Abstract
Hollow CoFe2O4 spheres consisted of CoFe2O4 nanoparticles were synthesized by a facile solvothermal treatment of an ethylene glycol solution of FeCl3 · 6H2O, CoCl2 · 6H2O, and NaAc at 200 °C in the presence of polyethylene glycol and oleic acid. The products were characterized by powder X-ray diffraction, transmission electron microscopy, selected area electron diffraction, high-resolution transmission microscopy, scanning electron microscopy. The magnetic properties were evaluated using a vibrating sample magnetometer. The probable mechanism of the formation of Hollow CoFe2O4 spheres was discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years considerable attention has been focused on hollow nanostructures owing to their higher specific surface area, lower density and better permeation, and potential applications in catalysts, chemical sensors, drug delivery, photonic crystal, low-density structural materials and in biotechnology [1]. Generally, there are two approaches for preparing such materials. One is based on the use of various removable templates including polymer latex spheres [2], silica sol–gel [3, 4], microemulsion droplets [5], liquid crystals [6], liquid droplets [7], surfactant vesicles [8], polymer micelles [9], polymer-surfactant complex micelles [10, 11], functional surfactant micelles [12], and metal nanoparticles [13]. For example, we have prepared hollow Ni nano/microstructures via hydrothermal treatment of alkaline solution of Ni(DS)2 and NaH2PO2 [12]. Another is based on the utilization of some physical phenomena, such as the Kirkendall effect or Ostwald ripening [14–17], For example, Alivisatos and co-workers synthesized hollow nanocrystals of cobalt oxide and chalcogenides through a Kirkendall effect [14]. Hollow structures also can be achieved by mild hydrothermal process [18, 19], γ-irradiation [20], ultrasonication [21]. Spinel ferrites (MFe2O4; M = Fe, Co, Ni, Mn, Zn) are among the most important magnetic materials and have been widely used in electronic devices, information storage, magnetic resonance imaging (MRI), and drug-delivery technology [22–27]. Because of the practical reasons mentioned above, the synthesis of nanostructured CoFe2O4 has also attracted considerable attention. Some methods for preparation of CoFe2O4 nanomaterials have been reported [28–38]. However, there are very few reports on the preparation of hollow CoFe2O4 spheres [39]. Chen et al. described a facile route for preparation of submicrometer ferrite/block copolymer hollow spheres. Herein, we report a method for the synthesis of CoFe2O4 hollow spheres via solvothermal treatment of an ethylene glycol solution of FeCl3 · 6H2O, CoCl2 · 6H2O and NaAc (sodium acetate) at 200 °C in the presence of oleic acid and polyethylene glycol (PEG). This method is simple and requires no expensive reagents.
Experimental section
All the regents were of analytical purity, and were used without further purification. Polyethylene glycol (average molecular weights (M w) of 400) oleic acid (OA), ethylene glycol (EG), and poly(vinyl pyrrolidone) (PVP) (average molecular weights (M w) of 40,000) were obtained from Shanghai Chemical Reagent Company.
The X-ray powder diffraction (XRD) pattern of the as-prepared products was collected on a Shimadzu XD-3A X-ray diffractometer with CuKα radiation (λ = 0.15147 nm). Transmission electron microscopy (TEM) images and selected area electron diffraction patterns (SAED) were obtained by employing JEOL JEM-200CX transmission electron microscope, using an accelerating voltage of 200 kV. Scanning electron microscopy (SEM) images were taken on a JEOL-JSM6360LA scanning electron microscope. Using a Lake Shore 7303–9309 vibrating sample magnetometer performed room-temperature magnetic characterization of the CoFe2O4 nanocrystals.
In a typical synthesis, FeCl3 · 6H2O (0.676 g, 2.5 mmol) and CoCl2 · 6H2O (0.278 g, 1.25 mmol) were dissolved in ethylene glycol (20 mL) to form a clear solution, followed by the addition of NaAc (1.8 g, 21.95 mmol), polyethylene glycol (1.0 mL) and oleic acid (2.0 mL). The mixture was stirred vigorously for 30 min and then sealed in a Teflon-lined stainless-steel autoclave (30 mL capacity). The autoclave was heated to and maintained at 200 °C for 8 h, and allowed to cool to room temperature. The black products were washed several times with water and absolute ethanol and dried at 60 °C for 3 h.
Results and discussion
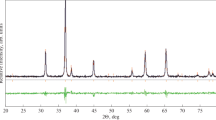
Figure 1 shows the power XRD pattern from the as-prepared product. The diffraction characteristic peaks are quite similar to those bulk CoFe2O4, which can be indexed as the cubic structure CoFe2O4 with lattice constants of a = 0.839 nm. This is in good agreement with the reported data (JCPDS File No 22-1086). No impurity peaks were observed, indicating that the nanomaterials obtained via our current synthetic methods consist of pure phases. However, the peaks were relatively broad compared with those of the bulk materials. Based on the calculation of Scherrer’s formula, the average particle diameter is about 15.5 nm.
The morphology and microstructure of the CoFe2O4 products were further examined with TEM, SAED, high-resolution transmission microscopy (HRTEM) and SEM. Figure 2a shows a typical TEM image of the CoFe2O4 products. The TEM image in Fig. 2a reveals that the products consist of many sphere particles and nanoparticles. The sphere particles have pale color regions in the central parts in contrast to dark edges, indicating that they are hollow spherical structure. The diameter of CoFe2O4 hollow spheres is in the range of 220–360 nm. The TEM image of a CoFe2O4 hollow sphere in Fig. 2b clearly reveals that the spherical shell is built up of numerous CoFe2O4 nanoparticles. The size of CoFe2O4 nanoparticles is in the range of 7–18 nm, and the average particle diameter is about 15 nm, which verifies the calculating result based on the XRD data. Figure 2c is a SAED of CoFe2O4 hollow spheres, suggesting that the CoFe2O4 spheres are polycrystalline. HRTEM image (Fig. 2d) also shows that the CoFe2O4 hollow sphere is multicrystalline and the fringe space is about 0.21 nm, close to the interplanar (400) distance of cubic structure CoFe2O4 (0.209 nm). The SEM image of CoFe2O4 products (Fig. 2e, f) further verifies that they have hollow spherical structure.
(a) TEM image of the CoFe2O4 hollow spheres and nanocrystals, (b) TEM image of a CoFe2O4 hollow sphere, (c) SAED image of the CoFe2O4 nanocrystals obtained in the typical synthesis, and (d) HRTEM image of a CoFe2O4 hollow sphere. (e) SEM image of the CoFe2O4 hollow spheres and nanocrystals. The inset is the SEM image of the broken CoFe2O4 hollow sphere (f) SEM image of a CoFe2O4 hollow sphere
In a typical synthesis, the usage of polyethylene glycol and oleic acid is 1.0 and 2.0 mL respectively. To learn more about the formation of CoFe2O4 hollow spheres, we also characterized the size and morphology of CoFe2O4 nanomaterials obtained from their synthesis using different amounts of polyethylene glycol and oleic acid. When the amount of oleic acid was kept at 2.0 mL, and the amount of polyethylene glycol was in the range of 2–3 mL, CoFe2O4 hollow spheres and nanoparticles were too obtained. Figure 3a shows the typical TEM images of CoFe2O4 hollow spheres and nanoparticles prepared in the presence of polyethylene glycol (2.0 mL) and oleic acid (2.0 mL), the diameter of CoFe2O4 hollow spheres are in the range of 150–420 nm and the nanoparticles in the range of 5–15 nm. When the usage of polyethylene glycol was kept at 1.0 or 2.0 mL, and the usage of oleic acid was decreased to 1.0 mL, we found that the products were CoFe2O4 hollow spheres and nanoparticles yet. Figure 3b shows the typical TEM images of CoFe2O4 hollow spheres and nanoparticles prepared in the presence of polyethylene glycol (2.0 mL) and oleic acid (1.0 mL), the diameter of CoFe2O4 hollow spheres and nanoparticles are in the range of 150–470 nm. When PVP (300 mg) was used instead of polyethylene glycol, the hollow spheres were also obtained (Fig. 3c). When the usage of oleic acid was kept at 2.0 mL and polyethylene glycol was not used, or only polyethylene glycol was used and no oleic acid, we found that the products were all CoFe2O4 nanoparticles and no CoFe2O4 hollow spheres. Figure 3d shows the typical TEM images of CoFe2O4 nanoparticles prepared in the presence of oleic acid (2.0 mL), the diameter of CoFe2O4 nanoparticles are in the range of 13–20 nm. The CoFe2O4 nanomaterials show excellent dispersibility in ethanol and poor dispersibility in water, which indicates that the surface of the products was covered by hydrophobic surfactants. Infrared spectroscopy analysis further verifies this conclusion (Fig. 4). The strong peak at 1,710 cm−1 belonging to ν s (OCO) stretching vibration mode of free oleic acid molecule is not found, and absorption peak at 1,550 cm−1 is appeared in infrared spectroscopy, which indicates that oleic acid molecules are bonded with CoFe2O4 nanoparticles (chemisorbed), and resulting in the changing of absorption peak location [40]. Weak peak at 1,100 cm−1 should belong to ν s (COC) stretching vibration mode of polyethylene glycol molecule. Strong peak at 2,921 cm−1 should be belonged to ν s (CH) stretching vibration mode of polyethylene glycol or oleic acid molecules. Thermogravimetric analysis (TG-DSC) also reveals the presence of polyethylene glycol and oleic acid molecules in the products. The TG-DSC for the product showed an initial weight loss of 15.09% from 190 to 430 °C mainly corresponding to the pyrolysis of PEG molecules, followed by another weight loss of 11.25% from 430 to 800 °C mainly for the decomposition of oleic acid molecules from the product, the remaining residue was CoFe2O4.
Based on the above facts, we speculate that the formation of CoFe2O4 nanoparticles and hollow spheres may be relevant to the little water/oil drops and spherical vesicles as soft structural templates as shown in Fig. 5. When the reaction temperature is raised, the crystalline water is released and polyethylene glycol, oleic acid molecules extend toward the water phase with their hydrophilic group, resulting in the formation of many little water/oil drops. FeCl3, CoCl2 react with NaAc in the water phase of little liquid drops, and form CoFe2O4 nanoparticles. Then, CoFe2O4 nanoparticles are assembled into hollow spheres via the spherical vesicles formed by oleic acid and polyethylene glycol/PVP molecules. The detailed formation mechanism of CoFe2O4 hollow spheres needs to be investigated further.
The magnetic properties of CoFe2O4 nanomaterials consisted of nanoparticles and hollow spheres obtained in the typical synthesis were investigated with a vibrating sample magnetometer. Figure 6 shows the magnetization curve measured at 300 K for the CoFe2O4 nanomaterials obtained in the typical synthesis. The saturation magnetization (M s), remanent magnetization (M r), and coercivity (H c) are ca. 58.94 emu g−1, 11.05 emu g−1, and 226 Oe for the CoFe2O4 nanomaterials at 300 K, respectively. The M s of the CoFe2O4 nanomaterials is lower than that of the corresponding bulk material (about 80 emu g−1). Compared with the H c value of bulk CoFe2O4 reported in literature (5,400 Oe) [41], the CoFe2O4 nanomaterials also exhibit smaller coercivity, which should be attributed to their nanostructure [42–43].
Conclusion
In summary, we have succeeded in synthesizing CoFe2O4 hollow spheres and nanoparticles by a facile solvothermal treatment of an ethylene glycol solution of FeCl3 · 6H2O, CoCl2 · 6H2O and NaAc at 200 °C in the presence of polyethylene glycol and oleic acid. Since it is a simple process, we believe that it can be applied to synthesize other metal oxides. Further research is under progress in our laboratory.
References
(a) Hu Y, Jiang XQ, Ding Y, Chen Q, Yang CZ (2004) Adv Mater 16:933; (b) Caruso F (2000) Chem Eur J 6:413
(a) Caruso F, Caruso RA, Möhward H (1998) Science 282:1111; (b) Wang L, Sasaki T, Ebina Y, Kurashima K, Wataanabe M (2002) Chem Mater 14:4827; (c) Caruso F, Shi X, Caruso RA, Susan A (2001) Adv Mater 13:740; (d) Radtechenko IL, Sukhorukov GB, Gaponik N, Koranowski A, Rogach AL (2001) Adv Mater 13:1684
Kim S, Kim M, Lee WY, Hyeon T (2002) J Am Chem Soc 124:7642
Wang D, Caruso F (2002) Chem Mater 14:1909
(a) Bao J, Liang Y, Xu Z, Si L (2003) Adv Mater 21:1832; (b) Walsh D, Lebeau B, Mann S (1999) Adv Mater 11:324
Braun PV, Stupp SI (1999) Mater Res Bull 34:463
(a) Fowler CE, Khushalani D, Mann S (2001) J Mater Chem 11:1968; (b) Fowler CE, Khushalani D, Mann S (2001) Chem Commun 2028; (c) Huang J, Xie Y, Li B, Liu Y, Qian Y, Zhang S (2000) Adv Mater 12:808; (d) Tartaj P, González-Carreño T, Serna CJ (2001) Adv Mater 13:1620
(a) Hubert DHW, Jung M, German AL (2000) Adv Mater 12:1291; (b) Schmidt HT, Ostafin AE (2002) Adv Mater 14:532
Liu T, Xie Y, Chu B (2000) Langmuir 16:9015
Zhang D, Qi L, Ma J, Cheng H (2002) Adv Mater 14:1499
Zhang D, Qi L, Ma J, Cheng H (2002) Adv Mater 14:300
Liu Q, Liu H, Han M, Zhu J, Liang Y, Xu Z, Song Y (2005) Adv Mater 17:1995
(a) Sun Y, Xia Y (2002) Science 298:2176; (b) Sun Y, Mayers B, Xia Y (2003) Adv Mater 15:641
Yin Y, Rioux RM, Erdonmez CK, Hughes S, Somorjai GA, Alivisatos AP (2004) Science 304:711
Yang JH, Qi LM, Lu CH, Ma JM, Cheng HM (2005) Angew Chem Int Ed 44:598
Liu B, Zeng HC (2004) J Am Chem Soc 126:16744
Li L, Chu Y, Liu Y, Dong L (2007) J Phys Chem C 111:2123
(a) Zhan J, Lin H, Mou C (2003) Adv Mater 15:621; (b) Chen D, Chen D, X, Jiao, Zhao Y (2003) J Chem Mater 13:2266
Wang X, Li Y (2003) Angew Chem Int Ed 42:3497
Hu Y, Chen J, Chen W, Lin X, Li X (2003) Adv Mater 15:726
Arul Dhas N, Suslick KS (2005) J Am Chem Soc 127:2369
Zhao W, Gu J, Zhang L, Chen H, Shi J (2005) J Am Chem Soc 127:8916
Caruso F, Spasova M, Susha A, Giersig M, Caruso RA (2001) Chem Mater 13:109
Hyeon T (2003) Chem Commun 927
Yu S, Yoshimura M (2002) Adv Funct Mater 12:9
Perez JM, Loughlin TO, Simeone FJ, Weissleder R, Josephson L (2002) J Am Chem Soc 124:2856
(a) Perez JM, Simeone FJ, Tsourkas A, Josephson L, Weissleder R (2004) Nano Lett. 4:119; (b) Yoon T-J, Kim JS, Kim BG, Yu KN, Cho M-H, Lee J-K (2005) Angew Chem Int Ed 44:1068
Sun S, Zeng H, Robinson DB, Ramoux S, Rice PM, Wang SX, Li G (2004) J Am Chem Soc 126:273
Song Q, Zhang ZJ (2004) J Am Chem Soc 126:6164
Deng H, Li X, Peng Q, Wang X, Chen J, Li Y (2005) Angew Chem Int Ed 44:2782
Hyeon T, Chung Y, Park J, Lee SS, Y.-W. Kim, Park BH (2002) J Phys Chem B 106:6831
Kang YS, Risbud S, Rabolt JF, Stroeve P (1996) Chem Mater 8:2209
Hong C-Y, Jang IJ, Horng HE, Hsu CJ, Yao YD, Yang HC (1997) J Appl Phys 81:4275
Fried T, Shemer G, Markovich G (2001) Adv Mater 13:1158
Tang ZX, Sorensen CM, Klabunde KJ, Hadjipanayis GC (1991) J Colloid Interface Sci 146:38
(a) Zhang ZJ, Wang ZL, Chakoumakos BC, Yin JS (1998) J Am Chem Soc 120:1800; (b) Liu C, Zou B, Rondinone AJ, Zhang ZJ (2000) J Phys Chem B 104:1141
Neveu S, Bec A, Robineau M, Talbol D (2002) J Colloid Interface Sci 255:293
Pileni MP, Moumen N (1996) J Phys Chem B 100:1867
Li X-H, Zhang D-H, Chen J-S (2006) J Am Chem Soc 128:8382
Davies KJ, Wells S, Upadhyay RV, Charles SW, O’Grady K, ElHilo M, Meaz T, Mørup S (1995) J Magn Magn Mater 149:14
Lee J, Park JY, Kim CS (1998) J Mater Sci 33:3965
Yan C-H, Xu Z-G, Cheng F-X, Wang Z-M, Sun L-D, Liao C-S, Jia J-T (1999) Solid State Commun 111:287
Ammar S, Helfen A, Jouini N, Fiévet F, Rosenman I, Villain F, Molinié P, Danot M (2001) J Mater Chem 11:186
Acknowledgements
We thank the National Natural Science Foundation of China (No. 20671045), the Natural Science Foundation of Education Department of Jiangsu Province (05KJB150023), the Natural Science Foundation of Jiangsu Province, and the Natural Science Foundation of Jiangsu Province Key Laboratory of Fine Petro-chemical Technology of Jiangsu Polytechnic University for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Q., Lai, L., Fu, X. et al. Solvothermal synthesis of CoFe2O4 hollow spheres. J Mater Sci 42, 10113–10117 (2007). https://doi.org/10.1007/s10853-007-2075-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-2075-y