhu, H.Q. Zhou, M. Liu, G.J. Xu, G. Ning, J. Mater. Sci. Mater. Electron. 20, 1135–1139 (2009)
N. Santha, T.K. Nideep, S.R. Rejisha, J. Mater. Sci. Mater. Electron. 23, 1435–1441 (2012)
M. Liu, H.Q. Zhou, H.K. Zhu, Z.X. Yue, J.X. Zhao, J. Cent, South Univ. 19, 2733–2739 (2012)
S.F. Wang, C.C. Chiang, Y.R. Wang, Y.F. Hsu, Jpn. J. Appl. Phys. 49, 021101 (2010)
S.H. Wang, H.P. Zhou, Mater. Sci. Eng. B 99, 597–600 (2003)
H.K. Zhu, H.Q. Zhou, M. Liu, P.F. Wei, G. Ning, J. Alloys Compd. 482, 272–275 (2009)
S. Rajesh, H. Jantunen, M. Letz, S. Pichler-Willhelm, Int. J. Appl. Ceram. Technol. 9, 52–59 (2012)
M. Liu, H.Q. Zhou, H.K. Zhu, Z.X. Yue, J.Z. Zhao, J. Wuhan Univ. Technol. Mater. Sci. Ed. 28, 1085–1090 (2013)
H. K. Zhu, M. Liu, H. Q. Zhou, L. Q. Li, A. G. Lv, Mater. Res. Bull. 42, 1137–1144 (2007)
X.H. Zhou, E.Z. Li, S.L. Yang, B. Li, B. Tang, Y. Yuan, S.R. Zhang, Ceram. Int. 38, 5551–5555 (2012)
S.O. Yoon, S. Kim, K.S. Kim, J.G. Park, S. Kim, Ceram. Int. 35, 1271–1275 (2009)
S. Arcaro, F.R. Cesconeto, F. Raupp-Pereira, A.P. Novaes de Oliveira, Ceram. Int. 40, 5269–5274 (2014)
A. Goel, D.U. Tulyaganov, A.M. Ferrari, E.R. Shaaban, A. Prange, F. Bondioli, J.M.F. Ferreira, J. Am. Ceram. Soc. 93, 830–837 (2010)
T. Zhang, Q. Zou, J. Eur. Ceram. Soc. 32, 4009–4013 (2012)
H.K. Zhu, M. Liu, H.Q. Zhou, L.Q. Li, A.G. Lv, J. Mater. Sci. Mater. Electron. 17, 637–641 (2006)
Acknowledgments
The authors gratefully acknowledge the financial support from the national high technology research and development program of China (863 programs) (No. 2013AA030701).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, JZ., Wu, XF., Xu, NX. et al. Crystallization, sinterability and dielectric properties of CaO–B2O3–SiO2 glass ceramics with Al2O3 additives. J Mater Sci: Mater Electron 26, 8899–8903 (2015). https://doi.org/10.1007/s10854-015-3571-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3571-5