Abstract
A novel blended solid polymer electrolyte comprising polyethylene oxide and polyvinylpyrrolidone polymers for blending and sodium nitrate (NaNO3) as ion conducting species has been optimized via standard solution-cast technique. XRD, FESEM, and FTIR were performed to obtain the information about the structural changes, morphology, and microstructural changes (polymer–ion and ion–ion interactions) of the solid polymer electrolyte films. The electrochemical impedance spectroscopy, linear sweep voltammetry, and i–t characteristics were performed to evaluate the ionic conductivity, voltage stability window, and ion transference number. The impedance study was done in a broad temperature range (40–100 °C). The DSC and TGA were used to obtain information about the thermal transitions and thermal stability of prepared films. The ion dynamics is further investigated by analyzing the complex permittivity, loss tangent, and complex conductivity. All the plots were fitted through established theoretical model/expressions in whole frequency window to obtain dielectric strength, ion conduction path behavior, and relaxation time. Transport parameters such as number density (n), mobility (μ), and diffusion coefficient (D) of mobile ions were obtained by three methods and compared satisfactorily. Lastly, a coherent mechanism for the migration of charge transport carriers within the solid polymer composites has been proposed based on the performed experimental outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In view of collective demand of energy worldwide and exhaustion of the traditional energy resources (fossil fuels, coal, etc.), it becomes mandatory to develop the clean and renewable (C&R) resources for application in the transport and portable electronic sectors. The most appropriate energy source is the Li-ion battery (LIB) technology, which is chosen due to its high specific energy density, broad electrochemical potential window, a prolonged service life, and no memory loss. However, the limited availability of the lithium metal and high price force the researchers to develop its alternative. Therefore, research in the field keeps trying parallel development of secondary batteries which could be able to fulfill the desire of Li-associated energy storage device and could be able to eliminate the hurdle associated with it. In this line, the most feasible and competitive element that has the potential to replace lithium (Li) is sodium (Na). It could be possible due to its high abundance (1000 times more than Li), low toxicity, lower price, voltage versus SHE (2.7 V), and no geographical boundaries. Recently, again there is huge work carrying on all over the world to develop sodium-ion batteries (SIB) [1,2,3,4].
As any rechargeable battery consists of four components as (1) cathode, (2) anode, (3) electrolyte, and (4) separator, during the transportation of charge carrier, ions are shuttled between the two electrodes via the electrolyte and separator prevents the physical contact of electrodes to avoid from short-circuiting. Electrolyte provides the path for conduction and plays a tremendous role during charging/discharging operation. It seems that electrolyte is a crucial component of any rechargeable battery system. The existing battery technology is based on the electrolyte consisting of organic/inorganic separator pored with ionic liquids (like LiPF6) that challenges the safety issues on every step in terms of volatility, leakage, reaction with electrodes, and flammability. In this way, it becomes mandatory to eliminate the above-said issues to fulfill the dream of a safe/stable secondary battery system [5].
Research and development of polymer electrolytes (PEs) have gained the interest of the scientific community as an alternative of the traditional liquid-based electrolytes due to advantages such as lightweight, flexibility, geometric stability, improved safety, and no leakage [6, 7]. Firstly, the gel polymer electrolyte (GPE) was developed by the addition of organic solvent as a plasticizer (such as EC, PC, DEC) in polymer salt matrix and displayed improved properties as compared to the liquid electrolyte. But, poor mechanical property and interfacial issues prevented their use for the safe battery system. Solid polymer electrolyte (SPE) films seem to be the best alternative and have the potential to eliminate all the above-said issues of the traditional and gel polymer electrolyte system. The major advantage with the SPE is simple and low-cost design strategy, flexibility, and miniaturization of devices that automatically lowers both cost and weight. As no liquid part is used, so all solid-state battery guarantees great safety with respect to existing one. The desirable properties for SPE are high ionic conductivity, broad operating voltage, and desirable mechanical, thermal and interfacial properties. One more important advantage of SPE become essential to mention here that it could be able to serve the dual purpose of electrolyte and separator in any such energy storage/conversion devices [7,8,9,10,11,12].
Some recently published works reported the exploration of SPEs for the application in battery to employ various compositions, i.e., poly (vinylidene fluoride-hexafluoropropylene) [P(VdF-co-HFP)] [13], polyethylene oxide (PEO) [14], polyvinylpyrrolidone (PVP) [15], PAN [16, 17], poly (vinyl alcohol)/poly (vinylpyrrolidone) (PVA/PVP) [18], PVA–PMMA [19], and PEO–PAN [20,21,22]. In aforementioned system combination, polyethylene oxide (PEO) gains more attention as a host polymer due to its wide range of properties like the good capability of complex formation with many salts, high ionic conductivity, and the presence of electron-rich ether group in polymer backbone (CH2–O–CH2). However, its semicrystalline nature results in low ionic conductivity and prevents its use in such application. In order to remove this drawback of PEO, there are various approaches (like polymer blending, cross-linking, etc.) have been adopted to suppress its crystallinity. In adopted approaches, polymer blending seems more convincing and effective, as polymer blending combined and improved the properties as compared with individual host polymer. The most common interaction existing in polymer blends is (1) hydrogen bonding, (2) dipole–dipole interactions, and (3) ionic interactions [23]. The best polymer for blending is the poly (vinylpyrrolidone) (PVP) due to high amorphous content and presence of rigid pyrrolidone group and carbonyl group (C=O). The former one promotes faster ion dynamics, while latter one promotes the possibility of complex formation with various inorganic salts [24,25,26,27].
Recently, a few investigations have been carried out to prepare solid polymer electrolyte using different sodium salts [26, 28,29,30,31,32,33]. Our group also investigated the blend of solid polymer electrolyte based on PEO–PVP + NaPF6. It demonstrated the improved electrochemical properties as compared to the other sodium salts [27]. To the best of our knowledge, there is no report on the preparation and characterization of blend solid polymer electrolyte with sodium nitrate salt in a wide range of composition. Hence, the present investigation is the extension of the investigation of different sodium salts in view of the exploration of appropriate and compatible solid polymeric separator required for such devices. Herein, we report the simple preparation of blend solid polymer electrolyte (BSPE) based on polyethylene oxide (PEO), polyvinylpyrrolidone (PVP)-blended matrix complexes with sodium nitrate (NaNO3) as aprotic salt. The structural, microstructural, morphological, electrochemical, transport and dielectric properties are investigated in detail followed by the correlation between the above results. On the basis of the experimental results, an ion-transport mechanism has been proposed to visualize the obtained results in a simple and logical manner.
Preparation of BSPE films
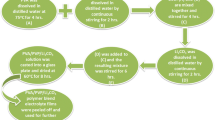
Blended solid polymer electrolyte has been prepared by the solution-cast technique. First of all, the polyethylene oxide (PEO; 0.4 g) and polyvinylpyrrolidone (PVP; 0.1 g) (Aldrich) are added with methanol (Aldrich) in the required wt%, and the solution is stirred till a homogenous solution is obtained. Figure 1a shows the interaction between the two polymers. Then, the salt is added in the stoichiometric ratio (O/Na = 2, 4, 6, 8, 10, 12, 14, 16, 20) to the blend polymer solution, and solution is stirred again till a transparent solution is obtained. Then the solution is cast in the Teflon Petri dishes and allowed to evaporate slowly followed by vacuum drying. Finally, a freestanding polymer film is obtained and kept is vacuum desiccator (with silica gel) for further characterization. The average thickness of the films was ~ 100 to 120 μm measured using micrometer (Digimatic Micrometer, Mitutoyo). Figure 1b shows the interactions between the blend polymer and salt.
Characterization techniques
The structural investigations were obtained by X-ray diffraction (XRD) (Malvern Panalytical) with CuKα radiation (λ = 1.54 Å) in the Braggs angle range (2θ) from 10° to 60°. The morphological properties are obtained using the field emission scanning electron microscopy (FESEM) (Carl Zeiss product). The microstructural and presence of interaction among the different component of the PNC are obtained through Fourier transform infrared (FTIR) spectroscopy (Model: Bruker Tensor 27, Model: NEXUS–870) recorded in absorbance mode in the wavenumber region 600–3000 cm−1 with a resolution of 4 cm−1.
The impedance spectroscopy has been performed using the electrochemical analyzer (CHI760, USA) in the frequency range of 1 Hz–1 MHz and temperature range of 40–100 °C (Temperature Controller; Marine India). An AC signal of 20 mV is applied across the cell configuration SS|BSPE|SS. Here, SS refers to stainless-steel electrodes. The activation energy (Ea) for ionic transport is estimated from the slope of the linear fit of the Arrhenius plot (log (σ/S cm−1) vs. 1000/T plot) represented by σ = σ0exp (− Ea/kT), where σ0 is the constant pre-exponential factor and Ea is the activation energy. The parameter T stands for the absolute temperature and k for the Boltzmann constant. Thermal properties are investigated by differential scanning calorimetry (DSC; Sirius 3500) and thermogravimetric analysis (TGA—SHIMADZU–DTG-60H). The ion transference number (tion) has been obtained by i–t characteristics by applying a fixed DC voltage of 10 mV across the SS|BSPE|SS cell. The linear sweep voltammetry (LSV) was performed to obtain the voltage stability window.
The impedance data is transformed into the dielectric format. Dielectric properties of the synthesized BSPEs are investigated in terms of the complex dielectric permittivity, loss tangent plot, and complex conductivity. We have also simulated the complex permittivity, loss tangent and complex conductivity plot via established theoretical expression in the complete frequency window to get a proper insight of the dielectric relaxation and its influence on the ion dynamics in the blend polymer matrix. The detail of the transformation is given in our previous report [34].
Results and discussion
X-ray diffraction (XRD) analysis
The XRD pattern of the PEO–PVP blend and with different salt contents over the range 2θ = 10°–60° is shown in Fig. 2. The fundamental peaks of the PEO are at 19° (corresponding to 120 planes) and at 23° (corresponding to 112, 032 planes) associated with the semicrystalline nature of PEO (Fig. 2a). The peaks are in good agreement with the previous reports. Several low-intense peaks of PEO are also observed at 14°, 21°, 27°, 29° [37]. The broad characteristics peaks of PVP are observed at 13° and 21°, and the absence of this in the blend polymer electrolyte confirms the blend formation [35,36,37,38]. Further addition of salt alters the peak position and intensity of the crystalline peaks (Fig. 2b–j). This indicates the complexation between the salt and blended polymer of the prepared thin polymeric separator. The peak broadening of PEO peak infers the enhancement of the amorphous content in it. The absence of any salt peak indicates the complete salt dissociation and is in agreement with the previous literature [36]. Addition of salt shifts the peak toward the lower angle side that infers the suppression of the crystalline part and is beneficial for the fast ion migration. From the XRD spectra, structural parameters interlayer d-spacing, and interchain separation (R) are obtained for getting insights into polymer salt complex formation. The interlayer d-spacing is obtained by Braggs law 2dsinθ = λ and (R) using the equation R = 5λ/8sinθ [27]. The increase in interchain separation and d-spacing for both the planes indicates the enhancement of the amorphous content owing to the structural modification (Table 1). It suggests (1) polymer blend formation, (2) polymer salt complexation and enhanced amorphous content, and (3) disruption of the crystalline arrangement of the polymer chain.
Field emission scanning electron microscopy (FESEM) analysis
The surface morphology of the prepared polymer films has been investigated by the FESEM. Figure 3 shows the FESEM micrographs for the pure PEO (Fig. 3a), PEO–PVP blend (Fig. 3b), PEO–PVP + O/Na = 14 (Fig. 3c), and PEO–PVP + O/Na = 4 (Fig. 3d). Pure PEO shows the rough morphology that is the characteristic of the crystalline nature of the polymer, while after polymer blending, morphology get drastically changed and disappearance of the rough nature reveals the enhancement of the amorphous phase. The smooth surface is favorable for fast ionic transport, and it depicts that polymer blending has occurred as anticipated in the premise. Figure 3c shows the morphology of the optimized polymer salt matrix, and changes in the morphology confirm that complexation has been occurred in between the salt and polymer. It also suggests the good miscibility between the polymer and salt. This is also in agreement with the XRD results. This smooth morphology and enhanced amorphous content could promote the faster ion migration [27, 39]. Figure 3d shows the morphology of the polymer salt system with higher salt content (O/Na = 4). The smooth nature of the film and overlapped nature suggests the complex formation which will support in lowering the interfacial resistance. However, the polymer film is seen to have an inhomogeneous morphology with some salt agglomeration (shown by a yellow circle) that reveals the inhomogeneous salt distribution and indicates the presence of an ion pair. Figure 3 e confirms the presence of salt in the polymer matrix (O/Na = 14) investigated in terms of EDS spectra. Further, to check the homogeneity of salt in a blend polymer matrix, elemental mapping of the Na, O, N is performed for polymer salt matrix (O/Na = 14) and confirmed the uniform salt dispersion in the complete polymer salt matrix. In brief, FESEM analysis discloses the (1) polymer blend formation, (2) polymer salt complexation occurrence, and (3) uniform dispersion of salt in an optimized system.
Fourier transform infrared spectroscopy (FTIR) analysis
Figure 4 depicts the FTIR absorption bands of PEO–PVP and PEO–PVP + NaNO3 in the wavenumber region 600–3000 cm−1. The variation in the characteristics groups of the polymer blend and with the addition of salt is shown by the dotted line. The band located at 842 cm−1 is assigned to the CH2 rocking mode of PVP, and a minor contribution from the C–O stretching mode is associated with PEO. The band located at 952 cm−1 is associated with C–O stretching vibration mode of PEO. The C–C stretching mode is located at 1060 cm−1. The fundamental band of the PEO is observed at 1120 cm−1 associated with the C–O–C stretching mode. The three important bands are: (1) at 1282 cm−1 associated with CH2 asymmetric twisting, (2) at 1348 cm−1 CH2 bending mode, and (3) at 1461 cm−1 associated with CH2 wagging mode. Two strong absorption bands are located at 1348 cm−1 and 1687 cm−1 which correspond to C–N stretching and C=O stretching, respectively. Then two important bands associated with the C–H stretching (symmetric and asymmetric) mode of PEO are located in the wavenumber range of 2700–3000 cm−1 [27, 32, 40, 41]. The characteristics IR mode of anion \( \left( {{\text{NO}}_{3}^{ - } } \right) \) is located at 680, and 1350 cm−1 in the polymer salt matrix [42]. To explore the interactions between the polymer and salt, the FTIR spectra are explained in the forthcoming section (Table 2).
Polymer–ion interaction
Figure 5 shows the magnified view of the (1) fingerprint region, 600–1500 cm−1, and (2) C–H st. mode, 2700–3000 cm−1. (1) fingerprint region Fig. 5 a shows the major absorbance bands at 842, 952, 1120, 1282, 1348, 1461 cm−1 associated with the CH2 rocking/C–O stretching, C–O stretching, C–O–C stretching, CH2 asymmetric twisting, CH2 bending mode, and CH2 wagging mode, respectively. It can be observed that even the addition of small amount of salt in all the blend polymer peaks shows a change in the peak intensity and shape [15, 26, 27, 29, 32]. This evidences that salt plays an effective role in altering the polymer blend system and interactions. The characteristics band of the PEO located at 1120 cm−1 shifts toward lower wavenumber side, and asymmetry in peak is observed. It suggests that the cation get coordinated with this and complexation between the polymer salts is confirmed. With the further change of salt content, this peak clearly shows the splitting in three peaks (Fig. 5b–j). This strongly evidences the polymer salt complexation, and peak broadening suggests the enhancement of the amorphous content. A new peak is observed with the addition of salt near 1240 cm−1 and is associated with the CH2 symmetric/asymmetric twisting mode of PEO which also shows noticeable changes with the addition of salt. The peaks located at 1348 and 1461 cm−1 show a reduction of the intensity and confirm that salt plays an effective role in alerting the interactions between the polymer and salt. (2) C–H st. mode; 2700–3000 cm−1 Addition of salt in the blend polymer matrix changes the peak shape located in wavenumber region of 2700–3000 cm−1. The appearance of shoulder peak (indicated by arrow) evidences the polymer salt complex formation. With the change of salt content, peak broadening is observed and PEO structure is altered due to the interaction of salt with the polymer.
It may be concluded from the above discussion and analysis that the addition of salt results in a reduction of intensity and peak broadening which infers the increase of disorder in the polymer matrix. This indicates the formation of a favorable environment for the cation migration which will be further confirmed by the impedance and the transport studies.
Ion–ion interaction
To further explore the present system, ion–ion interactions are studied by exploring the anion vibration mode (\( {\text{NO}}_{3}^{ - } \)) which is IR active, while the cation (Na+) is IR inactive. The \( {\text{NO}}_{3}^{ - } \) mode exhibits the D3h point group and four fundamental modes are observed, i.e., ν1 (\( A_{1}^{{\prime }} \)), ν2 (\( A_{1}^{{\prime \prime }} \)), ν3 (\( E^{\prime} \)), ν4 (\( E^{\prime} \)). The ν1 (symmetric stretching mode at 690/1049/1355 cm−1) mode is Raman active, ν2 mode (out-of-plane symmetric deformation mode at 830 cm−1) is IR active, and ν3 (antisymmetric stretching or degenerate mode at 1350 cm−1) + ν4 (antisymmetric deformation mode at 680 cm−1) are IR + Raman active [42]. So the peak located near the most intense band at 1350 cm−1 is deconvoluted due to the presence of dominant asymmetry using the Voigt area function (in Peak Fit software) to examine the free anion (\( {\text{NO}}_{3}^{ - } \)) and ion pair (Na+–\( {\text{NO}}_{3}^{ - } \)) contribution. The baseline correction was done prior to deconvolution. The deconvolution pattern gives two types of vibration modes due to asymmetry: one at lower wavenumber side is attributed to ‘free anion’ vibration (\( {\text{NO}}_{3}^{ - } \)), while the other at higher wavenumber side is attributed to the ‘ion pairs’ mode (Na+–\( {\text{NO}}_{3}^{ - } \)) (Fig. 6). A quantitative estimation of the fraction of free anion (FFA) and fraction of ion pair (FIP) was examined from the area of deconvoluted peaks assigned to specific ions using the following equation, \( {\text{FFA}} \left( \% \right) = \frac{{A_{\text{free}} }}{{A_{\text{free}} + A_{\text{pair}} }} \; {\text{and}}\;{\text{FIP}} \left( \% \right) = \frac{{A_{\text{pair}} }}{{A_{\text{free}} + A_{\text{pair}} }} \). Here, \( A_{\text{free}} \) is the area representing free ion peak and \( A_{\text{pair}} \) area of the peak representing as ion-pair peak [43, 44]. Table 3 summarizes the corresponding free ion area and ion-pair area along with peak position and value of correlation coefficient close to unity confirms the best fit.
It may be observed that the fraction of free ions varies with salt concentration and increases with salt concentration initially. Then at high content salt is not dissociated properly and ion-pair area is more as compared to the free ion area. As a high value of free ion area indicates the availability of more free ions for conduction, this directly infers the higher ionic conductivity which could be realized as discussed in the forthcoming discussion. The highest fraction of free anion is for the salt concentration which exhibits maximum conductivity (O/Na = 14). It evidences the proper salt dissociation and is attributed to the creation of the most favorable environment that provides balanced interactions between polymers and salt. It may be noted that corresponding to this concentration ion pair exhibits a minimum.
Electrochemical analysis
Impedance spectroscopic analysis
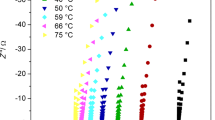
The effect of salt stoichiometric ratio on the polymer blend (PEO–PVP) has been investigated on the ionic conductivity via the complex impedance spectroscopy method in the temperature range 40–100 °C. (Cell assembly SS|SPE|SS is shown in inset.) Figure 7a shows the plot of imaginary part of impedance (Z″) against the real part of impedance (Z′) for varied salt concentrations in log–log presentation (Nyquist plot). The plot comprises two arcs: one lying at low-frequency region corresponds to the electrode–electrolyte interface and high-frequency region arc to the bulk phenomena. The dip in the plot corresponding to the minima in the imaginary part of impedance indicates the bulk resistance (Rb) value (real part of impedance on the x-axis). It may be noted that with the addition of salt nature of arc changes. The lowering in length of high-frequency arc suggests the lowering of resistivity with salt concentration. The low-frequency arc increases in length and indicates the enhanced contribution of the electrode polarization owing to the ion accumulation on the blocking SS electrodes [45,46,47,48,49,50]. It may be noticed from the inset plot that the minima in the imaginary part of the plot is lowest for O/Na = 14 based system (inset in Fig. 7a). The solid line in the plot is the best fit performed with ZSimpWin software, and perfect agreement between the experimental and fitted result is obtained. (The fitted circuit is shown in inset.) It suggests that this is the optimum system and exhibits the faster ion transport and also provides approximately evidence of the highest ionic conductivity. It is discussed further in the following section.
Electrical conductivity spectrum evaluation analysis
The electrical conductivity (σ) has been obtained from the CIS spectra using the equation \( \sigma = {\raise0.7ex\hbox{$t$} \!\mathord{\left/ {\vphantom {t {R_{\text{b}} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${R_{\text{b}} }$}} \times A, \) where ‘t’ is the thickness of the SPE thin film and Rb is bulk resistance, A is the contact electrode surface area of SPE. The Rb has been obtained from the dip in the plot. It is noticed from the plot that bulk resistance decreases with varying salt contents and is lowest for the O/Na = 14 salt concentration. The electrical conductivity is summarized for different salt concentrations in Table 4. The decrease in the bulk resistance implies the highest conductivity for this concentration (~ 3 × 10−5 S cm−1) and is attributed to the effective interaction between the ether group and Na+ cations in the polymer matrix. A variation of electrical conductivity is shown in Fig. 7b for the optimum concentration. It shows the disappearance of the high-frequency arc with temperature and the low-frequency arc is more dominating. It is indicated the thermal activation of charge carriers that trigger salt dissociation, and lowering of the bulk resistance infers the enhancement of the electrical conductivity. This is attributed to the enhanced polymer chain flexibility that facilitates the faster ion migration [49]. Figure 7c shows the variation of electrical conductivity for different salt concentrations and in the broad temperature range. The electrical conductivity increases with the temperature for all salt concentrations and evidences the faster ion migration in the polymer matrix.
Thermal activation energy measurement
Figure 8 shows the plot of log σ versus 1000/T for different salt concentrations. It shows an Arrhenius-type behavior, and it implies that the ion dynamics is linked with the thermal activation of charge carriers. The activation energy has been obtained by fitting the plot with Arrhenius equation; \( \sigma = \sigma _{0} {\text{e}}^{{ - E_{{{\rm a}}} /k_{T} }}, \) where σ is ionic conductivity, σo is a pre-exponential factor, Ea is activation energy, and ‘k’ is Boltzmann constant. The lowering of the activation energy suggests the highest ionic conductivity. The higher ionic conductivity is attributed to the reduction of polymer viscosity and hence increase in polymer flexibility. The thermal activation of charge carriers makes it easier for cation to jump to the next coordinating site, and simultaneous presence of enhanced free volume promotes faster ion migration. It also suggests the enhancement of the amorphous phase that is desirable requirement for the solid-state ionic conductor. The solid red line in the plot is the fitted Arrhenius plot [51,52,53].
The electrical conductivity is higher as compared to the other existing systems as summarized in Table 5. It confirms the suitability of the present system for the application in energy storage/conversion devices.
Ion-transport number analysis
The ion-transport number measurement for the blend solid polymer electrolyte is examined using Wagner’s DC polarization technique. Figure 9 shows the plot of polarization current against the time. Initially, the higher current is total current which is the totality of the ionic and electronic currents. This is followed by the steady state with the passage of time and corresponds to the electronic current. The SS electrodes prevent the flow of ions across the external circuit, and this blockage of ions only permits the flow of electronic current. The ion-transport number has been obtained using the equation, \( t_{\text{ion}} = (I_{\text{t}} - I_{\text{e}} )/I_{\text{t}} \times 100 \), and \( t_{\text{ion}} + t_{\text{e}} = 1 \). The high value of ion-transport number (close to unity) confirms the ionic nature of the polymer films and is summarized in Table 4 [56,57,58].
Electrochemical stability window analysis
Figure 10 shows the linear sweep voltammetry measurement, and electrochemical stability window (ESW) is obtained. ESW enables us to obtain safe operation limit of the electrolyte in the application [59]. The voltage stability window is improved with the addition of salt and is higher than 4 V for the optimized system that is in the desirable limit for the energy storage devices application [60, 61]. Table 4 summarizes the voltage stability window for BSPE.
Differential scanning calorimetry (DSC) analysis
Figure 11a–h shows the differential scanning calorimetric pattern of the blended polymer without salt and with different stoichiometric salt ratio freestanding solid films. It is important to note here that the lower value of the melting point (Tm) and crystallinity (Xc) clearly imply the enhanced polymer flexibility and favor the ion dynamics in terms of faster ion migration via coordinating sites of the polymer chain. It may be noted from Fig. 11a that the blended polymer without salt shows the melting peak located at 71.71 °C and with the addition of small amount of salt peak shifts toward lower temperature (Table 6). This evidences the polymer salt complex formation which is attributed to the interaction between the Lewis base ether group and Lewis acid cation. This also suggests the disruption of polymer chain arrangement and disorder produced in matrix enhances the ion mobility. With further addition of salt, peak shifts toward lower temperature and suggests the complexation between polymer and salt. The melting peak is lowest for the blend polymer system with the highest ionic conductivity (Fig. 11f). However, at high salt content again some shift toward high temperature is observed that may be associated with the poor polymer salt complexation [62].
In order to further corroborate the DSC analysis with the above-analyzed XRD results, the crystallinity of the without and with different content of salt, freestanding solid polymeric films has been analyzed and recorded systematically. It is very important and endorsement of the recorded XRD results in terms of the percentage amorphous content in the polymer after polymer salt complexation, as the evaluation of the percentage of crystallinity (\( \% X_{\text{c}} \)) is so crucial. Therefore, the percentage of crystallinity has been calculated using equation; \( X_{\text{c}} = \frac{{\Delta H_{\text{m}} }}{{\Delta H_{\text{m}}^{o} }} \times 100, \) where ΔHm is the melting enthalpy obtained from the DSC measurement and \( \Delta H_{\text{m}}^{o} \) is the melting enthalpy of pure 100% crystalline PEO (188 J/g) [63].
It is noticed from Fig. 11 that the area of the peak is the lowest (Fig. 11f) for the polymer salt system exhibiting highest ionic conductivity (Table 6). It is also clear that the crystallinity with the addition of salt decreases as compared to the polymer blend which infers the enhanced free volume. This increment of free volume size in the films certainly improves segmental motion of polymer chains that infers the faster cation migration from one coordinating site to another. The calculated results of all the samples are recorded in Table 6 confirming that the addition of salt plays an effective role in the enhancement of the amorphous content and polymer flexibility both (Fig. 11f). The simultaneous presence of both the achievement overall promotes the faster ion dynamics in the matrix. The above results are also in perfect agreement with the XRD, fraction of free ion, impedance study, and ion-transport parameters.
Thermogravimetric (TG) analysis
For safe operation of energy device, thermal stability checking is crucial before its use. The thermogravimetric (TG) curves of the blend polymer with and without salt are shown in Fig. 12. The initial weight loss is about 7.3%, 3.4% for the blend polymer (PEO–PVP, i.e., O/Na = 0) and blended polymer with salt (O/Na = 14), respectively. This is attributed to the evaporation of solvent and moisture content in BSPE films. At higher temperature, further weight loss is observed and at high temperature, (at 370 °C) weight loss is 10.33%, 8.22% for the blend polymer (without salt) and blend polymer with salt (O/Na = 14), respectively. It may be noted that the blend polymer electrolyte with salt shows the improved thermal stability inferred by low weight loss (shown by the arrow). Addition of salt slows down the process of polymer decomposition and reflects the enhanced stability range. After this temperature (i.e., 370 °C), rapid weight loss is observed that is associated with the polymer matrix decomposition followed by steady plot after 450 °C. It may be concluded that the high thermal stability of BSPE films (~ 400 °C) is suitable for the application in sodium-ion batteries operating at high temperature (than room temperature) [64].
Dielectric spectroscopy
Complex dielectric permittivity
The complex dielectric permittivity is expressed as \( \varepsilon^{*} = \varepsilon^{\prime } - j\varepsilon^{\prime \prime } \), where \( \varepsilon^{{\prime }} \) is the real part and \( \varepsilon^{{\prime \prime }} \) is imaginary part of the dielectric permittivity. The former one is associated with the polarizing ability or storage capacity of material and latter one corresponds to the energy loss [65]. The real part and imaginary part are expressed by the equation as \( \varepsilon^{\prime } = \frac{{ - Z^{\prime \prime } }}{{\omega C_{\text{o}} (Z^{\prime 2} + Z^{\prime \prime 2} )}}\; {\text{and}}\; \varepsilon^{\prime \prime } = \frac{{Z^{\prime } }}{{\omega C_{\text{o}} (Z^{\prime 2} + Z^{\prime \prime 2} )}} . \) Here, \( Z^{\prime} \) is real part of impedance, \( Z^{\prime\prime} \) is imaginary part of impedance, \( \omega = 6.28 \times f \) is angular frequency, and \( C_{\text{o}} = \varepsilon_{\text{o}} A/d \) is capacitance. The real and imaginary parts are also written in extended from as Eq. 1
where \( \varepsilon_{\text{s}} \) is dielectric constant for \( x \) → 0, \( \varepsilon_{\infty } \) is dielectric constant for \( x \) → ∞, \( x = \omega \tau \); \( \omega \) is angular frequency of applied field and \( \tau \) is average Debye relaxation time. Here, \( \alpha \) is distribution exponent of material sample.
Figure 13a shows the frequency-dependent real part of the dielectric permittivity against the frequency. The plot shows the decrease in dielectric constant with an increase in the frequency for all SPE systems. On close examination, it is noticed that the dielectric constant is higher for the polymer salt system as compared to the salt-free blend polymer films. This enhancement of the dielectric constant indicates the salt dissociation into cations and anions owing to the interactions between polymer and salt. The high value of the dielectric constant in the low-frequency window is due to the dominance of the electrode polarization region when a time-dependent electric field is applied. It results in the accumulation of the ions on the electrode–electrolyte interface due to the availability of sufficient time for ion diffusion [59, 65,66,67]. Now, when we move from left to right in the plot (low frequency to high frequency), the decrease in dielectric constant and change in slope at an intermediate frequency is observed. The fundamental reason behind this is the dominance of the dielectric relaxation processes in the polymer matrix. This results in the decrease in a number of ion accumulation which directly reduces the dielectric constant. Actually, in the high-frequency window, the rapid switching of field direction generates a negative environment for the dipole alignment and dipoles due to lack of the sufficient time for rotation/orientation on the application of field are unable to contribute in polarization or ion accumulation. The overall effect is blockage of ion diffusion and hence decrease in dielectric constant [68,69,70,71,72]. The high value of dielectric constant indicates the enhancement of the ionic conductivity and follows the relation \( \sigma_{i} = \mathop \sum \limits_{i} q_{i} n_{i} \mu_{i} \). The effective interactions between the polymer and salt result in better salt dissociation and higher ionic conductivity.
Figure 13b shows the frequency-dependent imaginary part of the complex dielectric permittivity, and solid lines in the plot are the best fit. It also shows the same trend as real part of complex permittivity. As in the polymer electrolytes, ions are the diffusion species that responds on the application of the field. When the field direction changes, the ion dynamics is as follows; when field direction is changed, the ion gets de-accelerated, followed by a steady state. Thereafter again ion gets accelerated in the opposite direction. This results in the generation of heat inside a matrix that is known as a dielectric loss. The decrease in dielectric loss at high salt content is due to ion association owing to the strong Coulomb interaction [73, 74].
Loss tangent analysis
The loss tangent plot shows the variation of the loss tangent against the frequency and is the ratio of imaginary part of permittivity to the real part of permittivity. The plot shows an increase in the loss with frequency, followed by a maximum at any particular frequency, and then again decrease in the high frequency (Fig. 14). The low-frequency region (left side of the peak) is associated with the dominated Ohmic component and maxima at a particular frequency (relaxation frequency) are observed where the frequency of applied electric field matches with the frequency of molecule rotation. At this frequency, maximum power transfer occurs. This is the high-frequency region which is associated with the dominated capacitive behavior [72, 75, 76].
The effect of salt concentration is investigated on the loss tangent peak. It is noticed that the addition of salt shifts the peak toward the high-frequency side. This indicates the lowering of relaxation time owing to the faster ion migration through the coordinating sites provided by polymer backbone. The highest frequency relaxation is corresponding to the polymer salts system which exhibits the highest conductivity. The reduction of relaxation time is attributed to the enhanced polymer chain flexibility which makes easier cation migration associated with the segmental motion of the polymer chain. Further to obtain more insights, the loss tangent plot is fitted with the proposed equation in our previous report [77]. The proposed equation is obtained by modifying the Debye equation which fails to fit the plot in low-frequency window [78]. The proposed equation agrees well with experimental results in whole frequency window. The equation is expressed as, \( \tan \delta = \left( {\frac{{\left( {r - 1} \right)}}{{r + x^{2} }}x} \right)^{\alpha } \), where r is the relaxation ratio (\( \varepsilon_{\text{s}} /\varepsilon_{\infty } \)), \( \varepsilon_{s} \) is static dielectric constant (\( x \) → 0), \( \varepsilon_{\infty } \) is dielectric constant (\( x \) → ∞), \( x = \omega \tau \); \( \omega \) is the angular frequency of applied field and \( \tau \) is Debye relaxation time (reciprocal of jump frequency in the absence of external electric field). The solid line is the best fit to the experimental results, and all shows perfect agreement with experimental results. The relaxation time obtained from the fitting is used to obtain transport parameters as summarized in the forthcoming section.
Complex conductivity analysis
The complex conductivity is expressed as, \( \sigma \left( \omega \right) = \sigma^{'} + i\sigma^{\prime\prime} \), where \( \sigma^{\prime} \left( { = \omega \varepsilon_{v} \varepsilon^{\prime\prime}} \right) \) is the real part of conductivity, \( \sigma^{\prime\prime} \) (\( = \omega \varepsilon_{v} \varepsilon^{\prime}) \) is the imaginary part of conductivity.
Sigma representation
Sigma representation plot comprises the imaginary part of complex conductivity (\( \sigma^{\prime\prime} \)) against the real part of complex conductivity (\( \sigma^{\prime} \)) with frequency as the variable parameter. The ideal plot shows a semicircle with two intercepts as: \( \sigma_{o} \), \( \sigma_{\infty } \) on x-axis, respectively [79]. The diameter of the semicircle shows inverse relationship with the relaxation time and is expressed as \( D = \frac{{\varepsilon_{v} \left( {\sigma_{o} - \sigma_{\infty } } \right)}}{2\tau }. \) When imaginary part of conductivity approaches to zero corresponding to two intercepts, (1) low-frequency intercept depicts DC conductivity (\( \sigma_{o} \)) and (2) high-frequency intercept provides \( \sigma_{\infty } \) (Fig. 15). The presence of semicircle in the plot confirms the Debye nature of the investigated system. The nature of plot remains same, but the spike in the plot and diameter changes with variations of salt concentrations are clearly visible. The diameter of the semicircle is highest for the optimized system having highest ionic conductivity. The highest diameter value deduces the lowest relaxation time for this concentration, which is in good agreement with the impedance results and loss tangent analysis [27, 80,81,82]. It may be concluded that the polymer salt matrix with O/Na = 14 concentration is optimized concentration for the further study for device applications.
Theoretical fitting of complex conductivity analysis
Figure 16a, b shows the frequency-dependent real and imaginary parts of complex conductivity. To study the salt concentration effect in the whole frequency, the corresponding expressions of the complex conductivity are as follows [81].
The real and imaginary parts of the conductivity can be written as:
The high-frequency Jonscher power law is also included in this equation as given. The real and imaginary parts of the conductivity are expressed as:
and
Here, all parameters have their usual meaning as earlier and both ‘n’ and ‘s’ have a value less than unity. For complete frequency window analysis, we replace \( \sigma_{\text{b}} \) in Eq. 3a by Eq. 4a and Eq. 3b by Eq. 4b, where \( C_{\text{dl}} \) is frequency-independent double-layer capacitance, \( \omega \) is the angular frequency, s and \( \alpha \) are exponent terms with value < 1 and \( C_{\text{b}} \) is the bulk capacitance of solid polymer electrolyte. The combined equations are used to fit the real and imaginary parts with the corresponding equation.
The real part of complex conductivity
The real part of conductivity against frequency comprises three regions: (1) low-frequency region associated with the electrode polarization region, (2) intermediate-frequency DC conductivity region, and (3) high-frequency dispersion region (Fig. 16a). The low-frequency region shows the increase in the conductivity with frequency, and at intermediate frequency long-range ion migration occurs associated with cation migration from one coordinating site to another. The DC conductivity is extracted from this region. At higher frequency, the ions possess short-range path and at any particular frequency transition from long range to short range, path occurs that is termed as hopping frequency. In the high-frequency window, two competitive phenomena occur: (1) unsuccessful hopping (ion jumps back to the previous coordinating site) and (2) successful hopping (coordinating site relaxation occurs when ion achieves new site) [82,83,84]. The blend polymer without salt comprises all three regions, and with varying salt concentrations the high-frequency regions disappear completely, while intermediate frequency regions get shortened. This indicates the shifting of hopping frequency toward the high-frequency window associated with faster ion migration. The real part of the complex conductivity is also fitted with the equations reported somewhere [81]. The solid red line is the fitted plot and agrees well with the experimental results in the whole frequency window.
The imaginary part of complex conductivity
Figure 16b shows the frequency-dependent imaginary part of complex conductivity, and the solid red line is the fitted result which is in agreement with the experimental result in whole frequency window. The imaginary part of complex conductivity decreases when we move from right to left and a dip in the plot is obtained corresponding to onset frequency (\( \omega_{\text{on}} \)). At this frequency, electrode polarization starts to build up and conductivity increases. With further decrease in frequency, peak in the plot is obtained corresponding to maximum frequency (\( \omega_{\hbox{max} } \)). At this frequency, maximum buildup of polarization occurs. With further decrease in frequency, conductivity decreases. The same has been reported in earlier studies also [85,86,87,88,89]. It is important to notice here that both onset frequency (\( \omega_{\text{on}} \)) and maximum frequency (\( \omega_{\hbox{max} } \)) shift toward high-frequency side and are highest for the polymer salt matrix having highest ionic conductivity. This infers that for this concentration comparatively faster ion migrations occur and are in agreement with the loss tangent plot which shows lowest relaxation time for this system.
Transport parameters
The improved electrochemical and voltage stability properties on the addition of salt in blend polymer matrix motivated us to evaluate the transport parameters also. The transport parameters play a crucial role in the smooth facilitation of the faster ion dynamics. The fundamental transport parameters are number density of charge carriers (n), ion mobility (\( \mu \)), and diffusion coefficient (D). The three methods are there to evaluate transport parameters, (1) Bandara and Mellander (B–M) approach, (2) impedance spectroscopy approach, and (3) FTIR method (Table 7). Till date, only one report is available that explored the detailed investigations and compared the transport parameters obtained by all three methods [90]. So, to strengthen the present investigation and to provide detailed analysis the transport parameters are evaluated by all methods. The mathematical equations used in evaluating the parameters are summarized in Table 7.
Figure 17 displays the variation of mobility (\( \mu \)), viscosity (η) and diffusion coefficient (D) of charge carriers against the different salt contents using three different approaches (Table 8). The increase in transport parameters is associated with the following reasons: (1) reduction of crystallinity, (2) increase in segmental motion of polymer chain, (3) decrease in polymer chain viscosity, and (4) high ion conductivity. It may be noted that the transport parameters are in correlation with the XRD, fraction of free ion and impedance study. The comparison of transport parameters is also in agreement with the literature. It may be concluded that the ion dynamics is strongly influenced by the addition of the salt.
Proposed mechanism
Two-peak percolation model/mechanism
An ion-transport mechanism is proposed on the basis of the experimental evidence obtained from the FESEM, FTIR, XRD and impedance results (Fig. 18). The role of polymer and salt is explored to understand the ion dynamics.
Stage-I As the polymer blending approach was adopted to develop new polymer electrolyte. When two polymers have dissolved, the presence of hydrogen bending results in the blend formation. The blend formation is also evidenced in the FESEM and XRD analysis. Now, when salt is added in the blend polymer matrix, the salt gets dissociated into cations and anions. Now, cation has two possibilities for coordination, (1) with electron-rich group of PEO and (2) electron-rich group of PVP. But it has been confirmed in the FTIR spectra that cation is going to coordinate with the PEO as the characteristics peak (–C–O–C–) of PEO shows noticeable changes. So, in the further mechanism, only PEO chain is shown for clear representation. So, the cation gets coordinating sites from the ether group of PEO and on the application of the field migrates via these coordinating sites. However, anion due to its bulky size remains in the immobilized state and attached with polymer backbone.
Stage-II When there is lower salt content in the polymer matrix, the salt gets dissociate completely into cations and anions. So, cation migrates via the coordinating sites and contributes to conduction. With further addition of salt dissociation more number of charges, carriers are available for conduction that is reflected in the maxima in the conductivity variation. This enhancement is in relation to the σ = neμ. This is also evidenced by the FTIR deconvolution of free ions. Now, when salt concentration is increased further (intermediate salt content), there is the tendency of the ion-pair formation and salt is not dissociated completely (shown by the green circle). This results in a decrease in the number of free ions and a decrease in conductivity is observed. With further increase in the salt content, another maximum in the conductivity is evidenced. This is associated with the additional contribution from the anions. As anion is attached with the polymer backbone and due to increased salt content, concentration gradient pushes the ion forward that also contributes to the conductivity. The increased disruption of the crystalline arrangement in the polymer matrix allows the anion movement via increased room for ion migration. Now, with the further addition of salt (high salt content), there is again a decrease in the conductivity. This decrease in the conductivity is due to the following reasons: (1) dominance of ion-pair formation as well as ion triplets, (2) cation may trap in between the salt aggregates (shown by the green circle), (3) polymer chain reorganization tendency may enhance due to insufficient salt dissociation. The simultaneous presence of these factors results in a decrease in the conductivity and poor segmental motion. It hinders the ion migration and is also evidenced by the FESEM which shows salt agglomeration at high salt content. It may be concluded that at an optimum concentration of salt–polymer matrix is able to provide a favorable environment for cation migration with balanced interactions. The enhanced improved ionic conductivity and other desirable properties of an electrolyte strengthen its candidature for the application in the sodium-ion batteries.
Conclusion
A novel blend solid polymer electrolyte has been synthesized using the blended polymer PEO and PVP and sodium salt (NaNO3) via standard solution-cast technique. The structural and microstructural changes have been analyzed through XRD and FESEM analysis. X-ray diffraction analysis indicates the reduction of crystallinity and increase in interchain separation evidence disruption of the covalent bonding between polymers chain which is favorable for faster ion migration. FTIR spectra confirm the complex formation and fraction of free anion increases with the addition of salt that is associated with the enhancement of a number of charge carriers. The blend polymer matrix with salt ratio O/Na = 14 exhibits the highest ionic conductivity 2.92 \( \times \;10^{ - 5} \;{\text{S}}\; {\text{cm}}^{ - 1} \) at room temperature and is the optimized system. This enhancement in the conductivity was attributed to the proper salt dissociation. The temperature dependence of conductivity follows the Arrhenius behavior, and conductivity increase with temperature is associated with enhanced polymer chain flexibility (2.90 \( \times \;10^{ - 4} \;{\text{S}}\;{\text{cm}}^{ - 1} \) at 100 °C). The ion transference number close to unity confirms the ionic nature of electrolyte, and broad voltage stability window (~ 4 V) is suitable for such applications. The decrease in melting temperature and crystallinity is evidenced from the DSC analysis which infers the increase in polymer chain flexibility which is in correlation with XRD and impedance analysis. The transport parameters have been evaluated by three methods: (1) Bandara and Mellander (B–M) approach, (2) impedance spectroscopy approach, and (3) FTIR method and are in good correlation with the literature and impedance results. The enhancement of the ion mobility and diffusion coefficient is in correlation with the impedance study. The thermal stability of the prepared film is up to 300 °C and is within a safe limit. The complex dielectric permittivity analysis evidences the enhancement of the dielectric constant with the addition of salt and indicates the enhancement of a number of free charge carriers. The loss tangent peak shifts toward higher-frequency side that implies the lowering of the relaxation time that suggests the faster polymer chain segmental motion. The sigma representation also evidences the lowering of relaxation time that is in correlation with time obtained from loss tangent analysis. The AC conductivity analysis shows an increase in DC conductivity with the addition of salt, and imaginary part of complex conductivity suggests the faster ion migration. The ion-transport mechanism has been proposed that highlights the role of polymer and salt. It may be concluded that the present BSPE has the potential to be used as an electrolyte for application in sodium-ion batteries.
References
Yue L, Ma J, Zhang J et al (2016) All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater 5:139–164
Hasa I, Hassoun J, Passerini S (2017) Nanostructured Na-ion and Li-ion anodes for battery application: a comparative overview. Nano Res 10:3942–3969
Yang Q, Zhang Z, Sun X-G et al (2018) Ionic liquids and derived materials for lithium and sodium batteries. Chem Soc Rev 47:2020–2064
Saykar NG, Pilania RK, Banerjee I, Mahapatra SK (2018) Synthesis of NiO–Co3O4 nanosheet and its temperature-dependent supercapacitive behavior. J Phys D Appl Phys 51:475501
Barbos JC, Dias JP, Lanceros-Méndez S, Costa CM (2018) Recent advances in poly(Vinylidene fluoride) and its copolymers for lithium-ion battery separators. Membranes 8:45
Sim LN, Sentanin FC, Pawlicka A et al (2017) Development of polyacrylonitrile-based polymer electrolytes incorporated with lithium bis (trifluoromethane) sulfonimide for application in electrochromic device. Electrochim Acta 229:22–30
Arya A, Sharma AL (2017) Polymer electrolytes for lithium ion batteries: a critical study. Ionics 23:494–540
Arya A, Sharma AL (2016) Conductivity and stability properties of solid polymer electrolyte based on PEO–PAN + LiPF6 for energy storage. Appl Sci Lett 2:72–75
Manuel Stephan A, Nahm KS (2006) Review on composite polymer electrolytes for lithium batteries. Polymer 47:5952–5964
Manthiram A, Yu X, Wang S (2017) Lithium battery chemistries enabled by solid-state electrolytes. Nat Rev Mater 2:16103
Yue L, Ma J, Zhang J et al (2016) All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater 5:139–164
Arya A, Sharma AL (2017) Insights into the use of polyethylene oxide in energy storage/conversion devices: a critical review. J Phys D Appl Phys 50:443002
Janakiraman S, Padmaraj O, Ghosh S, Venimadhav A (2018) A porous poly (vinylidene fluoride-co-hexafluoropropylene) based separator-cum-gel polymer electrolyte for sodium-ion battery. J Electroanal Chem 826:142–149
Zhang Z, Xu K, Rong X, Hu YS, Li H, Huang X, Chen L (2017) Na3.4Zr1.8Mg0.2Si2PO12 filled poly (ethylene oxide)/Na(CF3SO2)2N as flexible composite polymer electrolyte for solid-state sodium batteries. J Power Sources 372:270–275
Ravi M, Pavani Y, Kumar KK, Bhavani S, Sharma AK, Rao VN (2011) Studies on electrical and dielectric properties of PVP: KBrO4 complexed polymer electrolyte films. Mater Chem Phys 130:442–448
Sharma AL, Shukla N, Thakur AK (2008) Studies on structure property relationship in a polymer-clay nanocomposite film based on (PAN)8LiClO4. J Polym Sci Part B Polym Phys 46:2577–2592
Sharma AL, Thakur AK (2010) Improvement in voltage, thermal, mechanical stability and ion transport properties in polymer-clay nanocomposites. J Appl Polym Sci 118:2743–2753
Qiao J, Fu J, Lin R, Ma J, Liu J (2010) Alkaline solid polymer electrolyte membranes based on structurally modified PVA/PVP with improved alkali stability. Polymer 5:4850–4859
Rajendran S, Sivakumar M, Subadevi R (2004) Investigations on the effect of various plasticizers in PVA-PMMA solid polymer blend electrolytes. Mater Lett 58:641–649
Arya A, Sharma AL, Sharma S, Sadiq M (2016) Role of low salt concentration on electrical conductivity in blend polymeric films. J Integr Sci Technol 4:17–20
Arya A, Sharma S, Sharma AL, Kumar D, Sadiq M (2016) Structural and dielectric behavior of blend polymer electrolyte based on PEO–PAN + LiPF 6. Asian J Eng Appl Technol 5:4–7
Bhat C, Swaroop R, Arya A, Sharma AL (2015) Effect of nano-filler on the properties of polymer nanocomposite films of PEO/PAN complexed with NaPF6. J Mater Sci Eng B 5:418–434
Parameswaranpillai J, Thomas S, Grohens Y (2014) Polymer blends: state of the art, new challenges, and opportunities. In: Character Polymer Blends. Wiley
Zhang X, Takegoshi K, Hikichi K (1992) High-resolution solid-state 13C nuclear magnetic resonance study on poly (vinyl alcohol)/poly (vinylpyrrolidone) blends. Polymer 33:712–717
Polu AR, Kumar R, Rhee HW (2015) Magnesium ion conducting solid polymer blend electrolyte based on biodegradable polymers and application in solid-state batteries. Ionics 21:125–132
Kumar KK, Ravi M, Pavani Y, Bhavani S, Sharma AK, Rao VN (2011) Investigations on the effect of complexation of NaF salt with polymer blend (PEO/PVP) electrolytes on ionic conductivity and optical energy band gaps. Phys B Condens Matter 406:1706–1712
Arya A, Sharma AL (2018) Optimization of salt concentration and explanation of two peak percolation in blend solid polymer nanocomposite films. J Solid State Electrochem 22:2725–2745
Chapi S, Raghu S, Devendrappa H (2016) Enhanced electrochemical, structural, optical, thermal stability and ionic conductivity of (PEO/PVP) polymer blend electrolyte for electrochemical applications. Ionics 22:803–814
Kumar KK, Ravi M, Pavani Y et al (2014) Investigations on PEO/PVP/NaBr complexed polymer blend electrolytes for electrochemical cell applications. J Membr Sci 454:200–211
Chandra A, Agrawal RC, Mahipal YK (2009) Ion transport property studies on PEO–PVP blended solid polymer electrolyte membranes. J Phys D Appl Phys 42:135107
Koduru HK, Marino L, Scarpelli F, Petrov AG, Marinov YG, Hadjichristov GB, Iliev MT, Scaramuzza N (2017) Structural and dielectric properties of NaIO4–complexed PEO/PVP blended solid polymer electrolytes. Curr Appl Phys 17:1518–1531
Kumar KN, Kang M, Sivaiah K, Ravi M, Ratnakaram YC (2016) Enhanced electrical properties of polyethylene oxide (PEO) + polyvinylpyrrolidone (PVP): Li+ blended polymer electrolyte films with addition of Ag nanofiller. Ionics 22:815–825
Arya A, Nilesh Saykar G, Sharma AL (2018) Impact of shape (nanofiller vs. nanorod) of TiO2 nanoparticle on free standing solid polymeric separator for energy storage/conversion devices. J Appl Polym Sci 136:47361
Arya A, Sharma AL (2018) Effect of salt concentration on dielectric properties of Li-ion conducting blend polymer electrolytes. J Mater Sci Mater Electron 29:17903–17920
Sengwa RJ, Dhatarwal P, Choudhary S (2015) Effects of plasticizer and nanofiller on the dielectric dispersion and relaxation behaviour of a polymer blend based solid polymer electrolytes. Curr Appl Phys 15:135–143
Sreekanth T, Reddy MJ, Ramalingaiah S, Rao US (1999) Ion-conducting polymer electrolyte based on poly (ethylene oxide) complexed with NaNO3 salt-application as an electrochemical cell. J Power Sources 79:105–110
Jinisha B, Anilkumar KM, Manoj M, Abhilash A, Pradeep VS, Jayalekshmi S (2018) Poly (ethylene oxide)(PEO)-based, sodium ion-conducting, solid polymer electrolyte films, dispersed with Al2O3 filler, for applications in sodium ion cells. Ionics 24:1675–1678
Abdelrazek EM, Abdelghany AM, Badr SI, Morsi MA (2017) Structural, optical, morphological and thermal properties of PEO/PVP blend containing different concentrations of biosynthesized Au nanoparticles. J Mater Res Technol 7:419–431
Ma Y, Li LB, Gao GX, Yang XY, You Y (2016) Effect of montmorillonite on the ionic conductivity and electrochemical properties of a composite solid polymer electrolyte based on polyvinylidenedifluoride/polyvinyl alcohol matrix for lithium ion batteries. Electrochim Acta 187:535–542
Kesavan K, Mathew CM, Rajendran S, Ulaganathan M (2014) Preparation and characterization of novel solid polymer blend electrolytes based on poly (vinyl pyrrolidone) with various concentrations of lithium perchlorate. Mater Sci Eng B 184:26–33
Kesavan K, Mathew CM, Rajendran S (2014) Lithium ion conduction and ion-polymer interaction in poly (vinyl pyrrolidone) based electrolytes blended with different plasticizers. Chin Chem Lett 25:1428–1434
Bertolla L, Dlouhý I, Tatarko P, Viani A, Mahajan A, Chlup Z, Boccaccini AR (2017) Pressureless spark plasma–sintered Bioglass® 45S5 with enhanced mechanical properties and stress–induced new phase formation. J Eur Ceram Soc 37:2727–2736
Sharma AL, Thakur AK (2010) Polymer–ion–clay interaction based model for ion conduction in intercalation-type polymer nanocomposite. Ionics 16:339–350
Sharma AL, Thakur AK (2011) Polymer matrix–clay interaction mediated mechanism of electrical transport in exfoliated and intercalated polymer nanocomposites. J Mater Sci 46:1916–1931. https://doi.org/10.1007/s10853-010-5027-x
Das A, Thakur AK, Kumar K (2013) Exploring low temperature Li+ ion conducting plastic battery electrolyte. Ionics 19:1811–1823
Arya A, Sadiq M, Sharma AL (2018) Structural, electrical and ion transport properties of free standing blended solid polymeric thin films. Polym Bull. https://doi.org/10.1007/s00289-018-2645-y
Qian X, Gu N, Cheng Z, Yang X, Wang E, Dong S (2001) Impedance study of (PEO)10LiClO4–Al2O3 composite polymer electrolyte with blocking electrodes. Electrochim Acta 46:1829–1836
Tang R, Jiang C, Qian W, Jian J, Zhang X, Wang H, Yang H (2015) Dielectric relaxation, resonance and scaling behaviors in Sr3Co2Fe24O41 hexaferrite. Sci Rep 5:13645
Muchakayala R, Song S, Gao S, Wang X, Fan Y (2017) Structure and ion transport in an ethylene carbonate-modified biodegradable gel polymer electrolyte. Polym Test 58:116–125
Wu XL, Xin S, Seo HH, Kim J, Guo YG, Lee JS (2011) Enhanced Li+ conductivity in PEO–LiBOB polymer electrolytes by using succinonitrile as a plasticizer. Solid State Ionics 186:1–6
Nadimicherla R, Kalla R, Muchakayala R, Guo X (2015) Effects of potassium iodide (KI) on crystallinity, thermal stability, and electrical properties of polymer blend electrolytes (PVC/PEO: KI). Solid State Ionics 278:260–267
Ramesh S, Yahaya AH, Arof AK (2002) Dielectric behaviour of PVC-based polymer electrolytes. Solid State Ionics 152:291–294
Zhang J, Yue L, Hu P, Liu Z, Qin B, Zhang B, Yao J (2014) Taichi-inspired rigid-flexible coupling cellulose-supported solid polymer electrolyte for high-performance lithium batteries. Sci Rep 4:6272
Kumar K, Ravi M, Pavani Y, Bhavani S, Sharma AK, VVR NR (2012) Electrical conduction mechanism in NaCl complexed PEO/PVP polymer blend electrolytes. J Non Cryst Solids 358:3205–3211
Naveen Kumar P, Sasikala U, Sharma AK (2013) Investigations on conductivity and discharge profiles of novel (PEO + PEMA) polymer blend electrolyte. Int J Inno Res Sci Eng Tech 2:3575–3582
Fan L, Dang Z, Nan CW, Li M (2002) Thermal, electrical and mechanical properties of plasticized polymer electrolytes based on PEO/P (VDF-HFP) blends. Electrochim Acta 48:205–209
Deraman SK, Mohamed NS, Subban RH (2013) Conductivity and electrochemical studies on polymer electrolytes based on poly vinyl (chloride)-ammonium triflate-ionic liquid for proton battery. Int J Electrochem Sci 8:1459–1468
Laha P, Panda AB, Dahiwale S, Date K, Patil KR, Barhai PK, Das AK, Banerjee I, Mahapatra SK (2010) Effect of leakage current and dielectric constant on single and double layer oxides in MOS structure. Thin Solid Films 519:1530–1535
Chen D, Cheng J, Wen Y, Cao G, Yang Y, Liu H (2012) Impedance study of electrochemical stability limits for electrolytes. Int J Electrochem Sci 7:12383–12390
Sharma AL, Thakur AK (2013) Plastic separators with improved properties for portable power device applications. Ionics 19:795–809
Zhu P, Yan C, Dirican M, Zhu J, Zang J, Selvan RK, Wu N (2018) Li0.33La0.557TiO3 ceramic nanofiber-enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries. J Mater Chem A 6:4279–4285
Kim S, Park SJ (2007) Preparation and ion-conducting behaviors of poly (ethylene oxide)-composite electrolytes containing lithium montmorillonite. Solid State Ionics 178:973–979
Cimmino S, Di Pace E, Martuscelli E, Silvestre C (1990) Evaluation of the equilibrium melting temperature and structure analysis of poly (ethylene oxide)/poly (methyl methacrylate) blends. Die Makromolekulare Chemie Macromol Chem Phys 191:2447–2454
Anilkumar KM, Jinisha B, Manoj M, Jayalekshmi S (2017) Poly (ethylene oxide)(PEO)–Poly (vinyl pyrrolidone)(PVP) blend polymer based solid electrolyte membranes for developing solid state magnesium ion cells. Eur Polym J 89:249–262
Cole KS (1940) Dispersion and absorption in dielectrics I. Alternating current characteristics. J Chem Phys 9:341–351
Tripathi AK, Thakur A Shukla, Marx DT (2018) Dielectric, transport and thermal properties of clay based polymer-nanocomposites. Polym Eng Sci 58:220–227
Arya A, Sharma AL (2018) Structural, microstructural and electrochemical properties of dispersed-type polymer nanocomposite films. J Phys D Appl Phys 51:045504
Chilaka N, Ghosh S (2014) Dielectric studies of poly (ethylene glycol)-polyurethane/poly (methylmethacrylate)/montmorillonite composite. Electrochim Acta 134:232–241
Sharma AL, Thakur AK (2011) AC conductivity and relaxation behavior in ion conducting polymer nanocomposite. Ionics 17:135–143
Arya A, Sadiq M, Sharma AL (2018) Effect of variation of different nanofillers on structural, electrical, dielectric, and transport properties of blend polymer nanocomposites. Ionics 24:2295–2319
Bose P, Roy A, Dutta B, Bhattacharya S (2017) Decoupling of segmental relaxation from ionic conductivity in [DEMM][TFSI] room temperature ionic liquid incorporated poly (vinylidenefluoride-co-hexafluoropropylene) membranes. Solid State Ionics 311:75–82
Woo HJ, Majid SR, Arof AK (2012) Dielectric properties and morphology of polymer electrolyte based on poly (ɛ-caprolactone) and ammonium thiocyanate. Mater Chem Phys 134:755–761
Dhatarwal P, Sengwa RJ, Choudhary S (2019) Effectively improved ionic conductivity of montmorillonite clay nanoplatelets incorporated nanocomposite solid polymer electrolytes for lithium ion-conducting devices. SN Appl Sci 1:112
Teeters D, Neuman RG, Tate BD (1996) The concentration behavior of lithium triflate at the surface of polymer electrolyte materials. Solid State Ionics 85:239–245
Singh RJ (2012) Solid state physics. Dorling Kindersley, Noida
Chopra S, Sharma S, Goel TC, Mendiratta RG (2003) Structural, dielectric and pyroelectric studies of Pb1–XCaXTiO3 thin films. Solid State Commun 27:299–304
Arya A, Sharma AL (2018) Structural, electrical properties and dielectric relaxations in Na+-ion-conducting solid polymer electrolyte. J Phys Condens Matter 30:165402
Cao W, Gerhardt R (1990) Calculation of various relaxation times and conductivity for a single dielectric relaxation process. Solid State Ionics 42:213–221
Wei YZ, Sridhar S (1993) A new graphical representation for dielectric data. J Chem Phys 99:3119–3124
Kumar PS, Sakunthala A, Govindan K, Reddy MV, Prabu M (2016) Single crystalline TiO2 nanorods as effective fillers for lithium ion conducting PVdF-HFP based composite polymer electrolytes. RSC Adv 6:91711–91719
Roy A, Dutta B, Bhattacharya S (2016) Correlation of the average hopping length to the ion conductivity and ion diffusivity obtained from the space charge polarization in solid polymer electrolytes. RSC Adv 6(70):65434–65442
Shukla N, Thakur AK, Shukla A, Marx DT (2014) Ion conduction mechanism in solid polymer electrolyte: an applicability of almond-west formalism. Int J Electrochem Sci 9:7644–7659
Sharma AL, Thakur AK (2015) Relaxation behavior in clay-reinforced polymer nanocomposites. Ionics 21:1561–1575
Choudhary S, Sengwa RJ (2015) Structural and dielectric studies of amorphous and semicrystalline polymers blend-based nanocomposite electrolytes. J Appl Polym Sci 15:132
Wang Y, Sun CN, Fan F, Sangoro JR, Berman MB, Greenbaum SG, Zawodzinski TA, Sokolov AP (2013) Examination of methods to determine free-ion diffusivity and number density from analysis of electrode polarization. Phys Rev E Stat Nonlinear Soft Matter Phys 87:042308
García-Bernabé A, Rivera A, Granados A, Luis SV, Compañ V (2016) Ionic transport on composite polymers containing covalently attached and absorbed ionic liquid fragments. Electrochim Acta 213:887–897
Klein RJ, Zhang S, Dou S, Jones BH, Colby RH, Runt J (2006) Modeling electrode polarization in dielectric spectroscopy: ion mobility and mobile ion concentration of single-ion polymer electrolytes. J Chem Phys 124:144903
Arya A, Sharma AL (2018) Enhancement in dielectric properties of blend solid polymer electrolyte with variation of temp and salt concentration. Macromol Res. https://doi.org/10.1007/s13233-019
Fuentes I, Andrio A, Teixidor F, Viñas C, Compañ V (2017) Enhanced conductivity of sodium versus lithium salts measured by impedance spectroscopy. Sodium cobaltacarboranes as electrolytes of choice. Phys Chem Chem Phys 19:15177–15186
Arof AK, Amirudin S, Yusof SZ, Noor IM (2014) A method based on impedance spectroscopy to determine transport properties of polymer electrolytes. Phys Chem Chem Phys 16:1856–1867
Acknowledgements
One of the authors (Pritam) is thankful to CSIR, New Delhi for the award of JRF fellowship. AA is thankful to the Central University of Punjab, Bathinda, for providing fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pritam, Arya, A. & Sharma, A.L. Dielectric relaxations and transport properties parameter analysis of novel blended solid polymer electrolyte for sodium-ion rechargeable batteries. J Mater Sci 54, 7131–7155 (2019). https://doi.org/10.1007/s10853-019-03381-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03381-3