Abstract
Magnesium oxide (MgO) whiskers (with diameters of about 60–80 nm) formed on the surface of bulk polycrystalline MgB2 superconductor at a relative low temperature (720 °C) during in situ sintering process. The reaction between Mg and B powders begins at a temperature below melting point of Mg and maintains till about 750 °C. The residual Mg powders evaporate and react with trace oxygen to form MgO vapor as the temperature exceeds the melting temperature of Mg and a low supersaturation is required for the growth of MgO whiskers. The preformed MgB2 and MgO crystals act as substrates and the melted Mg powders on the surface of them serve as catalysts during the growth process of MgO whiskers. The growth process of MgO whiskers is dominated by a self-catalytic vapor–liquid–solid (VLS) mechanism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The research of crystal whisker has already made a significant contribution to the field of crystal growth and strength of solids. Due to their small dimensions, surface perfection, and high elastic strength, whiskers are undoubtedly of continuing interest for researchers. As a member of whiskers, magnesium oxide (MgO) whiskers show a large superiority for their high melting temperature, low density and high modulus of rupture [1–3].
Magnesium oxide whiskers are important functional materials, which can be widely used in the preparation of superconductive and spaceflight composites to improve their superconducting and mechanical properties [4]. In view of their superiority, many techniques have been developed to prepare MgO whiskers in various forms [5, 6]. However, both the high reaction temperature (>1,200 °C) and high cost limit the practical application of these methods. Therefore, it is essential to develop a method with mild temperature, low cost, and easy control to prepare high-quality MgO whiskers.
Very recently, considerable efforts have been made to develop low-temperature methods and control crystal sizes and morphologies. Since the discovery of superconducting MgB2 with a transition temperature of 39 K has stimulated great interest [7], many groups have put efforts on studying the decomposition and oxidation process of the MgB2. Meanwhile, MgO whiskers with different morphologies have been reported [8], which provide a new route to synthesize MgO whiskers at low temperature.
In this work, we proposed a new method of synthesizing MgO whiskers on the surface of bulk MgB2 sample during in situ sintering process at a relatively low temperature (about 720 °C), without using bulk MgB2 as precursor [9]. The growth process of bulk MgB2 and MgO whiskers, as well as growth mechanism of MgO whiskers on the surface of bulk MgB2 were discussed.
Experimental
The stoichiometrical mixture of amorphous boron powder (with a purity of 99%, grain size <25 μm) and magnesium powder (with a purity of 99.5%, grain size <0.1 mm) was put into an agate mortar. After adequately grinding, the mixed powder was pressed into cylindrical pellet (ø4 mm × 1.5 mm) using a metallic mold. Then, the pellet was heated and analyzed simultaneously using a high-resolution Differential Thermal Analysis (Netzsch DSC 404C) apparatus, which was protected in the flowing argon (with a flow rate of 10 L h−1) atmosphere at the ambient pressure. The pellet was heated up to 720 °C with a rate of 20 K min−1 and then cooled down to room temperature at a rate of 40 K min−1.
The phase structures of the prepared specimens were identified by a Rigaku D/max 2500 X-ray diffractometer with Cu Ka radiation. The typical microstructure of the sintered specimen was observed by JSM-6700F field emission scanning electron microscope with accelerating voltage of 10 KV.
Results and discussion
Formation of MgB2 during in situ sintering
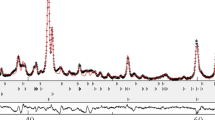
Figure 1 shows the measured DTA curve of the investigated sample, which is a mixture of Mg:B = 1:2 (dashed line). In order to make a comparison, pure Mg powder was also pressed into cylindrical pellet (ø4 mm × 1.5 mm) and then sintered in DTA apparatus. The heat flow signal of the specimen during heating was also recorded and illustrated in Fig. 1 (solid line). From these recorded curves, the reaction process of mixture of Mg:B = 1:2 can be identified distinctly. Reaction between Mg and B powder started at about 520 °C, and then an exothermic peak (peak 1 in Fig. 1) appeared until Mg melt at about 620 °C. The subsequent endothermic peak (peak 3 in Fig. 1), which is associated with the melting of Mg, is smaller than that of pure Mg powder (peak 4 in Fig. 1) and the corresponding peak value is prior to that of pure Mg simultaneously. This indicated that the reaction between Mg and B powders did not complete before Mg had melted, and the heat released from the combination reaction of Mg and B promoted the melt of residual Mg and partial offset of the decalescence of Mg melt. As residual Mg melted, part of them evaporated and the others reacted with residual B powder to form MgB2 till about 750 °C. The latter reaction between the melted Mg and B powder became stronger than the former as the flow of liquidus Mg not only increased the atomic diffusion rate, but also enlarged the contacting area of reactants.
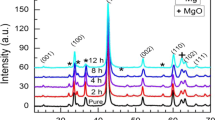
To identify the formation process of MgB2 phase, four different sintering temperatures, 530, 600, 650, and 720 °C, were selected according to successive heating DTA curve in Fig. 1. All samples were heated up to programmed temperatures with the same rate of 20 K min−1 and then cooled down immediately to room temperature with a rate of 40 K min−1. X-ray diffraction patterns of the four sintered samples obtained were illustrated in Fig. 2. It shows that the amount of MgB2 increased with raising the sintering temperature. However, the reactions between Mg and B powders at the temperature below and above the melting point of Mg were dominated by different mechanisms:
(1) Solid–solid reaction stage As the sample was sintered at a temperature below the melting point of Mg (530 or 600 °C), only a little amount of MgB2 formed in the sample, which could be identified from the X-ray diffraction patterns (see Fig. 2). The reaction between Mg and B powders at this stage belongs to a solid–solid reaction mechanism, which is controlled by interdiffusion of Mg and B atoms and the migrationrate of atoms is too slow to promote the growth of grain substantially at this stage.
(2) Solid–liquid–gas reaction mechanism When the sintering temperature increased up to 650 °C (the melting point of Mg), the amount of MgB2 increased remarkably for the reason that the molten Mg accelerated the atomic diffusion rate and enlarged the contacting area of reactants. The reaction between Mg and B powders belonged to a solid–liquid–gas reaction mechanism at this stage and the reaction would maintain till about 750 °C. Finally, polycrystalline MgB2 grains with regular hexagonal morphologies formed (see Fig. 3).
Besides, MgO with different morphologies formed accompanying MgB2 during the whole sintering process. Details about formation process and growth mechanism of MgO are discussed in below.
Formation of MgO with different morphologies
As seen from X-ray diffraction patterns in Fig. 2, the amount of MgO rose with the increase in sintering temperature for the reason that more and more Mg powders were oxidized. Since all starting materials are powders, they may contain a moderate amount of oxygen which cannot be completely degassed. Additionally, there might be other resources to introduce oxygen: (1) the desorption of oxygen physically adsorbed on the surfaces of Mg and B powders, as well as that in the alumina crucible; and (2) the leakage of air into the reaction system through joints. Wherever the oxygen comes from, its partial pressure has to be kept as low as possible to ensure a relatively low supersaturation of MgO vapor generated by reaction between the Mg vapor and trace O2, which is crucial to the formation of MgO whiskers.
At the initial stage of reaction between the Mg and B powders (around 530 °C), there was almost no MgO formed that could be detected by X-ray diffraction analysis (as indicated in Fig. 2(a)). As the temperature increased up to 600 °C, only a small amount of MgO was detected (as seen in Fig. 2(b)), which reveals that the oxidation of Mg powders was not fierce before Mg was melted.
When the temperature exceeded the melting point of pure Mg, the residual Mg powders began to melt and part of them evaporated to generate Mg vapor partial pressure. At this stage, the molten Mg not only reacted with the residual B to form MgB2 phase unceasingly, but also trace oxygen to form crystalline MgO phase. The formed crystalline MgB2 and MgO phases would act as substrate materials for the further growth of MgO whisker. Meanwhile, the Mg vapor reacted with trace oxygen in the system and formed MgO vapor. When MgO vapor is in the state of supersaturation (about 720 °C), it condensed onto the surface of solid substrates (MgB2 and MgO grains, Region A in Fig. 4(a)) and grew into highly anisotropic MgO whiskers (Region B in Fig. 4(a)). As seen from the high-magnification image of Region B in Fig. 4(a), MgO whiskers had diameters of about 60–80 nm and the tips were all foursquare (as seen in Fig. 4(b)), which present the facets of MgO crystalline plane. Furthermore, the EDS analysis result indicated that the tip of whisker was composed of the elements Mg and O and the atomic ratio of Mg:O is about 1:1 (as seen in Fig. 4(c)), which proved that the whiskers are pure MgO.
(a) SEM image of MgO whiskers formed on the surface of bulk polycrystalline MgB2 samples, where the MgO vapor reached a low supersaturation, at a temperature of 720 °C. (b) High-magnification image of Region B in (a), clearly visible small square particles at the tips of whiskers. (c) EDS spectrum of the end of whisker
Generally speaking, the mechanism for a growing crystal is mainly determined by the internal structure of given crystal, and affected by outer conditions such as temperature and supersaturation state. Namely, the final morphology is dependent on the intrinsic crystalline structure and outer growth conditions. For whisker growth, the control of supersaturation state is a prime consideration because there is good evidence that the degree of supersaturation determines the prevailing growth morphology [10]. A low supersaturation is required for the whisker growth whereas a medium supersaturation supports the growth of bulk crystals. At high supersaturation, powders are formed by homogeneous nucleation in the gas state. Under less supersaturation, however, 1D structure (such as whisker) is easy to grow (cf. Fig. 4(b)). This result is similar to that of a previous work that focused on the growth of Ga2O3, in which a low level of oxygen supersaturation facilitated the growth of thin 1D nanomaterials [11]. When the supersaturation is high enough, large saturation may activate secondary growth sites and heterogeneous nucleation on the side of the 1D structure, tending to produce thicker structure. Figure 5 illustrates the microstructure of the surface of pellet, which was sintered at 720 °C. It is found that a spot of bulk MgO crystal formed in the middle of the MgO whiskers indicated that the supersaturation of MgO was not homogeneous on the surface of the prepared pellet. From a thermodynamic point of view, the minimization of Gibbs energy suggested that the morphology assumed by a growing crystal is determined by the facets with minimum surface free energy and therefore cubic MgO tends to grow into a cubic structure (see Fig. 5).
Growth mechanism of MgO whisker
There are two widely accepted conventional mechanisms for the growth of whisker, viz. the vapor–liquid–solid (VLS) and the vapor–solid (VS) mechanisms. The conventional VLS mechanism is a catalyst-assisted process, in which the metallic particles act as metal catalysts presenting on the end of the whiskers. Generally, the noble metals and transition metals, such as Au, Fe, Co, and Ni, have proved to be effective catalysts to direct the VLS growth of 1D nanostructures including Si, GaAs, GaN, and so on [12–15]. Metals In, Sn, and Ga are also used as catalysts to grow GaN, ZnO, Si, and SiO2 1D nanostructures in VLS growth mechanism [16–20]. The selected catalysts mentioned above are all foreign metal elements for the 1D nanostructure materials and form the liquid eutectic alloy with 1D nanostructure materials under the growth temperature, which are basically required for conventional VLS mechanism. Though the growth of MgO nanowhiskers with the small particles capping the end was regarded as a typical sign of VLS process [21], no evidence has been given to support it.
Identifying from the Mg–MgO phase diagram (see Fig. 6) [22], no liquid eutectic alloy can be formed in the Mg–MgO system at the temperature above the melting point of Mg (about 650 °C). Only liquid Mg, which is one of the components of MgO whisker materials, can function as catalyst to prompt the growth of the MgO whiskers. As no external catalyst was introduced into the growth of MgO whiskers, the growth of the MgO whiskers in this work should not be attributed to a conventional VLS mechanism but a self-catalytic VLS process. The so-called self-catalytic growth was first reported in Ref. [23] and then some strong evidences for the mechanism were given in Refs. [24, 25] via observation of the growth of GaN and ZnO 1D nanostructures in Ga and Zn liquid droplets.
Partial phase diagram of Mg–MgO system [22]
In this work, however, there is a different view on the self-catalytic growth process of MgO whiskers from Ref. [23], in which the effects of formed MgO vapor on the growth of MgO whiskers were neglected. The growth of MgO whiskers was not only carried out by the absorption of oxygen in the inset flow, but also the deposition of the MgO vapor. A low supersaturation of MgO vapor is the crucial factor in the formation of MgO whiskers as described above. Referred to conventional VLS mechanism [26], a schematic illustration of the growth of MgO whisker by self-catalytic VLS mechanism was proposed in Fig. 7. It can be divided into three main stages: (1) nucleation, (2) deposition, and (3) axial growth. At the nucleation stage, the residual Mg particles on the surface of MgB2 substrates melted and acted as metal catalysts as the temperature increased till 650 °C. The molten Mg reacted with trace oxygen to form MgO nuclei at the liquid–solid interface. These sites are important because they would serve as the preferred sites for the further absorption of O2 and deposition of MgO whiskers. As MgO vapor reached its critical supersaturation with increasing temperature, the MgO vapor diffused through the fused Mg catalysts and precipitated onto the site of MgO nucleus at the liquid–solid interface. Then the anisotropic growth of MgO whiskers that occurred at only where the liquid metallic catalyst was present as the sticking coefficient is higher on liquid than solid surfaces. The fused Mg catalysts ultimately reacted with oxygen to form square MgO crown at the tip of MgO whiskers, which would cease the growth of the whiskers.
Conclusion
Magnesium oxide whiskers could be obtained on the surface of bulk polycrystalline MgB2 at a low temperature during in situ sintering process. The growth process of MgO whiskers is dominated by self-catalytic VLS mechanism. The formation and growth process could be divided into four steps: (1) Mg and B powders begin to react with each other to form polycrystalline MgB2 phase at a temperature below the melting point of pure Mg (650 °C) and the process will remain until about 750 °C along with the oxidation of Mg powders during the whole sintering process; (2) the preformed MgB2 and MgO crystals will act as substrates and the molten Mg powders on their surface will be taken as catalysts for the absorption of O2 and the deposition of MgO whiskers; (3) As the temperature exceeds the melting point of pure Mg, the residual Mg powders begin to evaporate and react with trace oxygen to form MgO vapor; and (4) when MgO vapor reached to a low supersaturation state, it condensed onto the surface of a solid substrate placed next to the MgB2 powders and grew into highly anisotropic MgO whiskers.
References
Hkanson DF, Hoover SM, Crowe CR (1985) J Mater Sci 20:4147. doi: https://doi.org/10.1007/BF00552410
Kondilis P, Mousdis G, Kordas G (1994) Phys C Supercond 235:467
Yuan YS, Wong MS, Wang SS (1995) Phys C Supercond 250:247
Yuan YS, Wong MS, Wang SS (1996) J Mater Res 11:1373
Kordas G (2000) J Mater Chem 10:1157
Saitoh H, Okada Y, Ohshio S (2002) J Mater Sci 37:4597. doi: https://doi.org/10.1023/A:1020696215411
Nagamatsu J, Nakagawa N, Muranaka T, Zenitani Y, Akimitsu J (2001) Nature 410:63
Delin Y, Hongwei S, Hongxia L et al (2003) Supercond Sci Technol 16:576–581
Yadong Y, Guangtao Z, Younan X (2002) Adv Funct Mater 12:293–298
Levitt AP (1997) Whisker technology, army materials and mechanics research center Watertown. Massachusetts, New York, London, p 37
Lee JS, Park K, Nahm S, Kim SW, Kim S (2002) J Cryst Growth 244:287
Duan XF, Lieber CM (2000) Adv Mater 12:298
Chen XL, Li JY, Cao YG, Lan YC et al (2000) Adv Mater 12:1432
Cui Y, Laucoln LJ, Gudiksen MS et al (2001) Appl Phys Lett 78:2214
Chen CC, Yeh CC, Chen CH et al (2001) J Am Chem Soc 123:2791
Chen CC, Yeh CC (2000) Adv Mater 12:738
Gao PX, Wang ZL (2003) Nano Lett 3:1315
Ding Y, Gao PX, Wang ZL (2004) J Am Chem Soc 126:2066
Pan ZW, Dai ZR, Ma C, Wang ZL (2002) J Am Chem Soc 124:1817
Sunkara MK, Sharma S, Miranda R et al (2001) Appl Phys Lett 79:1546
Chen YJ, Li JB, Han YS, Yang XZ, Dai JH (2002) J Cryst Growth 245:163–170
Bakulina VM, Tokareva SA, Latysheva EI (1970) J Struct Chem 11(1):150–151
Dang HY, Wang J, Fan SS (2003) Nanotechnology 14:738
Stach EA, Pauzauskie PJ, Kuykendall T et al (2003) Nano Lett 3:867
Jian JK, Wang C, Zhang ZH et al (2006) Mater Lett 60:3809
Wu Y, Yang P (2001) J Am Chem Soc 123:3165
Acknowledgements
The authors would like to thank the National Nature Science Foundation of China (granted No. 50401003), the Natural Science Foundation of Tianjin City (granted No. 07JCZDJC01200), the Keygrant Project of Chinese Ministry of Education (granted No. 707012) and Program for New Century Excellent Talents in University for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, Q., Liu, Y., Gao, Z. et al. Formation of MgO whiskers on the surface of bulk MgB2 superconductors during in situ sintering. J Mater Sci 43, 1438–1443 (2008). https://doi.org/10.1007/s10853-007-2306-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-2306-2