Abstract
Suzuki–Miyaura C–C coupling reactions were investigated with Pd/nitrogen-doped carbon nanotubes (Pd/N-CNTs) as a catalyst. Also, the same catalyst was examined for the solventfree oxidation of benzyl alcohol to benzaldehyde. Nitrogen-doped carbon nanotubes (N-CNTs) were synthesized from 1-ferrocenylmethyl(2-methylimidazole) and benzophenone via a chemical vapour deposition technique. Acetonitrile was used as a solvent and source of both carbon and nitrogen constituents of N-CNTs. Pd nanoparticles (Pd NPs) were successfully dispersed on N-CNTs via a metal organic chemical vapour deposition method. SEM, TEM, XRD, elemental analysis and ICP-OES measurements were used to characterize the nanomaterials. From the TEM analysis, it was observed that Pd NPs were spherical and with particle sizes ranging from 3 to 8 nm. For Suzuki C–C coupling reactions, phenylboronic acid, aryl halide, Pd/N-CNTs catalyst and a base (NaOAc, K2PO4, K2CO3, NaOH, Et3N and Na2CO3) were used. The optimized experiments indicate that K2CO3, as the base, and ethanol/water (1:1 v/v, 10 mL) mixture, as a solvent, are the best reaction conditions. The solventfree oxidation reactions of benzyl alcohol were also done with Pd/N-CNTs catalyst and benzyl alcohol as a substrate. In both sets of reactions, C–C coupling and oxidation, the increase in pyrrolic nitrogen species was found to be responsible for higher catalytic activities of Pd/N-CNT catalysts, and this was attributed to the ease of Pd NP dispersion on N-CNTs, relative to pristine CNTs. Also, the higher catalytic activity of Pd/N-CNTs could be ascribed not only to the smaller Pd NP size or surface area, but to also the surface properties and the nature of the support when compared with the undoped counterpart, Pd/CNTs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Efficient Suzuki coupling reaction of an aryl halide and boronic acids with less expensive substrates, and recyclable heterogeneous catalysts, is of economic importance for pharmaceutical and fine chemical industries. The selective oxidation of benzyl alcohol with the use of molecular oxygen is one of the favourable routes for a greener synthesis of benzaldehyde [1], i.e. relative to metal oxidants such as permanganates and chromates. Benzaldehyde is an important intermediate in organic synthesis due to its application in the pharmaceutical [2], cosmetic [3] and food packaging [4] industries, among other useful applications. On the other hand, palladium (Pd) metal has been intensively used as a catalyst in a wide range of catalytic processes, such as oxidation [5], hydrogenation [6, 7] and C–C coupling reactions [8, 9]. Pd metal catalyst has been found to be very useful, especially in homogeneous catalysis as it is relatively more selective, however, the use of homogenous Pd metal catalyst remains a challenge and uneconomical for large-scale preparation. Hence, heterogeneous catalysis is sometimes preferred. Under heterogeneous catalysis, Pd nanoparticles (Pd NPs) have been supported on several substrates such as zeolites [10], polymers [11] and metal oxides [12, 13] for improved catalytic activity.
Due to poor interactions between catalyst active sites and reactants, coupled with the unavailability of active site on some aforementioned supports, shaped carbon nanomaterials (SCNMs), such as carbon nanotubes (CNTs), carbon sphere (CS), carbon fibres (CF) and graphene, are among the most sought-after alternative supports. In the case of CNTs, they are known to enhance the deposition of active metal crystallites and regulate substrates access on supported metal nanoparticles [14]. Reports also show that CNTs demonstrate superior catalytic activity due to their enhanced thermal stability and larger surface area, which is tuneable when employed as catalyst support [15,16,17].
CNTs have been synthesized via several methods: namely, arc discharge [18], laser ablation [19] and CVD techniques [20]. Among these methods, the CVD technique is widely employed for CNTs synthesis, and this is due to their relatively high deposition rates, easy scale-up and relatively easy removal of impurities from gaseous precursors [21]. CNTs have been employed as a support for metal nanoparticles for several catalytic applications due to their unique structural and electronic properties [22, 23]. The introduction of heteroatoms such as nitrogen into the graphitic matrix to give N-CNTs improves their potential catalytic applications, due to improved bulk and surface properties [24]. Similarly, CNTs surface modification via acid treatment introduces oxygen-functionalized groups (i.e. hydroxyl, carbonyl, lactones and quinone) on the N-CNTs surface which act as additional anchoring sites (apart from the N-heteroatom) for Pd ions where the nucleation and growth of palladium nanoparticles (Pd NPs) are enhanced. Incorporation of these atoms (i.e. oxygen and nitrogen) introduces defects in CNTs [25]. These defects modify the dispersion, morphology, electronic structure, stability and surface area and ultimately enhance the catalytic performance of supported metal nanoparticles [26].
Pd nanoparticles as a catalyst have been widely employed for C–C coupling reactions; however, one of the setbacks in Pd heterogeneous catalysis is the Pd leaching aspect [27]. Heidenreich et al. [28] reported that Pd leaching could be prevented by fine-tuning of reaction conditions such as increasing reaction temperature and the introduction of reducing agents. However, this results in an increase in palladium particle size which leads to catalyst deactivation. Nevertheless, catalytic activities of CNTs as carbon support for Pd catalysts have been reported, especially for C–C coupling [29] and benzyl alcohol oxidation reactions [30]. However, there is not much work reported on the application of Pd/N-CNTs as supports for these reactions. Methods, such as electrodeposition [31] and metal–organic chemical vapour deposition (MOCVD) [32], have been employed for the deposition of Pd NPs on N-CNTs with latter approach still being the most efficient, cost-effective and environmentally friendly method [33].
There has been a lot of interest in Pd NPs supported on CNTs, and this is an ongoing research with continuous attempts towards modifying CNTs surface chemistry in order to improve the efficiency and greenness of the catalysts [34]. Interestingly, Pd NPs supported on N-CNTs have displayed promising catalytic activities in Heck coupling reactions [35], solventfree oxidation of benzaldehyde [36], Suzuki coupling [37] and hydrogenation reactions [38,39,40]. This has been associated with the type of nitrogen species incorporated [35, 41]. However, one of the major setbacks of Pd NPs anchored on N-CNTs is the uneven distribution of nanoparticles [42]. Hence, there is interest in achieving uniform dispersion of Pd NPs unto N-CNTs. To achieve better dispersion, oxygen functional groups such as carbonyl, carboxyl, lactone and quinone can be introduced in situ in the reactant mixture using water [43] or oxygen-containing aromatic compounds such as ethylbenzoate and benzaldehyde [44] to enhance N-CNTs wettability, thus leading to improved catalytic activity of N-CNTs. Also, the introduced surface oxygen functionalities can enhance the dispersion of Pd NPs by acting as the anchoring sites [27]. Duan et al. [45] reported the fabrication of Pd NPs supported on mesoporous N-CNTs with ultrafine well-dispersed Pd NPs of 2–3 nm diameter size by the impregnation method. The improved catalytic performance of the Pd/N-CNTs catalyst was attributed to a strong interaction between Pd NPs and pyridinic-N atoms incorporated into the N-CNTs [45].
Herein, we report for the first time the use of 1-ferrocenylmethyl(2-methylimidazole) as a catalyst for the synthesis of N-CNTs. Also, the effect of oxygen on the surface properties of N-CNTs during synthesis has been investigated. In this study, the effect of oxygen-treated N-CNTs on Pd NPs properties such as particle size, stability and its dispersion on the catalytic activity and selectivity, compared to CNTs and N-CNTs counterpart, was explored. The effect of the type and distribution of N atom species, i.e. pyridinic-N, pyrrolic-N or quaternary-N atoms on Pd NPs dispersion and also the stability of the Pd/N-CNTs was also investigated. The catalytic activity of the resulting Pd/N-CNTs catalysts was compared to that of Pd/CNTs in Suzuki coupling and solventfree benzyl alcohol oxidation reactions. Some comparisons were made with a few examples in the literature. Also, the recyclability of the synthesized heterogeneous catalysts was examined.
Experimental
Materials
Ferrocenecarboxaldehyde (98%) and benzophenone (99%) were supplied by Sigma-Aldrich. Palladium(II) acetyl acetate (Pd(acac)2) (99%), acetonitrile (99%), benzyl alcohol (98%), sodium borohydride (95%) and benzaldehyde (99%) were obtained from Merck Chemicals. All organic substrates were purchased from Sigma-Aldrich with 98–99% purity and were used as received without further treatment. Double-deionized water was used throughout the experiments.
Synthesis of 1-ferrocenylmethyl(2-methyl imidazole) catalyst
The general procedure described by Pan et al. [46] was followed in the synthesis of 1-ferrocenylmethyl (2-methyl imidazole). Briefly, ferrocenemethanol (1 mM) and 2-methyl-1H-imidazole (1.1 mM) mixture was refluxed in acetic acid for 6 h at 60 °C. The reaction was monitored on preparative TLC, with hexane/diethyl ether (v/v 2:1, 10 mL) as the eluent. Upon completion, it was neutralized and washed with 50% KOH in distilled water to remove excess acetic acid. The residue was chromatographed on a silica-packed column with CH2Cl2/MeOH (4:1) as the eluent. Yield: 68%; IR (cm−1) 703.82, 730.95, 896.20, 1264.38, 1422.24, 3055; 1H-NMR spectra (CDCl3) 2.27 (3H, s, CH3), 4.12 (9H, m, Cp), 4.73 (2H, s, CH2), 6.73 (1H, s, imidazole), 6.81 (1H, s, imidazole); 13C-NMR spectra (CDCl3): d (ppm) = 13.13, 23.75, 29.69, 45.84, 68.79, 77.30, 82.78, 118.99, 126.43, 128.799, 130.87, 143.94. LC–MS (C10H16FeN2) EI: [M + H+] m/z calc. 280.10022, found 281.10015.
Preparation of CNTs, N-CNTs and N-CNT-1%
CNTs were synthesized by dissolving ferrocene (0.25 g) in toluene (9.75 g) to make 10 g precursor solution, while N-CNTs were synthesized by pyrolyzing 1-ferrocenylmethyl(2-methyl imidazole) (0.25 g) in acetonitrile (9.75 g) at 850 °C (CVD method). In the case of N-CNT-1% synthesis, this was done by a mixing 1-ferrocenylmethyl(2-methyl imidazole) (0.25 g) and benzophenone (0.5 g) (oxygen source) in acetonitrile (9.25 g). The symbol 1% represents the weight percentage of oxygen from benzophenone in the reactant mixture. The CVD procedure and set-up were followed according to the procedure described in previous reports [47]. In a typical experiment, the reactant mixtures were injected by a syringe at a rate of 0.8 mL min−1 through the quartz tube placed in a muffle furnace. The mixture was swept through the tube by 10% hydrogen in argon carrier gas, at a rate of 100 mL min−1. After 30 min of reaction, the furnace was cooled to room temperature, and the product was collected from the reactor. Before use, the samples were calcined at 300 °C for 3 h to remove amorphous carbon and chemically treated by refluxing in 6M HNO3 under vigorous stirring at 120 °C for 24 h. Afterwards, the samples were washed with double-distilled water followed by ethanol and then dried at 100 °C. The resulting fluffy and black carbon materials obtained without and with benzophenone were labelled N-CNTs and N-CNTs-1%, respectively, and were characterized by TEM, SEM, SAED, XRD, Raman, ICP-OES, DSC/TGA and XPS.
Preparation of Pd/CNT, Pd/N-CNTs and Pd/N-CNTs-1% catalysts
The MOCVD technique, as outlined by previous reports [48], was employed for 0.02 mol% Pd loading onto CNTs and N-CNTs support. Pd(acac)2 (0.048 g) was mixed with 0.316 g of acid-treated CNTs, N-CNTs and N-CNTs-1%, followed by thorough grinding, using a pestle and mortar. The resulting mixture was transferred into a stainless steel MOCVD reactor. The sealed MOCVD reactor was evacuated with the aid of a vacuum pump maintained at a partial pressure of 2.1 × 10−2 mbar for 40 min. The MOCVD reactor was then inserted into a CVD muffle furnace and heated at 120 °C for 30 min. The temperature was then ramped up to 300 °C at a rate of 2 °C min−1 and thereafter maintained at 300 °C for 45 min. The obtained samples were kept under ambient condition and stored under an inert atmosphere for further applications.
Characterization
X-ray diffraction patterns were recorded on a Rigaku/Dmax RB using a graphite monochromatized high-density Cu Kα radiation (λ = 0.15406 Å). Fourier transform infrared (FTIR) was recorded by the use of KBr pellets with PerkinElmer spectrum RX1 FTIR spectrometer. Raman spectra were recorded on DeltaNu Advantage 532™ Raman spectrometer with a 1 mW laser power on the sample compartment. Spectra were obtained with five accumulations at 1 min each. Microstructure images were obtained by scanning electron microscopy (SEM) (JOEL JEM 1010) and transmission electron microscopy (TEM) (JOEL JSM 6100). Higher magnifications were obtained from high-resolution transmission electron microscope (HRTEM) operated at 200 kV. N2 adsorption–desorption isotherms and surface area of CNTs and N-CNTs were determined on a Micrometrics Tristar II surface area analyser. The Pd content of the catalysts was determined by inductively coupled plasma–optical emission spectroscopy (ICP–OES) (PerkinElmer Optima 5300 DV). The thermal stabilities of CNTs and N-CNTs were measured using a Q Series™ thermal analyser DSC/TGA (Q600). The H2-TPR experiments were conducted in a gas mixture containing 10% H2 in Ar by Micromeritics Autochem II chemisorption analyser (2920) with the flow rate of 30 mL/min. X-ray photoelectron spectroscopy (XPS) analysis was carried out on a KRATOS AXIS Ultra DLD equipped with Al Kα (1486 eV) X-rays, with X-ray power of 20 W and a beam diameter of 100 μm. The CasaXPS programme was employed in the analysis of XPS data. Proton NMR spectra were recorded on a 400 MHz Bruker Ultrashield spectrometer at room temperature using deuterated CDCl3 or DMSO as solvents. The Suzuki–Miyaura coupling reactions and solventfree oxidation of benzyl alcohol products were identified using GC-FID (Shimadzu 2010 gas chromatograph).
General procedure for Suzuki reaction
In a typical Suzuki reaction, phenylboronic acid (2.5 mmol), aryl halide (2.5 mmol), Pd catalysts (20 mg), K2CO3 (4 mmol) and EtOH–H2O (1:1, v/v, 10 mL) solvents were added unto a round-bottom flask equipped with a stirrer. The reaction mixtures were stirred at 60 °C for 10–30 min under reflux. Upon reaction completion, the reaction product was cooled and filtered through a 0.22-μm pore polycarbonate filter to separate the catalyst, and the filtrate was extracted with CH2Cl2 (10 mL) three times. The organic layer fractions were combined into one conical flask and dried with anhydrous sodium sulphate. Thereafter, the mixture was filtered, and the residue was concentrated by flash chromatography on silica gel (0.0063–0.20 mm) with hexane/ethyl acetate (10:1) eluent, leading to isolation of pure biphenyl product.
Solventfree oxidation of benzyl alcohol
The solventfree oxidation reactions of benzyl alcohol were carried out in a three-necked round-bottom flask (25 mL), pre-heated in an oil bath equipped with a condenser. Pd catalysts (20 mg) was added to benzyl alcohol (5.0 mL) and stirred at 500 rpm for each experiment. The mixture was heated under reflux at 110 °C for 3 h and flushed with pure oxygen at a flow rate of 20 mL/min. After the reaction, the products were cooled to room temperature and centrifuged to separate the catalysts. The products were analysed by a 30-m DB-1 capillary gas chromatography (GC) equipped with FID detector (Shimadzu 14B, FID detector) using n-dodecane as an internal standard. The column temperature was kept at 100 °C for 10 min and then raised to 200 °C at a heating rate of 10 °C/min. The conversion of benzyl alcohol and selectivity towards benzaldehyde product was calculated after three consecutive runs. The turn over frequency (TOF) for each catalyst was calculated using Eq. (1).
Recycling and hot filtration leaching tests
After each catalytic cycle, the catalyst was firstly washed sufficiently with distilled water, sequentially with ethanol and acetone and then finally with dichloromethane before being dried at 110 °C for 24 h. Further catalytic reaction was carried out using the dried black residue. Hot filtration leaching test was carried out for the Suzuki reaction between iodobenzene (2.5 mmol) and phenylboronic acid (2.5 mmol) under optimized conditions. After 30 min, half of the reaction mixture was centrifuged and filtered while hot at 2000 rpm. The reaction was allowed to continue under stirring for another 6 h. Subsequently, the reaction yield of the unfiltered and the filtered solution was monitored by GC analysis after sampling at a predetermined time.
Results and discussion
Characterization of catalysts
The structural information and the crystalline phase of the catalysts were investigated using an X-ray diffractometer. Figure 1a shows the XRD pattern of Pd/CNTs, Pd/N-CNTs and Pd/N-CNTs-1%. All the catalyst showed the diffraction characteristic peaks of Pd NPs. The diffraction peak at 26.35° was indexed to the (002) crystal face reflection of the graphitic carbon in CNTs and N-CNTs, while the peaks at 40.00, 46.75 and 68.18° were attributed to the (111), (200) and (220), respectively, hexagonal close-packed crystalline phase of Pd face-centred cubic (fcc) structure in Pd/N-CNTs and Pd/CNTs [49].
The Pd NPs crystallite sizes were estimated from Pd (111) peaks using the Scherrer equation given in Eq. (2):
where D = average diameter of the Pd/NPs in nm, λ = 0.154 nm (i.e. the wavelength of the X-rays), θ = diffraction angle of the Pd (111) reflection and β = full width at half maximum (FWHM) of the Pd reflection plane. The lattice d-spacing calculated for the Pd (111) plane by Bragg’s law for Pd/CNTs, Pd/N-CNTs and Pd/N-CNTs-1% was found to be 2.3152, 2.2530 and 2.3917 Å, respectively. According to the Scherrer’s method, the full width at half maximum (FWHM) of the diffraction peak is inversely proportional to the particle size [50]. The calculated palladium nanoparticle sizes of Pd/CNTs, Pd/N-CNTs and Pd/N-CNTs-1% were 9.5, 3.1 and 7.5 nm, respectively. This trend is comparable with the nanoparticle size obtained from the TEM analysis (Table 1). The ICP–OES analysis of the catalysts gave approximately 0.02 mol% Pd loading for all catalysts (Table 1).
The carbon framework and structural defect instigated by N-doping was further evaluated quantitatively by the Raman spectroscopy (Fig. 1b). This was done to evaluate the degree of disordered carbon structure caused by a defect on the graphitic carbon sheets. An intense D-band peak at ~ 1348 cm−1 and G-band at ~ 1590 cm−1 are ascribed to the disordered carbon and the graphitic carbon, respectively [51]. The ratio of D- to G-band intensity (ID/IG) was used to estimate the degree of distortions that exist in CNTs and N-CNTs. It was found that the ratio of ID/IG for Pd/CNTs (0.7583) was much less when compared to Pd/N-CNTs (0.9738) and Pd/N-CNTs-1% (0.8366). The lower ID/IG ratio demonstrates a more graphitic structure for Pd/CNTs, while on the other hand higher ID/IG ratio suggests a more successful N-doping in the increasing order of Pd/N-CNTs to Pd/N-CNTs-1%.
The SEM images of the Pd/N-CNTs showed a uniformly dispersed spherical Pd NPs indicative of a hierarchical porous network, often required in heterogeneous catalysis [52] (Fig. 2a). The morphology and the deposition of Pd NPs on CNTs and N-CNTs were clearly identified by the dark spots visible in Fig. 2b, c, respectively. Figure 2b, c shows a typical TEM and HRTEM images of Pd/N-CNTs-1%. TEM investigations revealed the average sizes to be 3.4, 7.3 and 8.7 nm, for CNTs, N-CNTs and N-CNTs-1%, respectively, and it also showed the distribution of the embedded Pd NPs. This result is consistent with particle sizes reported by Wang et al. [35]. Notably, the density of Pd NPs on N-CNTs-1% is profuse, and no nanoparticle aggregation is observed on the nanotube surface (Fig. 2b). The TEM result indicates that N-doping plays an essential role in Pd NPs dispersibility due to close interaction between Pd particles and defective N-CNTs than the graphitic CNTs [53]. The Pd atoms are deposited on the curvature of the wrinkled carbon sheets (Fig. 2c), suggestive of a functional interaction between Pd NP and N atom on N-CNT structure [53]. The crystallinity of Pd NPs anchored on the graphitic carbon was further determined by a selective area electron diffraction pattern (SAED, inset in Fig. 2c). The d-spacing (2.3917 Å) is consistent with the Pd (111) planes obtained from XRD analysis. Pd NPs consists of concentric rings, composed of bright discrete diffraction spots, which can be indexed to the (111), (200), (220) and (311) planes of fcc Pd. The SAED pattern of the as-prepared Pd nanoparticles shows their high crystallinity.
The chemical composition was investigated by FTIR (Fig. 3). The peaks at 2963 and 2843 cm−1 characterize the stretching vibration of the CH2 and CH3 groups, respectively, present in all samples [54]. The bands at 1530 and 1307 cm−1 are assigned to the sp2 C=C and aromatic C–N stretching bonds, respectively [55]. The bands at 1736, 1556 and 1227 cm−1 were assigned to C=O, C=N and C–O, respectively [56,57,58]. The peak observed at 2370 cm−1 was assigned to trapped CO2 stretching and was observed in all samples [56, 59]. Compared with Pd/CNTs, the appearance of C=N and C–N functional groups in Pd/N-CNTs indicates the doping of N atom into the graphitic carbon.
The bulk composition of Pd/CNTs, Pd/N-CNTs and Pd/N-CNTs-1% catalysts was analysed with an X-ray photoelectron spectroscopy (XPS). The full spectra survey (Fig. 4a) revealed the presence of C, O and Pd in Pd/CNTs, while C, N, O and Pd were detected in Pd/N-CNTs. The percentage composition of all surface elements from XPS analysis is given in Supplementary Table S1. C 1s peaks were deconvoluted into five individual peaks as shown in Fig. 4. The robust signals C 1s confirmed the presence of oxygen functionalization on the CNTs and N-CNT surfaces. Figure 4b shows the peaks at 286.6 (C-O), 288.7 (O–C=O) and 290.2 eV (C–O–C), which are ascribed to the oxygen-containing moieties [45, 60, 61]. The peaks 284.3, 285.1, 287.1, 283.9 and 291.5 eV are assigned to sp2 C, sp3 C, C=N, C–N and carbonates, respectively.
The total nitrogen content on N-CNTs and N-CNT-1% was 10.56 and 6.92 at.%, respectively (Supplementary Table S1). A similar result was reported by Koos et al. [62], where nitrogen contents in N-CNTs decrease as N/C ratio decreases. The N 1s peaks of the high-resolution spectrum (Fig. 4d; Supplementary Figure S5) were deconvoluted to three symmetrical peaks assigned to pyridinic (398.4 eV), pyrrolic (400.1 eV) and oxidized nitrogen-like N-oxides of pyridinic-N (404.2 eV) which is typical of N-doped CNTs [63, 64]. The analysis of the N 1s spectra conformed with Kotakoski et al. [65] simulation, which suggested that at higher nitrogen atom concentration, N atom might be introduced as pentagon defect corresponding to pyrrolic-N type, while incorporation of a lower amount of nitrogen into the CNTs hexagonal structure was ascribed to pyridinic-N type [66]. Although most studies argue that the type of nitrogen functionalities and N-doping level can be controlled or rather depend on synthesis condition [67,68,69,70], the introduction of oxygen-containing benzophenone into the reaction mixture can lead to substantial increase in the pyrrolic-N. This can be attributed to the formation of NOx resulting from the reaction of nitrogen with oxygen. On the other hand, also the emergence of pyridinic oxides is a result of the conversion of quaternary-N. This occurs due to highly reactive oxidation reaction induced by the presence of benzophenone dissolved in the reaction mixture for N-CNTs-1% [71].
XPS analysis also showed the chemical state of Pd particles on CNTs and N-CNTs surface. The interaction of the pyrrolic nitrogen of N-CNTs and Pd NPs of different sizes showed pairs of doublet overlapping peaks in Pd 3d XPS spectra, corresponding to Pd 3d5/2 and Pd 3d3/2 (Fig. 4c). The Pd 3d peaks with binding energies at 335.6 eV (Pd5/2) and 340.6 eV (Pd3/2) [72, 73] were attributed to Pd0, while peaks at 337.7 eV (Pd5/2) and 342.7 eV (Pd3/2) [74] were related to Pd2+ (Supplementary Table S2). The XPS data also revealed that Pd0 was predominant on the surfaces of the three catalysts with no noticeable Pd2+ ion, indicating that Pd2+ ion was reduced to Pd NPs. The weak Pd2+ peak observed at 336.3–336.9 eV was attributed to the formation of PdO due to sample exposure to air. The deposited Pd NPs were well anchored on N-CNTs with an average size of 3.4 and 7.3 nm for N-CNTs and N-CNTs-1%, respectively. This could be attributed to the high nitrogen density on the N-CNTs surface and enhanced anchoring of the Pd NPs, making the catalysts less prone to agglomeration and sintering on cooling during catalyst synthesis. Following the peak area integration of Pd 3d peaks, the total molar fraction of Pd is ~ 76% for Pd/N-CNTs, while Pd/N-CNTs-1% had a lower value of 56%. Detailed XPS results are accessible in Supporting Information Table S1 and Table S2.
For N-CNTs, the decrease in the pyridinic-N concentration with increased pyrrolic-N was observed upon introduction of oxygen into the reaction mix (Fig. 5; Supplementary Table S1). It is noteworthy that pyrrolic-N species were 70% in Pd/N-CNTs-1%, with such N species acting as anchoring site, thereby enabling the nucleation and growth of metal nanoparticles [75]. From the results, the increased percentage of pyrrolic-N is due to the dilution of the nitrogen source which largely affects the N species formation [76, 77]. Also, the observed increase in pyrrolic-N concentration for N-CNTs-1% could be attributed to the decrease in N/C ratio in the reaction mixture as precursor decomposition is assumed to occur at the surface of Fe NPs (i.e. top-growth model) [78]. The formation of pyrrolic-N and pyridinic-N on the outer shell of N-CNTs is due to the interaction of NH and CN species on catalyst surface followed by their surface or subsurface diffusion [79].
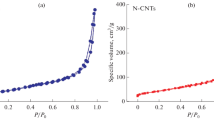
The surface area properties of the synthesized Pd/CNTs, Pd/N-CNTs and Pd/N-CNTs-1% catalysts were obtained from N2 adsorption–desorption isotherms as given in Table 2 with the typical isotherm shown in Fig. 6a. All samples showed type IV isotherm curves [80] with an H1 hysteresis loop between 0.50 and 0.99 P/Po, typical of mesoporous and macroporous structured material with narrow pore size distribution associated with a cylindrical-like porous material such as CNTs [81]. The oxidation of the CNTs and N-CNTs via acid treatments led to the opening of tube ends, which upon deposition of Pd metal, these openings were blocked by nanoparticles, thereby inhibiting N2 adsorption. This results in a decrease in the surface area of Pd/CNTs (133.87 m2g−1), Pd/N-CNTs (47.44 m2g−1) and Pd/N-CNTs-1% (87.50 m2g−1) catalysts [82, 83], relative to plain CNTs (135.42 m2g−1), N-CNTs (55.29 m2g−1) and N-CNTs-1% (98.46 m2g−1), respectively. A significant increase in surface area was observed for N-CNTs-1% upon introduction of benzophenone in the reaction mixture, due to increase porosity caused by oxygen activation of the disordered N-CNTs.
Pd/N-CNTs showed the highest pore volume of 1.361 nm followed by Pd/N-CNTs-1% (0.846 nm) and Pd/CNTs (0.402 nm) (Fig. 6b), which could be associated with variation in reactant stoichiometry and post-synthesis functionalization [84, 85].
The strong interaction between CNTs (and N-CNTs) support and Pd NPs stabilization was investigated by H2-TPR (Supplementary Figure S4). This interaction is evidenced by the reduction in temperature observed for the three catalysts in the H2-TPR experiment. The position, width and intensity of the peaks depend significantly on the carbon material synthesis and pre-treatment of the supports. From the H2-TPR profile, the hydrogen desorption peak at 50 °C could be attributed to interstitial Pd β-hydride. A sharp peak centred at 298 °C can be observed from Pd/CNTs and Pd/N-CNTs (Supplementary Fig. 4). The broad peak centred at 120 °C could be ascribed to the reduction of Pd2+ to Pd0 which is in support of observed results from the Pd 3d XPS spectra [86] (Fig. 5c).
Catalytic performance
Suzuki coupling reactions
A typical Suzuki coupling reaction was conducted using iodobenzene (2 mmol) and phenylboronic acid (2.5 mmol) as reactants, a base (4 mmol) and catalyst (20 mg). The solvent, base and temperature were varied to optimize the reaction conditions (Table 3). Table 3 summarizes the results obtained for quantitative yields of biphenyl compounds. High yields were obtained in protic solvents such as MeOH, EtOH and iPrOH (Table 3, entries 1, 2 and 12), while poor yields were obtained for aprotic solvents such as DMF, dioxane, acetonitrile and acetone (Table 3, entries 11 and 13–15). However, when H2O was introduced as a solvent, a gradual increase in reactivity was observed relative to aprotic solvents (Table 3, entry 3). An increase in the reactant solubility and reactivity was observed when solvent mixtures were employed (i.e. EtOH–H2O (1:1 v/v, 10 mL) and THF–H2O (1:1 v/v, 10 mL)) (Table 3, entries 5–10) similar to previous reports [87].
The advantage of the solvent mixture could be ascribed to the better solubility of the organic reactant in the organic base, which activates the phenylboronic acid and promotes reductive elimination reactions [88]. EtOH–H2O was found to be the solvent of choice than various bases such as K2CO3, NaOAc, KOH and Et3N. K2CO3 produces better yields compared to Na2CO3, NaOAc and Et3N bases. This could be attributed to the partial homogeneity of K2CO3 in the aqueous phase, leading to effective activation of phenylboronic acid towards boronate complex formation [89]. The yield noticeably reduced from 92 to 82% for Na2CO3 and Et3N, respectively (Table 3, entries 8 and 9).
Using the obtained optimum conditions, the catalytic efficiency of Pd catalysts in the coupling reaction between aryl halides (such as I and Br) and phenylboronic acid was investigated (Table 4). The reactions were rapid from 60 to 100 °C, beyond which an increase in the temperature did not increase the yield. Substituted aryl halide substrates with electron-withdrawing groups (–NO2, –COCH3 and –CF3) and electron-donating groups (–CH3, –H and –NH2) produced good yields ranging from 73 to 98% of biphenyl compounds. The highest yield with substituted aryl bromide was obtained using Pd/N-CNTs-1% catalyst within 10–30 min (Table 4, entries 2–10). Effect of substituents on the aryl halide has been reported for Suzuki reactions [90]. The lower yield obtained for electron-withdrawing substituent (–COOH) which facilitates the rate-limiting oxidative reaction could be attributed to increased acidity in the reaction mixture [90] (Table 4, entry 11). The sterically hindered ortho-substituted 2-bromophenol and 2-iodobenzotrifluoride proceeded with good yields but not with excellent yields as obtained for para-substituted aryl halide (Table 4, entries 12 and 14). Among substituted aryl halides, substituted bromobenzene gave higher yields (Table 4, entry 10) than substituted iodobenzene (Table 4, entry 2), even though iodobenzene shows greater reactivity than bromobenzene due to iodine being a better leaving group. Nonetheless, there are known exceptions where reverse catalytic activity was observed for alkyl iodide as a substrate for Suzuki coupling reactions. This could be attributed to the generation of iodide anion (I−) from alkyl iodide acting as a catalyst inhibitor during the catalytic reaction [91]. The excellent catalytic activity of Pd/N-CNTs-1% over Pd/N-CNTs and Pd/CNTs could be attributed to the smaller particles size of Pd NPs [92,93,94] and the strong interaction between Pd NPs and pyrrolic-N atom incorporated in the graphitic sp2 carbon, thereby preventing Pd leaching and agglomeration. From the results of TEM sample measurements (from images obtained) and values calculated from Scherrer equation, XRD (Table 1), the order of Pd particle size on CNTs, N-CNTs and N-CNTs-1% supports follows the order Pd/CNTs < Pd/N-CNTs-1% < Pd/N-CNTs, respectively. The yield of biphenyls obtained follows the order Pd/CNTs < Pd/N-CNTs < Pd/N-CNTs-1%. The inconsistency in the particle size trend and the yield suggest that the Suzuki coupling reaction was influenced not only by the change in particle sizes but by other factors such as surface properties and the nature of the support (amount of pyrrolic-N atom, XPS analysis) [33].

The efficiency of Pd/N-CNTs and Pd/N-CNTs-1% catalysts in this work is compared to those reported elsewhere for Suzuki coupling reactions of bromobenzene and phenylboronic acid [37, 38]. Table 5 shows that all reactions proceeded with better yields (> 90%) except a few with harsh reaction conditions and longer reaction times. From BET analysis, the surface area of Pd/CNTs, Pd/N-CNTs and Pd/N-CNTs-1% catalysts follows the order Pd/CNTs > Pd/N-CNTs-1% > Pd/N-CNTs. However, it was found that the yield of biphenyl follows the order Pd/CNTs < Pd/N-CNTs < Pd/N-CNTs-1%. This suggests that the coupling reaction is dependent not only of the surface area but also other factors such as surface properties including the pore volume [95, 96] and the nature of the support (amount of pyrrolic-N atom) [33].
Solventfree benzyl alcohol oxidation reactions
In our study, we extend the application of Pd/CNTs, Pd/N-CNTs and Pd/N-CNTs-1% catalysts in the solventfree oxidation reaction of benzyl alcohol. The catalytic activities of the Pd catalysts in the oxidation of benzyl alcohol in pure oxygen with a flow rate of 20 mL/min at a temperature of 110 °C were investigated. A blank reaction without any catalyst was conducted. A lower conversion of 3% (Table 6) was obtained, confirming that the reaction could not proceed in the absence of a catalyst. Also, the use of CNTs and N-CNTs as catalysts produced noticeable conversions of 15 and 35%, respectively (Fig. 7a). The oxidation reaction continued progressively as the catalytic activities increased in the order Pd/N-CNTs-1% > Pd/N-CNTs > Pd/CNTs, which correlates with an increase in the pyrrolic-N atom as observed in the XPS results. This increased catalytic activity is attributable to the effective enhancement of active surface oxygen by Pd NPs. For Pd/N-CNTs-1% and Pd/N-CNTs, about 98 and 96% conversion was achieved after 2 h of reaction, respectively, with a high TOF (Table 6). Nonetheless, a lower conversion was obtained for Pd/CNTs which could be due to the resulting agglomeration of Pd NPs which led to unfavourable larger Pd NPs size. The selectivity of Pd/N-CNTs-OT catalyst (96%) was found to be superior to Pd/N-CNTs which is reported in the literature [35] with larger particle size (Table 7, entries 5 and 7).
The catalytic strength of our catalyst for benzyl alcohol oxidation is comparable to other reported Pd-catalysed reactions (Table 7). The low selectivity observed for Pd/CNTs, Pd/N-CNTs and Pd/N-CNTs-1% catalysts after 30 min of reaction (Fig. 7b) is a result of (1) initial hydrogenolysis of benzyl alcohol towards the formation of toluene and (2) parallel oxidation reaction between benzyl alcohol and hydrogen [30]. However, further reaction of oxygen with hydrogen suppresses the hydrogenolysis reaction, thereby enhancing selectivity towards benzaldehyde formation after 120 min.
Influence of the catalyst preparation conditions on catalytic performance
From our experiments, it has been shown that N-CNTs and N-CNTs-1% are more efficient support than CNTs for Pd-catalysed reactions. For N-CNT, nitrogen atom usually increases the electron density on metal catalysts, thereby providing a suitable site for anchoring [71], leading to better dispersion and ultimately facilitating the adsorption of reactant and desorption of products [108,109,110]. The lone pair bonding electron and higher electronegativity of the substituted N atoms within the carbon support enhance the support basicity, hence promoting the desorption of nitrogen at low temperature [111]. Also, nitrogen doping modifies the Pd NPs active sites. During catalyst preparation, active Pd NPs crystallites react with both the pyrrolic- and the pyridinic-N atoms on nanotubes surfaces. We also presume that nitrogen atoms are uniformly spread inside the nanotubes.
The introduction of oxygen during the carbon support synthesis and chemical functionalization offers great opportunities in tailoring the surface properties of these materials with better porosity, thereby enhancing diffusion and mass transfer of substrates during catalytic reactions [112]. The presence of hydrogen decreases the amount of N atoms doped on CNTs due to the reaction of N and H radicals to form NH3 and HCN gases [113]. We, therefore, concluded that oxygen played a vital role by improving the nanotubes’ quality by scavenging the H radicals via the reaction (i.e. H + O2 → OH + O), as evidenced by the presence of a strong OH band obtained for N-CNTs-1% from the FTIR analysis (Fig. 4). The formation of OH radicals subsequently helps to suppress carbon etching at the growing edges, remove amorphous carbon (i.e. C + O → CO + O → CO2) and limit the supersaturation of carbon molecule during N-CNTs growth.
Surface properties of the CNTs and N-CNTs can be influenced by the amount of C–O functional group and the metal particle size. The Pd NPs size distribution on CNTs, N-CNTs and N-CNTs-1% is shown in Supplementary Figure S1. The Pd NPs size on the carbon materials follows the order: Pd/CNTs > Pd/N-CNTs > Pd/N-CNTs-1%. N-CNTs-1% produce good Pd particle dispersion due to the presence of a carbonyl functional group on the carbon surface. This was confirmed by O 1s peak of the three fitted components in the samples, namely: quinone and carbonyl group (C=O, 531.15 eV) [114]; ether and hydroxyl oxygen (C–O, 533.4 eV) [115, 116]; and carboxylic group oxygen (COOH, 534.4 eV) [117] from the XPS analysis. The increase in oxygen-containing fractions follows the trend: Pd/CNTs (11.37 at.%) < Pd/N-CNTs (12.72 at.%) < Pd/N-CNTs-1% (18.22 at.%). According to the FTIR and XPS results, carboxyl groups on the surface of N-CNTs-OT were capable of interacting with Pd NPs via covalent bonding [95]. Hence, the Pd/N-CNTs-OT catalyst played a critical role in preventing Pd NPs from leaching during the Suzuki coupling reaction and also in the solventfree benzyl alcohol oxidation, and this is due to the available binding sites (i.e. O–C=O, C–N, and C–O–C groups) available in N-CNTs-OT carbon support.
Catalyst recyclability
The catalytic activities of the three catalysts were studied under the optimized conditions. The recyclability of the catalysts was investigated for iodobenzene and phenylboronic acid as a test substrate by filtration, and the results are illustrated in Fig. 8. The catalysts were washed with ethanol and subsequently with acetone and dichloromethane and dried overnight in an oven at 373 K after each run. The catalytic properties of the three catalysts remained unchanged until after the fifth runs, with no loss in their selectivity and activity. The Pd/CNTs become less active with a drop in yield to 84% after the fourth run, while Pd/N-CNTs and Pd/N-CNTs-1% showed the highest stability with a 93% yield after the fifth run. Further TEM analysis of the catalysts after five runs showed well-dispersed Pd NPs (Supplementary Figure S5) with minimal agglomeration. Table 8 shows the recyclability of the catalyst after seven runs for benzyl alcohol oxidation. The Pd/N-CNTs-1% catalyst produced an outstanding conversion of 96% after seven recycles of reaction regeneration, signifying even Pd NPs dispersion, high stability and robust interaction between carbon support and Pd NPs, thus preventing not only agglomeration but also leaching.
Pd leaching test
Leaching of metal NPs is of great concern in heterogeneous catalysis, and to confirm the amount of metal leached, the hot filtration test method was employed to investigate whether the Pd/CNTs and Pd/N-CNTs acted as heterogeneous catalysts. Similar catalyst amounts and reaction conditions for Suzuki reaction were employed for the hot filtration experiment. After 20 min of reaction, the catalyst was filtered, and the filtrate was analysed through ICP-OES. For the reaction, 36% conversion was recorded, and no trace of Pd species was detected. The reaction was allowed to proceed until after 120 min. It was observed that the reaction was halted after catalyst removal, indicating no further conversion (Supplementary Figure S6). We suggested that Pd species from Pd/N-CNTs-1% catalyst remain active in the catalytic cycle (i.e. oxidative addition, transmetallation and reductive elimination) of Suzuki reaction (Supplementary Figure S7), thus preventing catalyst loss and permitting easy recovery. The observed stability, catalytic efficiency and reusability of Pd/N-CNTs and Pd/N-CNTs-1% catalyst make them a suitable candidate for C–C coupling reactions and oxidation reactions.
Further ICP-OES analysis was carried out after the fifth run with 4.56 wt% Pd content recorded for Pd/N-CNTs and Pd/N-CNTs-1%, indicating a strong interaction of Pd NPs with the active sites of the carbon support thereby thwarting leaching. A combination of the results of recyclability and the hot filtration test showed that the catalyst is genuinely heterogeneous and selective towards Suzuki coupling and oxidation of benzyl alcohol reactions without significant Pd leaching.
Conclusion
Pd/CNTs, Pd/N-CNTs and Pd/N-CNTs-1% catalysts were successfully synthesized by MOCVD techniques. Pd NPs sizes of 3.4, 7.3 and 8.7 nm were well dispersed on N-CNTs, N-CNTs-1% and CNTs, respectively. The catalysts possessed mesoporous surfaces with reasonable stability and excellent durability. The correlation of metal–carbon interaction, particle size distribution and the effect of pyrrolic-N atoms towards excellent catalytic activity and selectivity of Pd/N-CNTs-1% was successfully archived for Suzuki coupling and benzyl alcohol oxidation reactions. It must be stressed that 1% weight of oxygen atom, introduced by benzophenone, and seen from XPS result of Pd/N-CNTs-1%, is useful for applications where strong interfacial bonding of Pd NPs with carbon support is required.
References
Harada T, Ikeda S, Hashimoto F, Sakata T, Ikeue K, Torimoto T et al (2010) Catalytic activity and regeneration property of a Pd nanoparticle encapsulated in a hollow porous carbon sphere for aerobic alcohol oxidation. Langmuir 26:17720–17725
Koltunov KY, Walspurger S, Sommer J (2004) Superacid and H-zeolite mediated reactions of benzaldehyde with aromatic compounds and cyclohexane. The role of mono-and dicationic intermediates. Catal Lett 98:89–94
Della Pina C, Falletta E, Rossi M (2008) Highly selective oxidation of benzyl alcohol to benzaldehyde catalyzed by bimetallic gold–copper catalyst. J Catal 260:384–386
Canellas E, Aznar M, Nerín C, Mercea P (2010) Partition and diffusion of volatile compounds from acrylic adhesives used for food packaging multilayers manufacturing. J Mater Chem 20:5100–5109
Siebel A, Gorlin Y, Durst J, Proux O, Fdr Hasché, Tromp M et al (2016) Identification of catalyst structure during the hydrogen oxidation reaction in an operating PEM fuel cell. ACS Catal 6:7326–7334
Goszewska I, Giziński D, Zienkiewicz-Machnik M, Lisovytskiy D, Nikiforov K, Masternak J et al (2017) A novel nano-palladium catalyst for continuous-flow chemoselective hydrogenation reactions. Catal Commun 94:65–68
Saito Y, Ishitani H, Ueno M, Kobayashi S (2017) Selective hydrogenation of nitriles to primary amines catalyzed by a polysilane/SiO2-supported palladium catalyst under continuous-flow conditions. ChemistryOpen 6:211–215
Choudhary H, Jia J, Nishimura S, Ebitani K (2017) Surfactant-assisted Suzuki–Miyaura coupling reaction of unreactive chlorobenzene over hydrotalcite-supported palladium catalyst. Asian J Org Chem 6:274–277
Kim Y-O, You JM, Jang H-S, Choi SK, Jung BY, Kang O et al (2017) Eumelanin as a support for efficient palladium nanoparticle catalyst for Suzuki coupling reaction of aryl chlorides in water. Tetrahedron Lett 22:2149–2152
Choi J, Chan S, Yip G, Joo H, Yang H, Ko FK (2016) Palladium-zeolite nanofiber as an effective recyclable catalyst membrane for water treatment. Water Res 101:46–54
Choudhary M, Siwal S, Nandi D, Mallick K (2016) Catalytic performance of the in situ synthesized palladium–polymer nanocomposite. New J Chem 40:2296–2303
Gholinejad M, Bahrami M, Nájera C (2017) A fluorescence active catalyst support comprising carbon quantum dots and magnesium oxide doping for stabilization of palladium nanoparticles: Application as a recoverable catalyst for Suzuki reaction in water. Mol Catal 433:12–19
Freakley SJ, He Q, Harrhy JH, Lu L, Crole DA, Morgan DJ et al (2016) Palladium-tin catalysts for the direct synthesis of H2O2 with high selectivity. Science 351:965–968
Labulo AH, Martincigh BS, Omondi B, Nyamori VO (2017) Advances in carbon nanotubes as efficacious supports for palladium-catalysed carbon–carbon cross-coupling reactions. J Mater Sci 52:9225–9248. https://doi.org/10.1007/s10853-017-1128-0
Yue D, Liu Y, Shen Z, Zhang L (2006) Study on preparation and properties of carbon nanotubes/rubber composites. J Mater Sci 41:2541–2544. https://doi.org/10.1007/s10853-006-5331-7
Salvetat J-P, Bonard J-M, Thomson N, Kulik A, Forro L, Benoit W et al (1999) Mechanical properties of carbon nanotubes. Appl Phys A 69:255–260
Nam DH, Cha SI, Lee KM, Jang JH, Park HM, Lee JK et al (2016) Thermal properties of carbon nanotubes reinforced aluminum-copper matrix nanocomposites. J Nanosci Nanotechnol 16:12013–12016
Qiu H, Shi Z, Guan L, You L, Gao M, Zhang S et al (2006) High-efficient synthesis of double-walled carbon nanotubes by arc discharge method using chloride as a promoter. Carbon 44:516–521
Prasek J, Drbohlavova J, Chomoucka J, Hubalek J, Jasek O, Adam V et al (2011) Methods for carbon nanotubes synthesis. J Mater Chem 21:15872–15884
Maruyama T, Kondo H, Ghosh R, Kozawa A, Naritsuka S, Iizumi Y et al (2016) Single-walled carbon nanotube synthesis using Pt catalysts under low ethanol pressure via cold-wall chemical vapor deposition in high vacuum. Carbon 96:6–13
Zheng C, Huang L, Zhang H, Sun Z, Zhang Z, Zhang G-J (2015) Fabrication of ultrasensitive field-effect transistor DNA biosensors by a directional transfer technique based on CVD-grown graphene. ACS Appl Mater Interfaces 7:16953–16959
Chen D, Holmen A, Sui Z, Zhou X (2014) Carbon mediated catalysis: a review on oxidative dehydrogenation. Chin J Catal 35:824–841
Meemken F, Baiker A (2017) Recent progress in heterogeneous asymmetric hydrogenation of C=O and C=C bonds on supported noble metal catalysts. Chem Rev 117:11522–11569
Martin-Martinez M, Ribeiro RS, Machado BF, Serp P, Morales-Torres S, Silva AM et al (2016) Role of nitrogen doping on the performance of carbon nanotube catalysts: a catalytic wet peroxide oxidation application. ChemCatChem 8:2068–2078
Florea I, Ersen O, Arenal R, Ihiawakrim D, Messaoudi Cd, Chizari K et al (2012) 3D analysis of the morphology and spatial distribution of nitrogen in nitrogen-doped carbon nanotubes by energy-filtered transmission electron microscopy tomography. J Am Chem Soc 134:9672–9680
Xia W (2016) Interactions between metal species and nitrogen-functionalized carbon nanotubes. Catal Sci Technol 6:630–644
Old DW, Wolfe JP, Buchwald SL (1998) A highly active catalyst for palladium-catalyzed cross-coupling reactions: room-temperature Suzuki couplings and amination of unactivated aryl chlorides. J Am Chem Soc 120:9722–9723
Heidenreich RG, Krauter JG, Pietsch J, Köhler K (2002) Control of Pd leaching in Heck reactions of bromoarenes catalyzed by Pd supported on activated carbon. J Mol Catal A: Chem 182:499–509
Kim E, Jeong HS, Kim BM (2014) Studies on the functionalization of MWNTs and their application as a recyclable catalyst for C–C bond coupling reactions. Catal Commun 46:71–74
Yan Y, Jia X, Yang Y (2016) Palladium nanoparticles supported on CNT functionalized by rare-earth oxides for solventfree aerobic oxidation of benzyl alcohol. Catal Today 259:292–302
Maniam KK, Chetty R (2015) Electrochemical synthesis of palladium dendrites on carbon support and their enhanced electrocatalytic activity towards formic acid oxidation. J Appl Electrochem 45:953–962
He L, Weniger F, Neumann H, Beller M (2016) Synthesis, characterization, and application of metal nanoparticles supported on nitrogen-doped carbon: catalysis beyond electrochemistry. Angew Chem Int Ed 55:12582–12594
Ombaka LM, Ndungu PG, Kibet J, Nyamori VO (2017) The effect of pyridinic-and pyrrolic-nitrogen in nitrogen-doped carbon nanotubes used as support for Pd-catalyzed nitroarene reduction: an experimental and theoretical study. J Mater Sci 52:10751–10765. https://doi.org/10.1007/s10853-017-1241-0
Ding Y, Zhang L, Wu K-H, Feng Z, Shi W, Gao Q et al (2016) The influence of carbon surface chemistry on supported palladium nanoparticles in heterogeneous reactions. J Colloid Interface Sci 480:175–183
L-l Wang, L-p Zhu, N-c Bing, L-j Wang (2017) Facile green synthesis of Pd/N-doped carbon nanotubes catalysts and their application in Heck reaction and oxidation of benzyl alcohol. J Phys Chem Solids 107:125–130
Li M, Xu F, Li H, Wang Y (2016) Nitrogen-doped porous carbon materials: promising catalysts or catalyst supports for heterogeneous hydrogenation and oxidation. Catal Sci Technol 6:3670–3693
Zhang L, Dong W-H, Shang N-Z, Feng C, Gao S-T, Wang C (2016) N-Doped porous carbon supported palladium nanoparticles as a highly efficient and recyclable catalyst for the Suzuki coupling reaction. Chin Chem Lett 27:149–154
Zuo P, Duan J, Fan H, Qu S, Shen W (2018) Facile synthesis high nitrogen-doped porous carbon nanosheet from pomelo peel and as catalyst support for nitrobenzene hydrogenation. Appl Surf Sci 435:1020–1028
Ding S, Zhang C, Liu Y, Jiang H, Chen R (2017) Selective hydrogenation of phenol to cyclohexanone in water over Pd@ N-doped carbons derived from ZIF-67: role of dicyandiamide. Appl Surf Sci 425:484–491
Deng D-S, Han G-Q, Zhu X, Xu X, Gong Y-T, Wang Y (2015) Selective hydrogenation of unprotected indole to indoline over N-doped carbon supported palladium catalyst. Chin Chem Lett 26:277–281
Ombaka LM, Ndungu PG, Nyamori VO (2015) Pyrrolic nitrogen-doped carbon nanotubes: physicochemical properties, interactions with Pd and their role in the selective hydrogenation of nitrobenzophenone. RSC Adv 5:109–122
Chizari K, Janowska I, Houllé M, Florea I, Ersen O, Romero T et al (2010) Tuning of nitrogen-doped carbon nanotubes as catalyst support for liquid-phase reaction. Appl Catal A Gen 380:72–80
Amama PB, Pint CL, McJilton L, Kim SM, Stach EA, Murray PT et al (2008) Role of water in super growth of single-walled carbon nanotube carpets. Nano Lett 9:44–49
Futaba DN, Hata K, Namai T, Yamada T, Mizuno K, Hayamizu Y et al (2006) 84% catalyst activity of water-assisted growth of single walled carbon nanotube forest characterization by a statistical and macroscopic approach. J Phys Chem B 110:8035–8038
Duan X, Xiao M, Liang S, Zhang Z, Zeng Y, Xi J et al (2017) Ultrafine palladium nanoparticles supported on nitrogen-doped carbon microtubes as a high-performance organocatalyst. Carbon 119:326–331
He P, Du Y, Wang S, Cao C, Wang X, Pang G et al (2013) Synthesis, structure, and reactivity of ferrocenyl-NHC palladium complexes. Z Anorg Allg Chem 639:1004–1010
Oosthuizen RS, Nyamori VO (2012) Heteroatom-containing ferrocene derivatives as catalysts for MWCNTs and other shaped carbon nanomaterials. Appl Organomet Chem 26:536–545
Naidoo Q-L, Naidoo S, Petrik L, Nechaev A, Ndungu P (2012) The influence of carbon based supports and the role of synthesis procedures on the formation of platinum and platinum-ruthenium clusters and nanoparticles for the development of highly active fuel cell catalysts. Int J Hydrogen Energy 37:9459–9469
Saleh TA (2011) The influence of treatment temperature on the acidity of MWCNT oxidized by HNO3 or a mixture of HNO3/H2SO4. Appl Surf Sci 257:7746–7751
Kumar N, Yu Y-C, Lu YH, Tseng TY (2016) Fabrication of carbon nanotube/cobalt oxide nanocomposites via electrophoretic deposition for supercapacitor electrodes. J Mater Sci 51:2320–2329. https://doi.org/10.1007/s10853-015-9540-9c
Dubal DP, Chodankar NR, Caban-Huertas Z, Wolfart F, Vidotti M, Holze R et al (2016) Synthetic approach from polypyrrole nanotubes to nitrogen doped pyrolyzed carbon nanotubes for asymmetric supercapacitors. J Power Sources 308:158–165
Mao H, Shen Y, Zhang Q, Ulaganathan M, Zhao S, Yang Y et al (2016) Highly active and stable heterogeneous catalysts based on the entrapment of noble metal nanoparticles in 3D ordered porous carbon. Carbon 96:75–82
Arrigo R, Schuster ME, Xie Z, Yi Y, Wowsnick G, Sun LL et al (2015) Nature of the N–Pd interaction in nitrogen-doped carbon nanotube catalysts. ACS Catal 5:2740–2753
Dibandjo P, Bois L, Chassagneux F, Cornu D, Letoffe JM, Toury B et al (2005) Synthesis of boron nitride with ordered mesostructure. Adv Mater 17:571–574
Vanyorek L, Meszaros R, Barany S (2014) Surface and electrosurface characterization of surface-oxidized multi-walled N-doped carbon nanotubes. Colloids Surf A Physicochem Eng Asp 448:140–146
Misra A, Tyagi PK, Singh MK, Misra D (2006) FTIR studies of nitrogen doped carbon nanotubes. Diamond Rel Mater 15:385–388
Vinu A, Srinivasu P, Sawant DP, Mori T, Ariga K, Chang J-S et al (2007) Three-dimensional cage type mesoporous CN-based hybrid material with very high surface area and pore volume. Chem Mater 19:4367–4372
Mane GP, Talapaneni SN, Lakhi KS, Ilbeygi H, Ravon U, Al-Bahily K et al (2017) Highly ordered nitrogen-rich mesoporous carbon nitrides and their superior performance for sensing and photocatalytic hydrogen generation. Angew Chem Int Ed 56:8481–8485
Lazar G, Lazar I (2003) IR characterization of a C:H:N films sputtered in Ar/CH4/N2 plasma. J Non-Cryst Solids 331:70–78
Vikkisk M, Kruusenberg I, Ratso S, Joost U, Shulga E, Kink I et al (2015) Enhanced electrocatalytic activity of nitrogen-doped multi-walled carbon nanotubes towards the oxygen reduction reaction in alkaline media. RSC Adv 5:59495–59505
Tan X, Wu X, Hu Z, Ma D, Shi Z (2017) Synthesis and catalytic activity of palladium supported on heteroatom doped single-wall carbon nanohorns. RSC Adv 7:29985–29991
Koós AA, Dowling M, Jurkschat K, Crossley A, Grobert N (2009) Effect of the experimental parameters on the structure of nitrogen-doped carbon nanotubes produced by aerosol chemical vapour deposition. Carbon 47:30–37
Xie K, Xia W, Masa J, Yang F, Weide P, Schuhmann W et al (2016) Promoting effect of nitrogen doping on carbon nanotube-supported RuO2 applied in the electrocatalytic oxygen evolution reaction. J Energy Chem 25:282–288
Xiao M, Zhu J, Feng L, Liu C, Xing W (2015) Meso/macroporous nitrogen-doped carbon architectures with Iron carbide encapsulated in graphitic layers as an efficient and robust catalyst for the oxygen reduction reaction in both acidic and alkaline solutions. Adv Mater 27:2521–2527
Kotakoski J, Krasheninnikov A, Ma Y, Foster AS, Nordlund K, Nieminen RM (2005) B and N ion implantation into carbon nanotubes: insight from atomistic simulations. Phys Rev B 71:205408
Sjöström H, Stafström S, Boman M, Sundgren J-E (1995) Superhard and elastic carbon nitride thin films having fullerenelike microstructure. Phys Rev Lett 75:1336
Ewels C, Glerup M (2005) Nitrogen doping in carbon nanotubes. J Nanosci Nanotechnol 5:1345–1363
Chizari K, Sundararaj U (2014) The effects of catalyst on the morphology and physicochemical properties of nitrogen-doped carbon nanotubes. Mater Lett 116:289–292
Sharifi T, Nitze F, Barzegar HR, Tai C-W, Mazurkiewicz M, Malolepszy A et al (2012) Nitrogen doped multi walled carbon nanotubes produced by CVD-correlating XPS and Raman spectroscopy for the study of nitrogen inclusion. Carbon 50:3535–3541
Shan C, Zhao W, Lu XL, O’Brien DJ, Li Y, Cao Z et al (2013) Three-dimensional nitrogen-doped multiwall carbon nanotube sponges with tunable properties. Nano Lett 13:5514–5520
Latorre N, Romeo E, Cazana F, Ubieto T, Royo C, Villacampa J et al (2010) Carbon nanotube growth by catalytic chemical vapor deposition: a phenomenological kinetic model. J Phys Chem C 114:4773–4782
Li R, Zhang P, Huang Y, Zhang P, Zhong H, Chen Q (2012) Pd–Fe3O4@ C hybrid nanoparticles: preparation, characterization, and their high catalytic activity toward Suzuki coupling reactions. J Mater Chem 22:22750–22755
Chen X, Hou Y, Wang H, Cao Y, He J (2008) Facile deposition of Pd nanoparticles on carbon nanotube microparticles and their catalytic activity for Suzuki coupling reactions. J Phys Chem C 112:8172–8176
Radkevich V, Senko T, Wilson K, Grishenko L, Zaderko A, Diyuk V (2008) The influence of surface functionalization of activated carbon on palladium dispersion and catalytic activity in hydrogen oxidation. Appl Catal A Gen 335:241–251
Chen Y, Wang J, Liu H, Banis MN, Li R, Sun X et al (2011) Nitrogen doping effects on carbon nanotubes and the origin of the enhanced electrocatalytic activity of supported Pt for proton-exchange membrane fuel cells. J Phys Chem C 115:3769–3776
Hachimi A, Merzougui B, Hakeem A, Laoui T, Swain GM, Chang Q et al (2015) Synthesis of nitrogen-doped carbon nanotubes using injection-vertical chemical vapor deposition: effects of synthesis parameters on the nitrogen content. J Nanomater 16:425
Morjan I, Morjan I, Ilie A, Scarisoreanu M, Gavrila L, Dumitrache F et al (2017) The study of nitrogen inclusion in carbon nanotubes obtained by catalytic laser-induced chemical vapour deposition (C-LCVD). Appl Surf Sci 425:440–447
Hsiao C-H, Lin J-H (2017) Growth of a superhydrophobic multi-walled carbon nanotube forest on quartz using flow-vapor-deposited copper catalysts. Carbon 124:637–641
Bulusheva L, Okotrub A, Fedoseeva YV, Kurenya A, Asanov I, Vilkov O et al (2015) Controlling pyridinic, pyrrolic, graphitic, and molecular nitrogen in multi-wall carbon nanotubes using precursors with different N/C ratios in aerosol assisted chemical vapor deposition. Phys Chem Chem Phys 17:23741–23747
Sing KS, Williams RT (2004) Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt Sci Technol 22:773–782
ALOthman ZA (2012) A review: fundamental aspects of silicate mesoporous materials. Mater 5:2874–2902
Du J, Zhao R, Jiao G (2013) The short-channel function of hollow carbon nanoparticles as support in the dehydrogenation of cyclohexane. Int J Hydrogen Energy 38:5789–5795
Zhao Y, Li C-H, Yu Z-X, Yao K-F, Ji S-F, Liang J (2007) Effect of microstructures of Pt catalysts supported on carbon nanotubes (CNTs) and activated carbon (AC) for nitrobenzene hydrogenation. Mater Chem Phys 103:225–229
Tangestaninejad S, Moghadam M, Mirkhani V, Mohammadpoor-Baltork I, Ghani K (2009) Alkene epoxidation catalyzed by molybdenum supported on functionalized MCM-41 containing N–S chelating Schiff base ligand. Catal Commun 10:853–858
Kotal M, Bhowmick AK (2013) Multifunctional hybrid materials based on carbon nanotube chemically bonded to reduced graphene oxide. J Phys Chem C 117:25865–25875
Huang H, Leung DY (2011) Complete oxidation of formaldehyde at room temperature using TiO2 supported metallic Pd nanoparticles. ACS Catal 1:348–354
H-q Song, Zhu Q, X-j Zheng, X-g Chen (2015) One-step synthesis of three-dimensional graphene/multiwalled carbon nanotubes/Pd composite hydrogels: an efficient recyclable catalyst for Suzuki coupling reactions. J Mater Chem A 3:10368–10377
Xu Y, Wang T, He Z, Zhong A, Huang K (2016) Carboxyl-containing microporous organic nanotube networks as a platform for Pd catalysts. RSC Adv 6:39933–39939
Artok L, Bulut H (2004) Heterogeneous Suzuki reactions catalyzed by Pd (0)–Y zeolite. Tetrahedron Lett 45:3881–3884
Pourkhosravani M, Dehghanpour S, Farzaneh F (2016) Palladium nanoparticles supported on zirconium metal organic framework as an efficient heterogeneous catalyst for the Suzuki–Miyaura coupling reaction. Catal Lett 6:499–508
Primo A, Liebel M, Fo Quignard (2009) Palladium coordination biopolymer: a versatile access to highly porous dispersed catalyst for Suzuki reaction. Chem Mater 21:621–627
Arrigo R, Wrabetz S, Schuster ME, Wang D, Villa A, Rosenthal D et al (2012) Tailoring the morphology of Pd nanoparticles on CNTs by nitrogen and oxygen functionalization. Phys Chem Chem Phys 14:10523–10532
Deraedt C, Astruc D (2013) “Homeopathic” palladium nanoparticle catalysis of cross carbon–carbon coupling reactions. Acc Chem Res 47:494–503
Corma A, Garcia H, Leyva A (2005) Catalytic activity of palladium supported on single wall carbon nanotubes compared to palladium supported on activated carbon: study of the Heck and Suzuki couplings, aerobic alcohol oxidation and selective hydrogenation. J Mol Catal A: Chem 230:97–105
Alonso-Morales N, Ruiz-Garcia C, Palomar J, Heras F, Calvo L, Rodriguez JJ et al (2017) Hollow nitrogen-or boron-doped carbon submicrospheres with a porous shell: preparation and application as supports for hydrodechlorination catalysts. Ind Eng Chem Res 56:7665–7674
Bidabehere CM, García JR, Sedran U (2017) Transient effectiveness factor in porous catalyst particles. Application to kinetic studies with batch reactors. Chem Eng Res Des 118:41–50
Dong Y, Wu X, Chen X, Wei Y (2017) N-Methylimidazole functionalized carboxymethycellulose-supported Pd catalyst and its applications in Suzuki cross-coupling reaction. Carbohydr Polym 160:106–114
Dong W, Zhang L, Wang C, Feng C, Shang N, Gao S et al (2016) Palladium nanoparticles embedded in metal–organic framework derived porous carbon: synthesis and application for efficient Suzuki–Miyaura coupling reactions. RSC Adv 6:37118–37123
Kwon TH, Cho KY, Baek K-Y, Yoon HG, Kim BM (2017) Recyclable palladium–graphene nanocomposite catalysts containing ionic polymers: efficient Suzuki coupling reactions. RSC Adv 7:11684–11690
Veisi H, Azadbakht R, Saeidifar F, Abdi MR (2017) Schiff base-functionalized multi walled carbon nano tubes to immobilization of palladium nanoparticles as heterogeneous and recyclable nanocatalyst for Suzuki reaction in aqueous media under mild conditions. Catal Lett 147:976–986
Hajighorbani M, Hekmati M (2016) Pd nanoparticles deposited on isoniazid grafted multi walled carbon nanotubes: synthesis, characterization and application for Suzuki reaction in aqueous media. RSC Adv 6:88916–88924
Zhang A, Liu M, Liu M, Xiao Y, Li Z, Chen J et al (2014) Homogeneous Pd nanoparticles produced in direct reactions: green synthesis, formation mechanism and catalysis properties. J Mater Chem A 2:1369–1374
Yu L, Han Z (2016) Palladium nanoparticles on polyaniline (Pd@ PANI): a practical catalyst for Suzuki cross-couplings. Mater Lett 184:312–314
Ji R, Zhai S-R, Meng Y-Y, Xiao Z-Y, An Q-D (2017) Deposition of N-doped carbon layers inside acidic ZrSBA-15: significant enhancement of catalytic performance of Pd NPs toward benzyl alcohol aerobic oxidation. J Sol-Gel Sci Technol 84:180–191
Li Y, Huang J, Hu X, Lam FL-Y, Wang W, Luque R (2016) Heterogeneous Pd catalyst for mild solventfree oxidation of benzyl alcohol. J Mol Catal A: Chem 425:61–67
Hao Y, Wang S, Sun Q, Shi L, Lu A-H (2015) Uniformly dispersed Pd nanoparticles on nitrogen-doped carbon nanospheres for aerobic benzyl alcohol oxidation. Chin J Catal 36:612–619
Dimitratos N, Villa A, Wang D, Porta F, Su D, Prati L (2006) Pd and Pt catalysts modified by alloying with Au in the selective oxidation of alcohols. J Catal 244:113–121
Marco Y, Roldán L, Armenise S, García-Bordejé E (2013) Support-induced oxidation state of catalytic Ru nanoparticles on carbon nanofibers that were doped with heteroatoms (O, N) for the decomposition of NH3. ChemCatChem 5:3829–3834
Puthiaraj P, Pitchumani K (2014) Palladium nanoparticles supported on triazine functionalised mesoporous covalent organic polymers as efficient catalysts for Mizoroki–Heck cross coupling reaction. Green Chem 16:4223–4233
Chang F, Guo J, Wu G, Liu L, Zhang M, He T et al (2015) Covalent triazine-based framework as an efficient catalyst support for ammonia decomposition. RSC Adv 5:3605–3610
Bell TE, Zhan G, Wu K, Zeng HC, Torrente-Murciano L (2017) Modification of ammonia decomposition activity of ruthenium nanoparticles by N-doping of CNT supports. Top Catal 60:1251–1259
Tessonnier J-P, Rosenthal D, Hansen TW, Hess C, Schuster ME, Blume R et al (2009) Analysis of the structure and chemical properties of some commercial carbon nanostructures. Carbon 47:1779–1798
Hao Y, Qingwen L, Jin Z, Zhongfan L (2003) The effect of hydrogen on the formation of nitrogen-doped carbon nanotubes via catalytic pyrolysis of acetonitrile. Chem Phys Lett 380:347–351
Wang J, Huang R, Feng Z, Liu H, Su D (2016) Multi-walled carbon nanotubes as a catalyst for gas-phase oxidation of ethanol to acetaldehyde. Chemsuschem 9:1820–1826
Abdullahi I, Davis TJ, Yun DM, Herrera JE (2014) Partial oxidation of ethanol to acetaldehyde over surface-modified single-walled carbon nanotubes. Appl Catal A Gen 469:8–17
Shinde VM, Skupien E, Makkee M (2015) Synthesis of highly dispersed Pd nanoparticles supported on multi-walled carbon nanotubes and their excellent catalytic performance for oxidation of benzyl alcohol. Catal Sci Technol 5:4144–4153
Wilson D, Langell M (2014) XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature. Appl Surf Sci 303:6–13
Acknowledgements
This research was financially supported by the National Research Foundation (NRF) South Africa, Grant Number 103979. We are grateful to the School of Chemistry and Physics, University of KwaZulu-Natal (UKZN), for creating a conducive research laboratory for this work. Ayomide is grateful to Mrs Rashidat Labulo and Dr Moses Ollengo for proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Labulo, A.H., Omondi, B. & Nyamori, V.O. Suzuki–Miyaura reaction and solventfree oxidation of benzyl alcohol by Pd/nitrogen-doped CNTs catalyst. J Mater Sci 53, 15817–15836 (2018). https://doi.org/10.1007/s10853-018-2748-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2748-8