Abstract
CoFe2O4@Y2O3:Eu3+ magnetic–fluorescent bifunctional coaxial nanofibers have been successfully obtained via calcination of the [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers which were fabricated by coaxial electrospinning technique. The diameter of CoFe2O4@Y2O3:5 %Eu3+ magnetic–fluorescent bifunctional coaxial nanofibers was 133 ± 17 nm. Strong fluorescence emission peaks of Eu3+ in the CoFe2O4@Y2O3:Eu3+ coaxial nanofibers were observed and assigned to 5D0 → 7F1 (588 nm), 5D0 → 7F1 (593 nm), 5D0 → 7F1 (599 nm), 5D0 → 7F2 (612 nm) and 5D0 → 7F2 (630 nm) energy levels transitions of Eu3+ ions, and the predominant emission peak was located at 612 nm. Compared with CoFe2O4/Y2O3:Eu3+ composite nanofibers, CoFe2O4@Y2O3:Eu3+ magnetic–fluorescent bifunctional coaxial nanofibers simultaneously provided higher magnetism and fluorescent intensity. The color, photoluminescent intensity and magnetism of the coaxial nanofibers can be tuned via adjusting the diversity and content of fluorescent compounds and the content of magnetic compounds. Formation mechanism of CoFe2O4@Y2O3:Eu3+ coaxial nanofibers was also presented. The bifunctional magnetic–photoluminescent CoFe2O4@Y2O3:Eu3+ coaxial nanofibers have potential applications in many fields due to their excellent magnetism and fluorescence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Electrospinning is one of the most effective and convenient techniques to process viscous solutions or melts into continuous fibers with diameters ranging from micrometer to submicron or nanometer. Some inorganic and organic nanomaterials have been prepared by electrospinning technique [1–7]. Recently, coaxial electrospinning provides an alternative route to design and fabricate new functionalized composite nanofibers with core–shell structures [8, 9].
Nowadays, rare-earth-doped nanomaterials have been widely used in high performance luminescent devices, magnets and other functional materials [10, 11]. Y2O3 has received considerable attention when doped with rare earth ions owing to their interesting properties [12, 13]. Y2O3 doped with Eu3+ ions is an important phosphor with a variety of applications in many luminescent and optical devices. The interest in the synthesis of Y2O3:Eu3+ phosphor is motivated by the need to improve the luminescence efficiency of materials [14].
Recently, magnetic nanomaterials have attracted increasing interest of scientists owing to their practical and potential applications, such as biomacromolecules separation, catalyst separation and drug/gene delivery [15–18]. Among the magnetic materials, cobalt ferrite (CoFe2O4) has been studied intensively due to its significant properties, such as moderate saturation magnetization, remarkable chemical and thermal stabilities and good mechanical hardness. It has been broadly used as a traditional high-frequency low loss power converter, stress sensor and actuator [19–22].
Magnetic–photoluminescent bifunctional nanomaterials simultaneously possess excellent magnetism and luminescence, and have been applied in medical diagnostics and optical imaging. Presently, studies are mainly focused on the preparation, properties and applications of magnetic–photoluminescent bifunctional nanoparticles [23–25]. In order to obtain new morphologies of magnetic–fluorescent nanomaterials, the fabrication of one-dimensional magnetic–fluorescent nanomaterials is an urgent subject of study. Dong, et al. have prepared the magnetic–photoluminescent bifunctional composite nanofibers and nanoribbons via electrospinning [2, 5, 8]. It has been proved that the existence of magnetic nanomaterials will greatly decrease the luminescence of rare earth compounds if magnetic nanomaterials directly blend with the rare earth luminescent compounds [26–28]. Therefore, if the strong luminescence of the magnetic–fluorescent bifunctional nanomaterials is to be achieved, rare earth compounds must be effectively isolated from magnetic nanomaterials in order to avoid direct contacting. The peculiar nanostructure of coaxial nanofibers can help to realize this academic idea if magnetic nanomaterials are only dispersed in the core of the coaxial nanofibers and rare earth luminescent compounds are merely dispersed in the shell of the coaxial nanofibers, and thus it is expected that the coaxial nanofibers exhibit excellent magnetic and fluorescent properties.
To the best of our knowledge, there have been no reports on the preparation of CoFe2O4@Y2O3:Eu3+ magnetic–fluorescent bifunctional coaxial nanofibers. Herein, we employ the electrospinning technique to prepare CoFe2O4@Y2O3:Eu3+ coaxial nanofibers with magnetic–photoluminescent bifunction. The morphology, structure, luminescence, magnetism and formation mechanism of the CoFe2O4@Y2O3:Eu3+ coaxial nanofibers were investigated in detail, and some meaningful results were obtained.
2 Experimental sections
2.1 Chemicals
Polyvinyl pyrrolidone (PVP, Mw = 130 0000) and N,N-dimethylformamide (DMF) were purchased from Tianjin Tiantai Fine Chemical Reagents Co. Ltd., HNO3 and NaOH were bought from Beijing Chemical Company. Y2O3 (99.99 %), Eu2O3 (99.99 %), Fe(NO3)3·9H2O and Co(NO3)2·6H2O were bought from Sinopharm Chemical Reagent Co. Ltd. All chemicals were of analytical grade and directly used as received without further purification. Y(NO3)3·6H2O and Eu(NO3)3·6H2O were prepared by dissolving Y2O3 and Eu2O3 in HNO3, followed by crystallizing from the solution through evaporating the water from the solution by heating.
2.2 Preparation of CoFe2O4 nanofibers and CoFe2O4/Y2O3:Eu3+ composite nanofibers via electrospinning
In a typical procedure of preparing spinning solution of CoFe2O4 nanofibers, 1 mmol of Fe(NO3)3·9H2O, 0.5 mmol of Co(NO3)2·6H2O and 2.2 g of PVP were dissolved into 15.8 g of DMF.
In order to perform the comparative experiment, CoFe2O4/Y2O3:Eu3+ composite nanofibers were also prepared. The spinning solution for preparing CoFe2O4/Y2O3:Eu3+ composite nanofibers was acquired as follows: 0.95 mmol of Y(NO3)3·6H2O, 0.05 mmol of Eu(NO3)3·6H2O, 1.5 g of PVP, 10 g of DMF and 1.5 g of CoFe2O4 nanofibers were mixed together.
These two spinning solutions were stirred for 24 h to form homogeneous mixture solutions for further electrospinning. The spinning solutions were injected into the traditional single axial electrospinning setup. Subsequently, the spinning solutions were electrospun under the high-voltage of 15 kV, and the distance between the spinneret and collector was fixed at 18–20 cm. The room temperature was 20–24 °C and the relative humidity was 30–40 %. In this way, the electrospun [(Co(NO3)2 + Fe(NO3)3)]/PVP composite nanofibers and CoFe2O4/[(Y(NO3)3 + Eu(NO3)3)]/PVP were fabricated on the collector. CoFe2O4 nanofibers and CoFe2O4/Y2O3:Eu3+ composite nanofibers can be obtained when the relevant composite nanofibers were annealed in air at 900 °C for 4 h and 700 °C for 4 h with the heating rate of 1 °C min−1, respectively.
2.3 Fabrication of magnetic–luminescent coaxial nanofibers via coaxial electrospinning
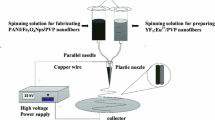
In the preparation of core spinning solution, 1.3 g of CoFe2O4 nanofibers was added into 8.7 g of DMF, and dispersed ultrasonically for 20 min, then 1.3 g of PVP was dissolved into the above solution under stirring for 12 h.
A mixed solution of 0.95 mmol of Y(NO3)3·6H2O, 0.05 mmol of Eu(NO3)3·6H2O, 1.5 g of PVP and 8.5 g of DMF was prepared as the shell spinning solution with Eu3+ molar percentage of 5 %. Other series of CoFe2O4@Y2O3:x%Eu3+ [x = 1, 3, 7, x stands for molar ratio of Eu3+ to (Y3++Eu3+)] coaxial nanofibers were also prepared by the similar procedure except for different percentage of rare earth nitrates in the spinning solution.
A homemade coaxial electrospinneret was used in this study. As shown in Fig. 1, the core spinning solution was injected into the inner plastic syringe while the shell spinning solution was loaded into the outer one. The spinning processing parameters were the same as they were in the above electrospinning process. [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers were successfully prepared via the coaxial electrospinning. CoFe2O4@Y2O3:Eu3+ coaxial nanofibers were obtained after annealing the relevant composite coaxial nanofibers in air at 700 °C for 4 h with the heating rate of 1 °C min−1.
2.4 Fabrication of Y2O3:5 %Eu3+ powders by hydrothermal method
In order to compare the fluorescent properties of CoFe2O4@Y2O3:Eu3+ coaxial nanofibers and conventional fluorescent powders, Y2O3:5 %Eu3+ powders were synthesized according to the traditional method as described in the Ref. [12].
In the typical procedure of preparing the Y2O3:5 %Eu3+ powders, 0.95 mmol of Y2O3 and 0.05 mmol of Eu2O3 were dissolved in dilute HNO3. After heating, a clear solution was obtained. The residual HNO3 was removed by evaporation of the water together with the acid for several times and the final pH value was controlled to be around 5. The precipitate was formed by adding 10 wt % of NaOH aqueous solution to the above mixture with stirring at room temperature. The pH value of the solution was controlled to 8. The resulting suspensions were further stirred for 20 min at room temperature. Finally, the milky solutions were transferred to Teflon-lined autoclaves for hydrothermal treatment at 120 °C for 24 h. The resulting precipitates were first ground into powders, heated to 600 °C at a rate of 5 °C min−1, and then maintained at that temperature for 2 h. The diameter of Y2O3:5 %Eu3+ powders is ca. 1–2 μm.
3 Characterization
The samples were identified by an X-ray powder diffractometer (XRD, Bruker D8 FOCUS) with Cu Kα radiation. The operation voltage and current were kept at 40 kV and 20 mA, respectively. The morphology and internal structure of samples were observed by a field emission scanning electron microscope (FESEM, XL-30) and a transmission electron microscope (TEM, JEM-2010), respectively. The fluorescent properties of samples were investigated by a Hitachi fluorescence spectrophotometer F-7000. Then, the magnetic performance of samples was measured by a vibrating sample magnetometer (VSM, MPMS SQUID XL). All measurements were performed at room temperature.
4 Results and discussion
4.1 Characterizations of the structure and morphology
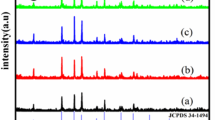
The phase compositions of CoFe2O4 nanofibers, [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers and CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers are characterized by means of XRD analysis, as shown in Fig. 2. It can be seen that XRD patterns of the as-prepared CoFe2O4 nanofibers are readily indexed to those of the pure cubic spinel of CoFe2O4 (PDF#22-1086), and no characteristic diffraction peaks are observed for other impurities, as indicated in Fig. 2a. Figure 2b reveals that [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers contain CoFe2O4 and the diffraction peak of the amorphous PVP (2θ = 22.2°) can also be observed. It is seen from Fig. 2c that XRD patterns of CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers are conformed to the cubic phase with primitive structure of Y2O3 (PDF#73-1334) and the cubic spinel structure of CoFe2O4 (PDF#22-1086), indicating that CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers are composed of crystalline CoFe2O4 and Y2O3:5 %Eu3+.
Figure 3 shows FESEM images of CoFe2O4 nanofibers, [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers and CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers, respectively. CoFe2O4 nanofibers have coarse surface, as shown in Fig. 3a. It is found from Fig. 3b that [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers are extremely smooth. As seen from Fig. 3c, the diameter of CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers is decreased owing to decomposition and volatilization of PVP and decomposition of rare earth nitrates, and the CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers have relatively coarse surface. The diameters of CoFe2O4 nanofibers, [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers and CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers are 81 ± 11, 333 ± 46 and 133 ± 17 nm under the confidence level of 95 %, respectively, as demonstrated in Fig. 4.
The TEM images of [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers and CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers are presented in Fig. 5. As revealed in Fig. 5, an obvious coaxial structure can be seen in the [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers and CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers, and the CoFe2O4 nanofibers are clearly observed in the core of the coaxial nanofibers. The diameters of the [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers and CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers are 310–320 nm and 120–130 nm, respectively, which are in good agreement with those of FESEM analyses.
4.2 Fluorescent properties analysis
In order to obtain the optimum ratio of Eu3+ to (Eu3++Y3+), a series of CoFe2O4@Y2O3:x%Eu3+ [x = 1, 3, 5, 7] coaxial nanofibers are fabricated by coaxial electrospinning process. The excitation and emission spectra of CoFe2O4@Y2O3:x%Eu3+ [x = 1, 3, 5, 7] coaxial nanofibers and CoFe2O4/Y2O3:5 %Eu3+ composite nanofibers are revealed in Figs. 6 and 7. Broad excitation bands extending from 200 nm to 275 nm are observed when monitoring wavelength is 612 nm in the samples. The peak at 236 nm assigned to the charge transfer band of O2−–Eu3+ can be also identified. Characteristic emission peaks of the Eu3+ observed in the samples are ascribed to the energy levels transitions of 5D0 → 7F1 (588 nm), 5D0 → 7F1 (593 nm), 5D0 → 7F1 (599 nm), 5D0 → 7F2 (612 nm) and 5D0 → 7F2 (630 nm), and the emission peak at 612 nm is the predominant emission peak. Obviously, the luminescence intensity of CoFe2O4@Y2O3:x %Eu3+ [x = 1, 3, 5, 7] coaxial nanofibers increases with the increase of the concentration of Eu3+ ions from the beginning, and reaches a maximum value with the Eu3+ concentration of 5 %, and then decreases with further increasing in Eu3+ concentration, indicating that the optimum doping molar concentration of Eu3+ ions is 5 %.
To illustrate the advantages of the coaxial nanostructure of the magnetic–fluorescent bifunctional nanofibers, the CoFe2O4/Y2O3:5 %Eu3+ composite nanofibers, as a contrast sample, are also fabricated by mixing core spinning solution and shell spinning solution together at the mass ratio of 1:1 followed by electrospinning via the traditional single-nozzle electrospinning method. From the comparison between CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers and CoFe2O4/Y2O3:5 %Eu3+ composite nanofibers, it is found that both excitation peaks intensity and emission peaks intensity of the CoFe2O4/Y2O3:5 %Eu3+ composite nanofibers are much more weaker than those of the CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers, and this weak fluorescent emission intensity makes the CoFe2O4/Y2O3:5 %Eu3+ composite nanofibers impractical in fluorescent performance. From the UV–vis diffuse reflectance spectrum of CoFe2O4 nanofibers illustrated in Fig. 8, it is observed that the CoFe2O4 nanofibers can absorb visible light (400–700 nm) and much more strongly absorb ultraviolet light (<400 nm), the result is in good agreement with the references [29, 30]. Therefore, both the exciting light (236 nm) and emitting light (588–630 nm) can be absorbed by dark-colored CoFe2O4. As illustrated in Fig. 9, because the CoFe2O4 nanofibers are distributed over the whole parts of the CoFe2O4/Y2O3:5 %Eu3+ composite nanofibers, the exciting light has to pass through many CoFe2O4 nanofibers to reach the Y2O3:Eu3+ fluorescence center. In this process, a large part of the exciting light has been absorbed by the CoFe2O4 nanofibers, and thus the exciting light is much weakened before it reaches the Y2O3:Eu3+. Similarly, the emitting light emitted by the Y2O3:Eu3+ also has to pass through the CoFe2O4 nanofibers, which is severely weakened by the absorption of the CoFe2O4 nanofibers. Thus, the heavy loss in fluorescent emission intensity of CoFe2O4/Y2O3:5 %Eu3+ composite nanofibers results from the strong light absorption of the dark-colored CoFe2O4 to the exciting light and emitting light when CoFe2O4 and Y2O3:Eu3+ are blended together. As to the CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers, the CoFe2O4 nanofibers only disperse in the core of the coaxial nanofibers so that the exciting light and emitting light almost will not be affected by the CoFe2O4 nanofibers. The discussion about the effect of magnetic compounds on luminescence performance in coaxial nanofibers and composite nanofibers has been reported in the Ref. [33], and our above research result is similar to the result reported this literature.
For comparison, the emission spectra of the CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers and Y2O3:5 %Eu3+ powders under the same excitation conditions (in order to avoid the experimental errors) are shown in Fig. 10a. It is known that the defects as quenching centers have severe drawback in luminescence intensity for nanomaterials because they provide nonradiative recombination centers for electrons and holes [11]. As seen from Fig. 10a, the CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers have stronger emission intensity than that of Y2O3:5 %Eu3+ powders due to the fact that coaxial nanofibers have less surface defects than Y2O3:5 %Eu3+ powders.
The luminescent decay curves of CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers and Y2O3:5 %Eu3+ powders, as shown in Fig. 10b, are used to calculate the lifetime and to investigate the luminescence dynamics of these samples. The samples are excited by 236 nm and monitored by 612 nm, and the curves can be well-fitted into a double-exponential function as I = A1exp(−t/s1) + A2exp(−t/s2), in which s1 and s2 are the fast and slow components of the luminescence lifetimes, A1 and A2 are the fitting parameters. The average lifetime values of CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers and Y2O3:5 %Eu3+ powders are 1.51 and 1.47 ms, respectively.
According to the above results, the CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers have stronger fluorescent intensity and longer luminescence lifetimes compared with the Y2O3:5 %Eu3+ powders.
4.3 CIE analysis
Generally, color is represented by the Commission Internationale de L’Eclairage (CIE) chromaticity coordinates and color ratios. The chromaticity coordinates and color ratios have been calculated from the emission spectra. The chromaticity coordinates of CoFe2O4@Y2O3:Eu3+ coaxial nanofibers and CoFe2O4/Y2O3:5 %Eu3+ composite nanofibers are indicated in Fig. 11. The coordinates (X, Y) of samples are (0.515, 0.317), (0.539, 0.331), (0.545, 0.342), (0.556, 0.356) and (0.476, 0.301), which correspond to CoFe2O4@Y2O3:x%Eu3+ [x = 1, 3, 5, 7] coaxial nanofibers and CoFe2O4/Y2O3:5 %Eu3+ composite nanofibers, respectively. Remarkably, the emission colors of samples are red owing to the strong emission intensity originating from 5D0 → 7F2 (612 nm) energy levels transitions of Eu3+ ions. According to the above results, it can be found that the emission colors of CoFe2O4@Y2O3:Eu3+ coaxial nanofibers can be tuned by adjusting the concentrations of Eu3+ ions, which are considered to be promising candidates for application in LEDs.
4.4 Magnetic performance
The typical hysteresis loops for CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers, [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers and CoFe2O4/Y2O3:5 %Eu3+ composite nanofibers are shown in Fig. 12, and the saturation magnetizations of them are listed in Table 1. As shown in Table 1, the saturation magnetization of the CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers, [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers and CoFe2O4/Y2O3:5 %Eu3+ composite nanofibers are 16.1 , 14.6 and 11.4 emu g−1, respectively.It can be seen that the saturation magnetization of the CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers is higher than that of [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers and CoFe2O4/Y2O3:5 %Eu3+ composite nanofibers. It is well known that the saturation magnetization of a magnetic composite material depends on the mass percentage of the magnetic substance in the magnetic composite material [27, 28], thus, the saturation magnetization of the coaxial nanofibers can be tuned via adjusting the content of magnetic compounds.
Combined with the fluorescent performance analyses, the CoFe2O4@Y2O3:5 %Eu3+ coaxial nanofibers have better fluorescent intensity and magnetic property compared with the CoFe2O4/Y2O3:5 %Eu3+ composite nanofibers. Based on the above experimental results, we can safely conclude that our academic ideas described in the introduction are successfully realized via coaxial electrospinning.
Compared with the magnetic–fluorescent bifunctional core–shell nanoparticles reported in the references [31, 32], CoFe2O4@Y2O3:Eu3+ coaxial nanofibers is a new kind of magnetic–fluorescent bifunctional nanomaterials with special morphology. It has obvious advantages owing to its length-diameter ratio, unique optical and magnetic properties. These excellent properties may make the coaxial nanofibers to possess the potential applications in the fields of medical diagnostics, drug target delivery and bioimaging [8, 33].
4.5 Formation mechanism for CoFe2O4@Y2O3:Eu3+ coaxial nanofibers
Formation mechanism for CoFe2O4@Y2O3:Eu3+ coaxial nanofibers is proposed on the basis of the above experimental results, as shown in Fig. 13. CoFe2O4 nanofibers are ultrasonically dispersed into DMF, then PVP is dissolved into the above solution to form core spinning solution. Y(NO3)3·6H2O, Eu(NO3)3·6H2O and PVP are mixed with DMF to form shell spinning solution with certain viscosity. [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers are fabricated via coaxial electrospinning. PVP acted as template during the formation of [CoFe2O4/PVP]@[(Y(NO3)3 + Eu(NO3)3)/PVP] composite coaxial nanofibers. The core of the coaxial nanofibers only remains CoFe2O4 nanofibers owing to decomposition and volatilization of PVP during the calcination process. Simultaneously, the nitrates are decomposed and oxidized to NO2, Y3+ and Eu3+ are oxidized to form Y2O3:Eu3+ nanocrystallite, many nanocrystallites are combined into nanoparticles, and these nanoparticles are mutually connected to generate Y2O3:Eu3+ shell of the coaxial nanofibers.
5 Conclusions
In summary, uniform bifunctional magnetic–photoluminescent CoFe2O4@Y2O3:Eu3+ coaxial nanofibers are successfully prepared by the coaxial electrospinning. The diameter of the coaxial nanofibers is 133 ± 17 nm. Compared with the CoFe2O4/Y2O3:Eu3+ composite nanofibers, CoFe2O4@Y2O3:Eu3+ coaxial nanofibers simultaneously possess both higher fluorescent intensity and saturation magnetization. The color, photoluminescent intensity and magnetism of the coaxial nanofibers can be tuned via adjusting the diversity and content of fluorescent compounds and the content of magnetic compounds. Besides, other multifunctional nanomaterials such as electrical–magnetic, photoluminescent–electrical and photoluminescent–electrical–magnetic twi- or trifunctional nanostructures can be designed and fabricated based on this novel design conception and construct technology.
References
H. Zhang, H.W. Song, B. Dong, L.L. Han, G.H. Pan, X. Bai, L.B. Fan, S.Z. Lu, H.F. Zhao, F. Wang, J. Mater. Chem. 112, 9155–9162 (2008)
G.Q. Gai, L.Y. Wang, X.T. Dong, S.Z. Xu, J. Mater. Sci. 48, 5140–5147 (2013)
S. Sinha-Ray, D.D. Pelot, Z.P. Zhou, A. Rahman, X.F. Wu, A.L. Yarin, J. Mater. Chem. 22, 9138–9146 (2012)
M. Miyauchi, J.J. Miao, T.J. Simmons, J.W. Lee, T.V. Doherty, J.S. Dordick, R.J. Linhardt, Biomacromolecules 11, 2440–2445 (2010)
W.W. Ma, X.T. Dong, J.X. Wang, W.S. Yu, G.X. Liu, J. Mater. Sci. 48, 2557–2565 (2013)
C. Song, X.T. Dong, Optoelectron. Adv Mater RC 6, 225–229 (2012)
C. Song, X.T. Dong, Optoelectron. Adv Mater RC 5, 1296–1300 (2011)
Q.L. Ma, J.X. Wang, X.T. Dong, W.S. Yu, G.X. Liu, J. Xu, J. Mater. Chem. 22, 14438–14442 (2012)
T.H. Hwang, Y.M. Lee, B.S. Kong, J.S. Seo, J.W. Choi, Nano Lett. 12, 802–807 (2012)
D. Li, J.X. Wang, X.T. Dong, W.S. Yu, G.X. Liu, J. Mater. Sci. 48, 5930–5937 (2013)
L.Y. Yang, J.X. Wang, X.T. Dong, G.X. Liu, W.S. Yu, J. Mater. Sci. 48, 644–650 (2013)
Peter A. Tanner, L.S. Fu, Chem. Phys. Lett. 470, 75–79 (2009)
Y.X. Zhang, S.S. Pan, X.M. Teng, Y.Y. Luo, G.H. Li, J. Phys. Chem. C 112, 9623–9626 (2008)
L.Z. Tong, J.H. Shi, D.M. Liu, Q.H. Li, X.Z. Ren, H. Yang, J. Phys. Chem. C 116, 7153–7157 (2012)
W.W. Wang, J.L. Yao, J. Phys. Chem. C 113, 3070–3075 (2009)
M.Y. Zhu, G.W. Diao, J. Phys. Chem. C 115, 18923–18934 (2011)
Y.F. Zhu, Y. Fang, S. Kaskel, J. Phys. Chem. C 114, 16382–16388 (2010)
L. Wang, Y. Yu, P.C. Chen, D.W. Zhang, C.H. Chen, J. Power Sour. 183, 717–723 (2008)
J.C. Fu, J.L. Zhang, Y. Peng, J.G. Zhao, G.G. Tan, N.J. Mellors, E.Q. Xie, W.H. Han, Nanoscale 4, 3932–3936 (2012)
C. Cannas, A. Musinu, D. Peddis, G. Piccaluga, Chem. Mater. 18, 3835–3842 (2006)
Q. Liu, L.F. Lai, X.J. Fu, F.P. Zhu, J.H. Sun, H.G. Rong, M.Y. He, Q. Chen, Z. Xu, J. Mater. Sci. 42, 10113–10117 (2007)
Z.L. Wang, X.J. Liu, M.F. Lv, P. Chai, Y. Liu, J. Meng, J. Phys. Chem. B 112, 11292–11297 (2008)
X.Y. Wang, F. He, F. Tang, N. Ma, L.D. Li, Colloids Surf. A physicochem. Eng. Aspects 392, 103–109 (2011)
H.G. Wang, L. Sun, Y.P. Li, X.L. Fei, M.D. Sun, C.Q. Zhang, Y.X. Li, Q.B. Yang, Langmuir 27, 11609–11615 (2011)
H.X. Peng, G.X. Liu, X.T. Dong, J.X. Wang, W.S. Yu, J. Xu, Powder Technol. 215–216, 242–246 (2012)
H.G. Wang, Y.X. Li, L. Sun, Y.C. Li, W. Wang, S. Wang, S.F. Xu, Q.B. Yang, J. Colloid Interface Sci. 350, 396–401 (2010)
Q.L. Ma, W.S. Yu, X.T. Dong, J.X. Wang, G.X. Liu, J. Xu, J. Nanopart. Res. 14, 1203–1209 (2012)
Q.L. Ma, J.X. Wang, X.T. Dong, W.S. Yu, G.X. Liu, Chem. Eng. J. 222, 16–22 (2013)
Z.R. Zhu, X.Y. Li, Q.D. Zhao, Y. Shi, H. Li, G.H. Chen, J Nanopart Res. 13, 2147–2155 (2011)
C.J. Li, J.N. Wang, B. Wang, J.R. Gong, Z. Lin, Mater. Res. Bull. 47, 333–337 (2012)
X.Z. Ren, J.H. Shi, L.Z. Tong, Q.H. Li, H. Yang, J. Nanopart. Res. 15, 1738–1747 (2013)
Y.M. Jia, Z.H. Zhou, Y.B. Wei, Z. Wu, H.H. Wang, J.R. Chen, Y.H. Zhang, Y.S. Liu, Smart Mater. Struct. 22, 125014–125019 (2013)
Q.L. Ma, J.X. Wang, X.T. Dong, W.S. Yu, G.X. Liu, J. Nanopart. Res. 16, 2239–2248 (2014)
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (NSFC 50972020, 51072026), Specialized Research Fund for the Doctoral Program of Higher Education (20102216110002, 20112216120003), the Science and Technology Development Planning Project of Jilin Province (Grant Nos. 20130101001JC, 20070402, 20060504), the Science and Technology Research Project of the Education Department of Jilin Province during the eleventh five-year plan period (Under Grant No. 2010JYT01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bi, F., Dong, X., Wang, J. et al. Coaxial electrospinning preparation and properties of magnetic–photoluminescent bifunctional CoFe2O4@Y2O3:Eu3+ coaxial nanofibers. J Mater Sci: Mater Electron 25, 4259–4267 (2014). https://doi.org/10.1007/s10854-014-2158-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-2158-x