Abstract
Ex situ and in situ bulk MgB2 superconducting materials have successfully been produced by the simple amorphous boron and nano-amorphous boron powders with the processes of ball milling, pressing, and annealing. The superconducting properties and diffusion behavior of MgB2 samples after nickel (Ni) coating process have been characterized and compared to the microstructure and performance of uncoated (bare) MgB2 bulk sample. One surface of MgB2 superconductor sample was coated with a thin Ni layer of about 50 − 60μm thickness using vapor deposition techniques in versatile high vacuum coater, then every sample was annealed at temperatures between 923 and 1123 K for 1 h. The role of annealing temperature on physical, electrical, superconducting, and structural characterizations of bare and Ni-coated ex situ/in situ MgB2 bulk superconductors has been studied using X-ray diffraction and dc electrical resistivity versus temperature measurements. Finally, the Ni diffusion coefficients (DNi = D0exp(E/kBT)) are calculated in the temperature range to calculate the required minimum activation energy value for the Ni atoms/ions into the ex situ MgB2 crystal structure for the first time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Superconductivity is directly related to the electrical resistance of a material and when the temperature is below a certain value (known as the critical transition temperature), the electrical resistance value is completely 0. The superconductivity phenomenon was experimentally discovered by H. K. Onnes in 1911 and initial studies began with resistance measurements of pure metals (lead, gold, etc.) at the liquid helium temperature [1, 2]. Since the discovery of the phenomenon, many metals and alloys have been examined in detail and observed to have superconducting properties at a low temperature range of 20 − 30 K. In 1953, Jones et al. [3] and Russell et al. [4] reported that the MgB2 phase was chemically formed by the interaction of Mg and B, and the superconducting properties were discovered in 2001 by Akumutsu et al. [5]. The ex situ and in situ methods in the superconducting material preparation techniques take the first place to survey the fundamental characteristic properties such as the micro- and macrostructural properties [6], mechanical performance behaviors [7], and superconductivity properties of materials [8, 9].
In order to improve the above properties mentioned for the MgB2 material, a number of techniques as regards nanosize chemical addition [10, 11], substitution [12, 13], evaporation [14, 15], and changing preparation conditions [16, 17] have widely been used in the literature. Among the studies, the serious enhancements in the characteristic features of Bc2 and in-field Jc parameters of MgB2 were observed with the aid of Ni nanoparticles inserted in the MgB2 crystal structure [18, 19]. Similarly, in the literature, it is possible to encounter the positive effects of Ni inclusions on the dc electrical resistance, superconductivity, bulk density, energy barrier formation, texturing, crystallinity, phase volume fraction, surface morphology, vortex lattice period, flux pinning strength, microstructural [20, 21], grain boundary coupling problem, connection between the superconducting grains, key design mechanical performance, and mechanical characteristic properties of Bi-containing superconducting materials [22, 23].
Besides, it was noted that the existence of Ni nanoparticles in the crystal core decreased the structural problems including the grain misorientations, omnipresent flaws (stress raisers/crack initiation sites), lattice distortions/strains, porosity, cracks, voids, and defects (point, line, planar, or bulk) in the superconducting crystal structure. Thus, the penetration of optimum Ni additives into the superconducting system strengthens remarkably the mechanical durability and ideal flexural strength due to the stabilization of durable tetragonal phase. In this respect, we examine extensively the role of Ni inclusions embedded in the MgB2 superconducting crystal lattice on the main characteristic features to be able to present some beneficial effects of Ni coating so that a new MgB2 matrix with the improved features can widely be used in much more application fields such as the application-oriented material science and technological [24, 25], engineering, industrial, magnetic energy storage, and large-scale areas [26, 27]. Moreover, it is to be mentioned here that to the best of our knowledge so far there has been a few researches on the phase expansion of metal doped/coated Mg-B alloy, but no detailed information on the formation process of secondary (foreign) phases in the crystal structure, diffusivity mechanism of coating metal (especially Ni metal ions), and related characteristic properties.
In our research for this paper, the significant changes in the structural and electrical properties of ex situ and in situ MgB2 polycrystalline superconducting materials prepared at the temperature range of 923 − 1123 K for the duration of 1 h with the nickel coating process are investigated by means of powder X-ray diffraction and dc electrical resistivity versus temperature measurements. Moreover, we determine the diffusion coefficients of Ni ions for the ex situ MgB2 superconducting samples using the lattice cell parameters and Fick’s law [28]. At the same time, the temperature-dependent diffusion coefficient, being interested in the relation between the Ni diffusion coefficient and annealing temperature, is computed by use of the diffusivity rates determined. Also, the required minimum activation energy (Q) for the diffusion of Ni impurities into the ex situ bulk MgB2 crystal structure is calculated by the Arrhenius equation, being one of the most striking point deduced from this work [29].
2 Experimental Details
Ex situ and in situ MgB2 superconducting samples were produced by using the solid-state reaction method. The commercially available MgB2 ex situ powder with 99% purity was mixed homogeneously in argon (Ar) gas atmosphere with agate ball milling for 3 h. Throughout the mixing, the agate ball-powder mass ratio was determined to be 4:1. After mixing process of powders, we performed manual mixing in an agate mortar inserted in the glow box at Ar gas atmosphere to obtain a completely homogeneous mixture of powders. All the ex situ and in situ MgB2 samples were handled without exposure to air in a little glove box under Ar gas. The in situ MgB2 superconducting bulks were prepared from the magnesium (Mg) powder (PVZ Atomized Magnesium, purity 99%, particle sizes ∼ 74 − 149μm), amorphous boron (B) powder (PVZ Boron, purity 95 − 97%, particle sizes < 1μm), and amorphous nano-B (PVZ Nano Boron, purity 99%, particle sizes < 250nm) obtained from Pavezyum Chemicals, Turkey [30]. The mixture powders are then pressed into the rectangular bars of 25×4×2mm3 by using a hydraulic press with an applied load of 400 MPa at room temperature.
MgB2 superconducting bulks are coated by the evaporation of Ni metal using EDWARDS-AUTO 306 under the 10− 4 Pa vacuum condition. An about 50 − 60μm thick coated layer was deposited by the evaporation process of Ni (a 15-cm long Ni wire with a diameter of 0.1 mm and weight of 0.01 g) only on one of the wide faces of ex situ and in situ bulk samples. Heat treatments of bulks exposed to the Ni coating are carried out for the duration of 1 h at five different annealing temperature values such as 923 K, 973 K, 1023 K, 1073 K, and 1123 K under high-purity Ar gas atmosphere of 5 − 10 bars. The Ni-coated ex situ MgB2 superconducting samples were named to be ENi923, ENi973, ENi1023, ENi1073, and ENi1123 according to the annealing temperatures. In the same way, the Ni-coated in situ MgB2 superconducting samples produced at the same conditions are presented as INi923, INi973, INi1023, INi1073, and INi1123.
The first part of the current study investigates the effects of the Ni coating process on superconducting and structural properties of MgB2 superconducting bulks. We record the experimental resistivity temperature curves in the temperature range of 20 − 45 K with the aid of the four-point contact method by using Helium closed-cycle cryostat system (CRYO Industries) with the provision for the vacuum. Powder X-ray diffraction patterns of ex situ and in situ Ni-coated MgB2 samples were obtained by a Rigaku Multiflex+XRD in the range 2𝜃 = 10 − 90∘ at a scan speed of 5∘ min− 1 and step increment of 0.02∘ at room temperature. At the second part, the experimental measurements were performed in eight steps by removing the thin layers of about 20 − 25μm (totally a depth of 195 − 205μm) from the Ni-coated surface of ex situ MgB2 samples to determine the differentiation of lattice constant parameter c values.
3 Result and Discussion
3.1 X-Ray Diffraction Examination
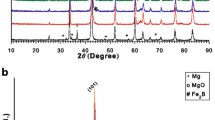
Before the X-ray diffraction analyses of in situ and ex situ MgB2 samples, the Al2O3 substrates were coated four times to verify the Ni coating with a thickness range of 50 − 60μm on the surface. One can see the X-ray diffraction patterns for the Ni-coated Al2O3 substrate and MgB2 materials in Fig. 1.
According to the characteristic diffraction peaks in the XRD diffractograms, the Al2O3 substrate presents three main peaks at two angles of 14∘, 21∘, and 42∘ [6]. The XRD patterns of multi-coated Ni ions on the Al2O3 substrates were examined at a range of 2𝜃 = 10 − 90∘, and the main peak of Ni thin film was at 2𝜃 = 44.5∘ in the (111) plane. In this way, it has been confirmed that the Ni thin film coating in the homogeneous and adjustable thickness can be achieved on a desired surface by using a vapor deposition technique in the versatile high vacuum coater. Since the thickness of each Ni-coated Al2O3 substrate is about 50μm, it is calculated that the second step is 100μm, the third step is 150μm, and the last one is about 200μm. As illustrated in Fig. 1, no changes in the X-ray diffraction patterns are observed in the highly dense Ni coatings with a uniform deposit thickness of around 50 and 200μm.
The XRD results of ex situ and in situ MgB2 superconducting bulk samples (pre/post annealing and Ni coating) are shown in Fig. 2a and b. As shown in Fig. 2a, non-annealing/bare (ExBr-NonAnnealing), non-annealing/Ni-coated (ExNi-NonAnnealing), annealing/bare (ExBr-Annealing), and annealing/Ni-coated (ExNi-Annealing) ex situ MgB2 bulk samples were drawn with black, red, blue, and pink lines, respectively. In more detail, the commercial MgB2 powder (without any heat treatment process) gives its own main phase in the angle of 2𝜃 = 42.5∘ (black line in Fig. 2a) and characteristic peak of free (unreacted) Mg atoms.
The commercial MgB2 powder was pressed by load to form in the bulk shape, one surface of which was exposed to the Ni coating process. One can encounter in Fig. 2a (red line) that the characteristic Ni peak appears at 44.5∘ (marked with a star) in the diffraction patterns of material. After the ex situ MgB2 sample was annealed at 1073 K for 1 h, the peaks belonging to the Mg2Ni metal alloy [31] were found to emerge at 20∘, 40∘, and 45∘ in the Ni-coated MgB2 sample (ExNi-Annealing). The presence of Ni particles in the coated samples produced at different heat treatment conditions (at 923, 973, 1023, 1073, and 1123K for 1 h) was determined within the coating thickness of 50 − 60μm. This clearly shows that the ex situ Ni-coated MgB2 bulk samples are sufficiently homogeneous, and the atoms/ions of Ni metal are diffused into the MgB2 crystal structure because of the metal–metal alloy with Ni and Mg. The XRD experimental measurements produced that only the c-axis parameters of MgB2 lattice drop in change (inclusive of the experimental error) upon the Ni coating.
Similar to Fig. 2a, the X-ray diffraction patterns of in situ MgB2 samples are shown in Fig. 2b, where non-annealing/bare (InBr-NonAnnealing), non-annealing/Ni-coated (InNi-NonAnnealing), annealing/bare (InBr-Annealing), and annealing/Ni-coated (InNi-Annealing) in situ MgB2 bulk samples were drawn with black, red, blue, and pink lines, respectively. Unlike the commercial MgB2 powder, only the atoms belonging to Mg and B in the X-ray diffraction patterns of non-annealing/bare in situ MgB2 samples were detected as given in Fig. 2b (black and red lines). While the Mg and B may probably originate from the initial in situ MgB2 powder, the MgB2 phase formation is due to heat treatment, as seen in (blue line) Fig. 2b. As observed in the ex situ MgB2 samples, in situ MgB2 samples also had a Mg-Ni alloy between Mg and Ni with the coating after it was annealed at 1073 K for 1 h. From both the XRD patterns of MgB2 samples, it was observed that the predominant phase was composed of MgB2 phase and there was a minor amount of the impurity of MgO marked with x. The Ni coatings are closely integrated with the in situ MgB2 bulk samples. We used Debye-Scherrer method [32] to calculate the lattice parameters (a and c) of the ex situ and in situ bulks, and the values of all lattice parameters are numerically tabulated in Table 1. The crystallite size of the ex situ and in situ MgB2 samples is found to be within the angstrom scale by using the Scherrer equation [33]. It is found that the Ni-coated MgB2 samples sintered at 1123 K for 1 h had the broadest grain size (292.05 Å) and the differences between Ni-coated and bare samples are visible.
The lattice parameter a of Ni-coated and bare ex situ MgB2 samples sintered at all the temperatures is approximately around 3.08 Å. Nevertheless, the lattice parameter c of ex situ samples varies between 3.529 and 3.543 Å; here, only Ni coated ex situ MgB2 samples exhibit a bit larger c parameter. It can be mentioned here that the Ni atoms migrate to the crystal matrix of MgB2 and make the metal–metal alloys (Mg2Ni) with the free Mg atoms in the structure causing lattice parameter c to amplify. The changes in the lattice cell and particle size parameters of all the (Ni-coated and bare) in situ MgB2 bulk samples are not systematic with annealing temperatures in comparison with those of ex situ MgB2 bulk samples. At the same time, the bulk densities of in situ MgB2 samples are found to be smaller than those of ex situ MgB2 samples.
3.2 Experimental Electrical Resistivity Measurement Results
In this part of work, we analyzed the influence of Ni ions embedded in the ex situ and in situ MgB2 crystal structures via the variation of dc electrical resistivity values versus the temperature between 25 and 45 K. The experimental electrical resistivity results measured (annealed temperature at 1073 K/1 h) are graphically represented in Fig. 3. The critical temperatures ascertained from the curves are found to be about 38.42 K, 37.97 K, 38.27 K, and 37.36 K for the Ex-Bare, ENi1073, In-Bare, and INi1073 samples, respectively. As illustrated in the inset of Fig. 3, the conductivity is directly related to the annealing temperature values of the samples. It is apparent from the figure that the critical transition temperature is observed to slightly decrease for the ex situ and in situ Ni-coated MgB2 samples as compared to that of bare samples. It is clear that the electrical resistance values of Ni-coated samples decrease suddenly as a result of differentiation in the conducting mechanism in the MgB2 crystal system. The electrical and superconducting characteristic parameters as regards the \(T_{\mathrm {c}}^{\text {offset}}\), ΔT, Tc, and ρ300K values of bare and Ni-coated MgB2 samples are given in Table 2.
It is visible from the table that the Ex-Bare sample has the highest Tc value among the ex situ MgB2 samples while the ENi1123 sample exhibits the lowest Tc value. However, the In-Bare bulk material obtains the highest Tc value whereas the INi1123 sample presents the smallest Tc value among the in situ MgB2 samples. The \(T_{\mathrm {c}}^{\text {offset}}\) of Ex-Bare sample is equal to \(T_{\mathrm {c}}^{\text {offset}}\) value of In-Bare sample indicating the same current-carrying capability under the same temperature conditions. Accordingly, it can be nature to confirm that the Ni coating plays a little negative role on the critical transition temperature parameters for the MgB2 materials. Moreover, according to the ρ −T curves in the figure, the In-Bare sample with the smallest resistivity at 40 K and room temperature (ρ300K = 41.4μΩcm) exhibits a sharp superconducting transition width of ΔT = 0.56K. However, the presence of Ni ions in the MgB2 crystal structure leads to slight increase of not only the resistivity values, but also of the superconducting transition width. It is necessary to underline that the negative effects arise from the formation of Mg2Ni phase in the MgB2 crystal matrix. Furthermore, the other parameters such as the sintering temperature, sintering duration, coating material (Ni metal), and intra-interface interaction may affect the superconducting properties (Tc, Jc, \(T_{\mathrm {c}}^{\text {onset}}\), \(T_{\mathrm {c}}^{\text {offset}}\), etc.).
3.3 Calculation of Diffusion Coefficient and Activation Energy of Ni-Coated Ex Situ MgB2 Samples
In this part of study, we examine the diffusion coefficient (DNi) and activation energy values of nickel metal ions in the ex situ MgB2 superconductors at the sintering temperature range of 923 − 1123 K while the in situ MgB2 samples are not suitable to make the diffusion coefficient calculations due to the densities and coated surface smoothness problems. Active diffusivity in the solid material [34] has to be considered together with the porous microstructure [35] for the determination of DNi and D0. As it is well known that the measurements of lattice parameters from the XRD patterns and energy-dispersive X-ray fluorescence (EDXRF) [22, 36] after the successive removal of thin layers from the specimen surface are the most commonly used methods for the calculation of diffusion fast-rate (coefficient) in the superconductor materials. In the present study, we used the XRD method to describe the diffusion fast-rate of Ni ions into the ex situ MgB2 samples. Throughout the study, we defined the changes in lattice cell parameter c values after the successive removal of thin layers (∼ 25μm) from the coated surfaces. One can encounter the changes of lattice constant parameter Δc/c0 (c0 is the lattice parameter of the Ex-Bare sample) with respect to the depths of the superconducting samples in Fig. 4.
It is received that the Ni diffusion is transported from a fixed source to a semi-infinite solid [37], given by the following formula:
here, the diffusion length of \(2\sqrt {D t}\) and \(\text {erf}(x/2\sqrt {Dt})\) represent the error function \(y=x/2\sqrt {Dt}\)
where N0 = N(0,t) is the constant concentration on the sample surface, N(x,t) refers to the impurity concentration at the distance x from the surface, D expresses the diffusion coefficient, and t is the diffusion sintering duration. The diffusion coefficients were calculated with the assistant of fitting method for the Ni-coated materials sintered at five different temperatures between 923 and 1123 K, and the calculations were numerically listed in the inset of Fig. 4. It is apparent from the figure that the Ni diffusivity in the MgB2 superconductor bulk samples is directly related to the diffusion sintering temperature. For all the ex situ MgB2 superconducting bulk samples produced in this study, the lattice cell parameter c initially decreases rapidly with the thin-layer removal thickness (ranging from 25 to 200 μm) from the coated surfaces. This is attributed to the fact that the diffusion of Ni impurities into the MgB2 sample happens through the defects and grain boundaries. As for the numerical parameters, the diffusion coefficient is found to increase systematically from 4.1578× 10− 10 to 7.1186× 10− 9cm2/s with the rise of the annealing temperature from 923 to 1123 K. The practical consequence based on the findings is that the Ni coating is effective for the MgB2 material at a surface area of about 100–125 μm.
The experimental results show that the DNi diffusion coefficients into the MgB2 at temperatures between 923 and 1123 K can be expressed by Arrhenius relationship as can be seen in Fig. 5 to calculate the temperature-dependent Ni diffusion rate and required minimum activation energy (Q) for the diffusion of Ni impurities into the ex situ bulk MgB2 crystal structure. In this regard, based on the Arrhenius equation, the temperature-dependent Ni diffusion rate is calculated to be about 2.6785 × 10− 9 exp(1.013eV) at the surface of ex situ bulk MgB2 superconducting material for the first time. The result of temperature-dependent Ni diffusion rate relies on the fact that the minimum activation energy value is 1.013 eV for the diffusion of transition metal Ni atoms/ions into the bulk MgB2 crystal lattice. According to the combination of low temperature-dependent DNi and Q values, this may be concerned to the fact that the Ni atoms/ions migrate through the lattice defects.
4 Conclusions
Bulk superconducting ex situ and in situ MgB2 samples have successfully been prepared from Mg, B, and MgB2 powders by ball milling at the room temperature. We have found that the Tc and ρ300K values decrease with the Ni coating on the surfaces of MgB2 bulk samples. Conversely, it is noted that the ρ300K parameters tend to increase with the enhancement in the sintering temperature of Ni-coated samples. Likewise, the lattice cell c and grain size parameters are observed to be larger for the Ni-diffused samples as compared to those of bare samples. Additionally, the Ni diffusion into ex situ bulk MgB2 superconducting materials is examined by the changes in lattice constant parameter (Δc/c0) along with the depth profile. It is found that the variation of Δc/c0 decreases with the increment in the thin-layer removal thickness from the coated surface. The diffusion coefficients calculated are obtained to increase monotonously from 4.1578 × 10− 10 to 7.1186 × 10− 9cm2/s with the enhancement of sintering temperature. Similarly, the required minimum activation energy value for the Ni atoms/ions into the ex situ MgB2 crystal structure is determined by means of the Arrhenius equation. The results illustrate that the temperature-dependent Ni diffusion rate is noted to be DNi = 2.6785 × 10− 9 exp(1.013eV/kBT) of Ni atoms/ions at the surface of the ex situ bulk MgB2 superconducting material for the first time. As a conclusion, the MgB2 superconducting samples with high and convenient performances can be produced by sintering at nominal temperatures for extended duration time provided that the other parameters of production process are optimized.
References
Onnes, H.K.: Commun. Phys. Lab. Univ. Leiden 12, 120 (1911)
Onnes, H.K.: Proceedings of the KNAW 13, 1910 (1911)
Jones, M.E., Marsh, R.E.: J. Am. Chem. Soc 76, 870 (1953)
Russell, V., Hirst, R., Kanda, F., King, A.J.: Acta Crystallogr. 6, 870 (1953)
Nagamatsu, J., Nakagawa, N., Muranaka, T., Zenitani, Y., Akimitsu, J.: Nature 410, 63 (2001)
Ulgen, A.T.: J. BAUN Inst. Sci. Technol. 19(3), 121 (2017)
Karaboga, F., Ulgen, A.T., Yetis, H., Akdogan, M., Pakdil, M., Belenli, I.: Mater. Sci. Eng. A. 721, 89 (2018)
Ertekin, E., Gecer, S., Yanmaz, E., et al.: J. Supercond. Nov. Magn. 30, 3549 (2017)
Al, H., Aksu, E., Gencer, A.: J. Supercond. Nov. Magn. 30, 2735 (2017)
Tan, K.Y., Tan, K.L., Tan, K.B., et al.: J. Supercond. Nov. Magn. 24, 2025 (2011)
Ma, Z., Liu, Y.C., Hu, W.P., Gao, Z.M., Yu, L.M., Dong, Z.Z.: Scr. Mater. 61, 836 (2009)
Erdem, O., Abdioglu, M., Guner, S.B., Celik, S., Kucukomeroglu, T.: J. Alloys. Compds. 727, 1213 (2017)
Alghamdi, F.S., Shahabuddin, M., Alzayed, N.S., et al.: J. Supercond. Nov. Magn. 31, 1119 (2018)
Ulgen, A.T., Belenli, I.: J. Supercond. Nov. Magn. 30, 3367 (2017)
Dogruer, M., Yildirim, G., Ozturk, O., et al.: J. Supercond. Nov. Magn. 26, 101 (2013)
Olutaş, M., Kiliç, A., Kiliç, K., et al.: J. Supercond. Nov. Magn. 25, 753 (2012)
Drozd, V.A., Gabovich, A.M., Gierlowski, P., Pekala, M., Szymczak, H.: Phys. C 402, 325 (2004)
Novosel, N., Galic, S., Pajic, D., Skoko, Z., Loncarek, I., Mustapic, M., Zadro, K., Babic, E.: Supercond. Sci. Technol. 26, 105024 (2013)
Guner, S.B., Zalaoglu, Y., Turgay, T., Ozyurt, O., Ulgen, A.T., Dogruer, M., Yildirim, G.: J. Alloys Comp. 772, 388–398 (2019)
Al, H.: J. Mater. Sci. Mater. Electron. 29(19), 16157–16165 (2018)
Terzioglu, R., Aydin, G., Soylu Koc, N., et al.: J. Mater. Sci. Mater. Electron. https://doi.org/10.1007/s10854-018-0497-8 (2018)
Zalaoglu, Y., Terzioglu, C., Turgay, T., Yildirim, G.: J. Mater. Sci: Mater. Electron. 29, 3239 (2018)
Yildirim, G.: J. Alloy. Compd. 745, 100 (2018)
Buckel, W., Kleiner, R.: Superconductivity: Fundamentals and Applications, 2nd edn. Wiley-VCH Verlag, Weinheim (2004)
Xu, H.H., Cheng, L., Yan, S.B., Yu, D.J., Guo, L.S., Yao, X.: J. Appl. Phys. 111, 103910 (2012)
Yildirim, G.: J. Alloy. Compd. 699, 247–255 (2017)
Werfel, F.N., Floegel-Delor, U., Rothfeld, R., Riedel, T., Goebel, B., Wippich, D., Schirrmeister, P.: Supercond. Sci. Technol. 25, 014007 (2012)
Fick, A.: Ueber diffusion. Ann. Phys. 170(1), 59 (1855)
Arrhenius, S.: Zeitschrift fur physikalische Chemie 4(1), 96 (1889)
Faraboa, F., Yetiş, H., Akdoan, M., et al.: J. Supercond. Nov. Magn. 31, 1359 (2018)
Chen, X.J., Xia, T.D., Liu, X.L., et al.: J. Alloys Comput. 426, 123 (2006)
Taylor, A., Sinclair, H.: Proc. Phys. Soc. 57(2), 126 (1945)
Patterson, A.L.: Phys. Rev. 56(10), 978 (1939)
Heitjans, P., Kärger, J. (eds.): Diffusion in Condensed Matter—Methods, Materials, Models. Springer, Berlin (2005)
Grathwohl, P.: Diffusion in Natural Porous Media, vol. 1. Springer Science and Business Media, Berlin (2012)
Dogan, O., Ertugrul, M., Cevik, U., Bacaksiz, E., Tirasoglu, E., Kobya, A.I., Erdogan, H.: X-Ray Spectrom. 32, 363 (2003)
Abdullaev, G.B., Dzhafarov, T.D.: Atomic Diffusion in Semiconductor Structures, 2nd edn. Harwood (1987)
Funding
This work is supported by the Scientific and Technological Research Council of Turkey (Project no. 117F263) and in part by Sirnak University Research Fund Grant No. 2017.03.02.01.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ulgen, A.T. Evaluation of Superconducting Properties and Diffusion Behavior of Ex Situ and In Situ Bulk MgB2 Materials with Ni Coating. J Supercond Nov Magn 32, 2383–2389 (2019). https://doi.org/10.1007/s10948-019-5000-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-019-5000-0