Abstract
UC781, a very potent HIV-1 non-nucleoside reverse transcriptase inhibitor with extreme hydrophobicity and poor water solubility, is under development as a topical vaginal microbicide product to prevent HIV transmission. In this study, the thermodynamic behavior of the interaction between UC781 with three cyclodextrins (CDs): β-cyclodextrin (βCD), hydroxypropyl-β-cyclodextrin (HPβCD) and methyl-β-cyclodextrin (MβCD), was investigated using a reversed-phase HPLC method. A mobile phase consisting of acetonitrile: H2O (30:70) solution containing various CD concentrations was used. The retention time at different temperatures was determined to evaluate the inclusion process. The influence of βCDs on the solubility and hydrophobicity of UC781 was characterized by retention time values. The results showed that the inclusion capacity of cyclodextrins follows the order MβCD > βCD > HPβCD. An enthalpy–entropy compensation effect was also observed. In addition, the results revealed that the change of ΔH is greater than that of ΔS. These results suggested that the complexation of UC781 with βCDs is an enthalpy driven process. The modification on β-cyclodextrin will influence the inclusion process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclodextrins (CDs) are cyclic (α-1,4)-linked oligosaccharides with different numbers of α-d-glucopyranose units [1]. Cyclodextrins are produced from the enzymatic degradation of starch by bacteria [2, 3]. α-Cyclodextrin (αCD) comprises six glucopyranose units, β-cyclodextrin (βCD) seven such units, and γ-cyclodextrin (γCD) eight such units. The inner diameter of the relative hydrophobic cavity is approximately 4.7–5.3, 6.0–6.5, and 7.5–8.3 Å for αCD, βCD, and γCD, respectively [4, 5]. Thus, each cyclodextrin has its own ability to form host–guest inclusion complexes by partially or fully incorporating specific guest molecules with appropriate dimensions into the hydrophobic cavities of cyclodextrins. The chemically modified βCDs, particularly hydroxypropyl-β-cyclodextrin, have received considerable attention due to their pharmaceutical properties and safety profile as compared to the other βCDs [6]. Their application in pharmaceutical research is known to bring about an enhancement of the solubility [4, 6], chemical stability [1, 4], permeability [7], and bioavailability [8, 9] as novel drug carriers.
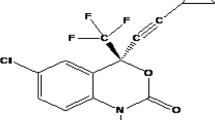
The thiocarboxanilide UC781, (N-[4-chloro-3-(3-methyl-2-butenyloxy) phenyl]-2-methyl-3-furancarbothioamide) (Fig. 1), is a tight-binding HIV-1 non-nucleoside reverse transcriptase inhibitor (NNRTI) that is an extremely potent inhibitor of HIV-1 in cell culture [10–12]. It belongs to the class of (thio)carboxanilide derivatives, the prototype of which (UC84) was originally reported as an antiviral agent by Bader et al. [13]. UC781 can neutralize several laboratory and clinical strains of HIV-1, including both syncytium- and non syncytium-inducing phenotypes [10]. It has also been shown to inhibit HIV-1 strains which are resistant to nucleoside RT inhibitors with potency similar to that for inhibition of wild-type virus [14]. Furthermore, UC781 targets HIV-1 RT and is effective against a variety of NNRTI resistant HIV-1 strains. Importantly, high resistance to UC781 is only obtained when more than one mutation occurs in the NNRTI binding pocket [12]. However, the extremely high hydrophobicity and low solubility (<30 ng/mL) of UC781 poses a huge challenge in formulation development of UC781.
A β-cyclodextrin based drug delivery system has been developed in our laboratory to enhance the aqueous solubility of UC781 [15]. The thermodynamic properties are important for the understanding and processing method optimization of βCD complex development. Several physical–chemical methods have been used to characterize the thermodynamic properties of inclusion complexes including UV spectroscopy [16, 17], NMR spectroscopy [17], as well as liquid chromatographic methods, such as thin layer chromatography [18] and high performance liquid chromatography (or High Pressure Liquid Chromatography HPLC) system [19, 20]. The difference on retention characteristics between complexed drug and neat drug [21, 22] can be used to evaluate their thermodynamic properties.
The aim of this paper is to examine the inclusion and thermodynamic properties of UC781 with natural and modified βCDs using HPLC method and to compare the complexation ability of UC781 with these different βCDs. This HPLC method is advantageous in that it requires only a limited amount of material and full evaluation can be completed quickly.
Materials and methods
Materials
UC781 for these studies was initially provided by Biosyn Co. Ltd. (Huntington, PA). However the licensing rights to this drug were transferred to CONRAD who provided subsequent supply as needed. βCD (MW 1134), MβCD (MW: ~1320; mean degree of substitution: 1.7–1.9), and HPβCD (MW: ~1380 and mean degree of substitution: 0.8) were purchased from Sigma-Aldrich (St Louis, MO). All other reagents used were of reagent grade and all solvents were of HPLC grade. Milli-Q water was used to prepare buffer solutions and other aqueous solutions.
Experimental
HPLC analysis for UC781 was briefly described as follows: a Waters HPLC system equipped with an auto injector model Waters 717, and a Waters 2487 dual wavelength (λ) absorbance detector at 300 nm were used (Waters Corporation, Milford, MA). Separation of compound in the experiment was achieved by using an Alltech ODS-C8 column (4.6 mm i.d. × 7.5 mm, 5 μm, Columbia, MD). A mobile phase of acetonitrile (ACN)/milliQ water (30:70 v/v) containing different CD concentrations at a flow rate of 1.0 mL/min was used. Temperature (from 25 to 50 °C) was controlled using a water bath within a range of ±0.1 °C. UC781 was dissolved in ACN at a concentration of 3 μg/mL. 10 μL of the UC781 solution was injected for the thermodynamic study. The retention time of UC781 was recorded for thermodynamic parameters calculations.
Retention time of UC781 was determined using HPLC method. K1:1 (complexation constant between drug and cyclodextrin) is calculated based on the capacity factor change described in the results section. Thermodynamic properties, such as ΔG, ΔH, and ΔS were calculated according to Van’t Hoff equation.
HPLC theoretical treatment of UC781-cyclodextrins inclusion process
HPLC is a separation technique using column chromatography to separate, identify, and quantify compounds. Retention time (tR) of drugs in HPLC varies depending on the interactions between the stationary phase, the molecules being analyzed, and the solvent(s) used. In a given HPLC system, tR can be thought of as the character of the drug itself, reflecting the interaction between drug and the stationary phase.
In addition to tR, another important parameter used in this study is “capacity factor” (Kc). Historically, a slightly different retention parameter, Kc was introduced by the analogy based on the liquid partitioning theory and is widely accepted in chromatographic practice. Kc is dimensionless and independent of any geometrical parameters of the column or HPLC system. It can be considered a thermodynamic characteristic of the solid phase-compound-mobile phase system [23]. Kc is defined by Eq. 1 [24]
where s is the solid phase and m is the mobile phase; Ms is the mass of analyte in the solid phase, Mm is the mass of analyte in the mobile phase; t is the retention time of drug (tdrug) and mobile phase (tm).
In a given mobile phase, the following equilibrium can be reached between drug and solid phase. The mass balance can be expressed as Eq. 2
where S is the solid phase of column; K1 is the binding constant for drug and solid phase;[Drug] is free drug in mobile phase; and [Drug−S] is drug binding to solid phase. When CD is added into the mobile phase, equilibrium can occur between the drug and CD. This equilibrium can be expressed as Eq. 3
K1:1 is the complexation constant of the drug and cyclodextrin; the total drug in the HPLC system can be given by Eq. 4
here [Drug]Total is total drug in HPLC; [Drug−S] is drug in solid phase; [Drug]m is free drug in mobile phase; and [Drug−CD] is drug in complexed for with cyclodextrin in mobile phase. Combining Eqs. 4 and 1 obtain Eqs. 5 and 6
the drug concentration and retention time are now correlated with each other through Kc. Here, [Drug] equals to free drug in mobile phase [Drug]m. Re-arranging the expression gives an Eqs. 7 and 8
Thus, plotting \( \frac{{t_{m} }}{{{\text{t}}_{\text{drug}} - t_{m} }} \) versus [CD] will results in a linear relationship between the tdrug \( \left( {\frac{{t_{m} }}{{{\text{t}}_{\text{drug}} - t_{m} }}} \right) \) and βCD concentration ([CD]) where the slope = \( \frac{{K_{1:1} }}{{[S] \cdot K_{1} }} \) and intercept = \( \frac{1}{{[S] \cdot K_{1} }} \). K1:1 under the experimental conditions can be calculated using Eq. 9
Results and discussion
Inclusion behavior of βCDs with UC781
The effect of CD concentration on UC781 retention time is shown in Table 1. A significant decrease in UC781 tR was observed with increasing βCDs concentration in the mobile phase for all types of cyclodextrin. Both HPβCD and MβCD exhibit better solubility properties than native βCD in mobile phase which greatly enhance the complexation ability with UC781. These results indicate that the hydrophobicity of UC781 controls its retention on column and complexation with βCDs in mobile phase. In addition, amount by which the UC781 retention time was reduced was observed to be greater with changes in MβCD concentration as compared to those with observed with changes in HPβCD concentration. This result demonstrates a stronger complexation ability of MβCD with UC781 than that of HPβCD. The effect of βCD on retention time was not compared with that of MβCD and HPβCD due to its limited solubility in mobile phase.
The effect of βCDs on the lipophilicity change of UC781
The partition coefficient is a parameter that is used to describe the degree of lipophilicity of a compound typically expressed as logP specifically; it is experimentally determined by evaluating the distribution of a compound between two immiscible phases (such as octanol and water). Drug molecules with larger logP values are more lipophilic and tend to be less able to be dissolved into water. This parameter is useful in estimating distribution and permeability of the drug within the body [25–27]. However, the traditional shake-flask method [28, 29], does not provide a rapid determination of lipophilicity. In addition, this method requires a large amount of compound when the compound is very lipophilic. A HPLC method was used to rapidly determine logP from Kw [30, 31]. Kw is the capacity factor when the mobile phase is pure water. It has been demonstrated that the extrapolated logarithm of the capacity factor for a pure aqueous eluent (log kw) gives the best correlation with logP [32]. Moreover, the relationship between the logarithm of the capacity factor (log Kc) and log Kw can be expressed through Eq. 10
where A is the slope, C is the volume fraction of organic solvent in mobile phase and log Kw the intercept of the regression curve. In these studies, the CD concentration change was used instead of the change in organic solvent for the calculation of log Kw since it was desired to determine the impact of CD on lipophilicity. The log Kw is a fixed value obtained from Eq. 10 in these studies. Therefore, the slope values obtained from linear regression can be compared to evaluate the complexation ability of the three βCDs with UC781.
Figure 2 represents the logarithm of the Kc of UC781 as a function of CD concentration in the mobile phase. In the presence of βCDs in the mobile phase, log Kc of UC781 decreased with increasing CD concentration. Linear relationships between log Kc and CD concentration in the mobile phase were observed, indicating a classical reverse phase elution mechanism [20, 33]. The absolute value of slopes (Table 2) obtained in the linear regression treatment followed a rank order of MβCD > βCD > HPβCD, which also corresponds with the retention time changes shown in
Table 1. This phenomenon can be explained by the alteration of hydrophobic interaction between UC781 and the solid phase in the HPLC column, which is induced by the complexation of UC781 with CD in the mobile phase. Furthermore, the substitution of different groups on βCD can cause steric interactions between gust molecules and βCD. The 2-hydroxypropyl groups may occlude the entrance for the guest molecules which hinder the complexation ability [34, 35]. The cyclodextrin cavity is lengthened upon methylation of the OH(2) and OH(6) groups of the cyclodextrin rim without significant distortion of the ring, and this would enhance the complexation ability of MβCD [36, 37]. The results also indicate that MβCD has a better ability to form a complex with UC781 under these experimental conditions.
The effect of temperature on the inclusion process of UC781 with βCDs
The effect of temperature on the inclusion constants of UC781 with different βCDs is given in Fig. 3. ΔG values were calculated from the inclusion constants and are listed in Fig. 4. K1:1 and absolute value of ΔG change decreased with increasing temperature. The results reveal that higher temperature is unfavorable for the inclusion process, which can be explained by extensive movement of molecules at high temperature leading to the disassociation of the cyclodextrin complex.
Inclusion process thermodynamics
All ΔG values shown in Fig. 4 are negative, which suggests that the inclusion process can proceed spontaneously. Given the ΔG values, other thermodynamic parameters can be obtained using the Van’t Hoff equation. Enthalpy (ΔH) and entropy (ΔS) changes for complexes were calculated as following:
The slope and the intercept of the plot of ΔG/T versus 1/T are ΔH and –ΔS, respectively. Figure 5 represents the plot of ΔG/T versus 1/T for the three βCDs. The ΔH and ΔS values are shown in Table 3. Negative values for ΔH mean that the inclusion process is exothermal and low temperature is suitable for the inclusion process. A relatively large negative ΔH and small negative ΔS suggests that the inclusion process of UC781 with βCDs is primarily an enthalpy driven process indicating that Van der Waals force is the major driving force in this complex formation [4, 38].
Enthalpy–entropy compensation is the phenomenon in which the change in enthalpy is offset by a corresponding change in entropy resulting in a smaller net free energy change. It is believed to play an important role in reactions in solution [39, 40]. The enthalpy-entropy compensation relationship has been observed in these βCDs complex [41]. The enthalpy–entropy compensation is also attributed to the interactions with water molecules in the βCD cavity [42]. In this study, the plot of ΔH versus ΔS shows a straight line (Fig. 6). The slope is the compensation temperature, T = 304 K. It demonstrates that the inclusion process also displays an enthalpy–entropy compensation effect [43].
Conclusions
Different mechanisms of complex formation were widely investigated for decades. The formation of cyclodextrins complex may involve several driving forces: Van der Waals interactions, hydrophobic interactions, exclusion of cavity-bound high-energy water, hydrogen bonding, electrostatic interactions, etc. [4, 44]. The thermodynamic properties of complexation are considered important for governing the complex formation between cyclodextrin and guest molecule. The enthalpy and entropy changes of the complex formation can be utilized to analysis the major driving force for the formation of cyclodextrin complex in spite of the anthalpy–entropy compensation. Although, the extent of complex formation mainly depends on the chemical properties of the guest molecule itself. Many auxiliary methods can be used to further enhance the formation of complex, such as change the species and concentration of cyclodextrins, temperature, procedures of complex formation, pH, co-solvent, etc. which can reflect the changes on the thermodynamic properties [4].
The thermodynamic behavior of the complexation between UC781 and three different βCDs was investigated systematically using a HPLC method utilizing retention difference between UC781 and its complex. The inclusion ability and hydrophilicity enhancement effects of βCDs on UC781 were found to follow the rank order of MβCD > βCD > HPβCD which can be explained by sterically influence caused by different substitution groups. The thermodynamic behavior of UC781 and all the βCDs studied were shown to be an enthalpy-driven processes suggesting that Van der Waals forces plays an important role in the complexation. Enthalpy–entropy compensation was also observed for complexation of UC781 with βCDs indicating a replacement of water molecules in the cyclodextrin cavity with UC781.
The HPLC method provides rapid evaluation of the thermodynamics of inclusion and requires minimal amount of material. The results from this study increase our understanding of the mechanism of complexation of UC781 with βCD and its derivatives and offer useful information which can be utilized in the formulation development of a cyclodextrin based drug delivery system for UC781. Additionally, the HPLC method developed in these studies provides a novel means for evaluating other hydrophobic drug candidates and their complexation with βCDs.
References
Davis, M.E., Brewster, M.E.: Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discovery 3, 1023–1035 (2004). doi:10.1038/nrd1576
Biwer, A., Antranikian, G., Heinzle, E.: Enzymatic production of cyclodextrins. Appl. Microbiol. Biotechnol. 59(6), 609–617 (2002)
Larsen, K., Duedahl-Olesen, L., Christensen, S.J.H., Mathiesen, F., Pedersen, L., Zimmermann, W.: Purification and characterization of a cyclodextrin glycosyltransferase from Paenibacillus sp. F8. Carbohydr. Res. 310(3), 211–219 (1998)
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 85(10), 1017–1025 (1996)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98(5), 1743–1753 (1998)
Stella, V.J., Rajewski, R.A.: Cyclodextrins: their future in drug formulation and delivery. Pharm. Res. 14(5), 556–567 (1997)
Loftsson, T., Másson, M., Sigurdsson, H.H.: Cyclodextrins and drug permeability through semi-permeable cellophane membrane. Int. J. Pharm. 232(1–2), 35–43 (2002)
Nasongkla, N., Wiedmann, A.F., Bruening, A., Beman, M., Ray, D., Bornmann, W.G., Boothman, D.A., Gao, J.: Enhancement of solubility and bioavailability of β-lapachone using cyclodextrin inclusion complexes. Pharm. Res. 20(10), 1626–1633 (2004)
Wong, J.W., Yuen, K.H.: Improved oral bioavailability of artemisinin through inclusion complexation with β- and γ-cyclodextrins. Int. J. Pharm. 227(1–4), 177–185 (2001)
Balzarini, J., Pelemans, H., Aquaro, S., Perno, C., Witvrouw, M.D.D.S., Clercq, E.D., Karlsson, A.: Highly favorable antiviral activity and resistance profile of the novel thiocarboxanilide pentenyloxy ether derivatives UC-781 and UC-82 as inhibitors of human immunodeficiency virus type 1 replication. Mol. Pharmacol. 50(2), 394–401 (1996)
Barnard, J., Borkow, G., Parniak, M.A.: The thiocarboxanilide nonnucleoside UC781 is a tight-binding inhibitor of HIV-1 reverse transcriptase. Biochemistry 36(25), 7786–7792 (1997)
Buckheit, R.W.J., Snow, M.J., Fliakas-Boltz, V., Kinjerski, T.L., Russell, J.D., Pallansch, L.A., Brouwer, W.G., Yang, S.S.: Highly potent oxathiin carboxanilide derivatives with efficacy against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus isolates. Antimicrob. Agents Chemother. 41(4), 831–837 (1997)
Bader, J.P., McMahon, J.B., Schultz, R.J., Narayanan, V.L., Pierce, J.B., Harrison, W.A., Weislow, O.S., Midelfort, C.F., Stinson, S.F., Boyd, M.R.: Oxathiin carboxanilide, a potent inhibitor of human immunodeficiency virus reproduction. Proc. Natl. Acad. Sci. USA. 88(15), 6740–6744 (1991)
Borkow, G., Arion, D., Wainberg, M.A., Parniak, M.A.: The thiocarboxanilide nonnucleoside inhibitor UC781 restores antiviral activity of 3′-azido-3′-deoxythymidine (AZT) against AZT-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 43(2), 259–263 (1999)
Yang, H., Parniak, M.A., Isaacs, C.E., Hillier, S.L., Rohan, L.C.: Characterization of cyclodextrin inclusion complexes of the anti-HIV non-nucleoside reverse transcriptase inhibitor UC781. AAPS J 10(4), 606–613 (2008)
Jullian, C., Cifuentes, C., Alfaro, M., Miranda, S., Barriga, G., Olea-Azar, C.: Spectroscopic characterization of the inclusion complexes of luteolin with native and derivatized beta-cyclodextrin. Bioorg. Med. Chem. 18(14), 5025–5031 (2010)
Zielenkiewicz, W., Terekhova, I.V., Wszelaka-Rylik, M., Kumeev, R.S.: Thermodynamics of inclusion complex formation of hydroxypropylated α- and β-cyclodextrins with aminobenzoic acids in water. J. Therm. Anal. Calorim. 101(1), 15–23 (2010)
Guo, X., Shuang, S., Wang, X., Dong, C., Pan, J., Aboul-Enein, H.Y.: Comparative study on the inclusion behaviour of cyclodextrin derivatives with venoruton and rutin by thin layer chromatography. Biomed. Chromatogr. 18(8), 559–563 (2004)
Morin, M., Verreault, S., Mailloux, A., Frechette, J., Chatigny, S., Painchaud, Y., Beaudry, P.: Inclusion characterization in a scattering slab with time-resolved transmittance measurements: perturbation analysis. Appl. Opt. 39(16), 2840–2852 (2000). doi:62574[pii]
Clarot, I., Cledat, D., Battu, S., Cardot, P.J.: Chromatographic study of terpene derivatives on porous graphitic carbon stationary phase with beta-cyclodextrin as mobile phase modifier. J. Chromatogr. A 903(1–2), 67–76 (2000)
Love, L.J.C., Arunyanart, M. (eds.): Cyclodextrin mobile-phase and stationary-phase liquid chromatography. ACS Symposium Series 297 (1986)
Kai, G., Pan, J., Yuan, C., Yuan, Y.: Separation of madecassoside and madecassic acid isomers by high performance liquid chromatography using β-cyclodextrin as mobile phase additive. Bull. Korean Chem. Soc. 29(3), 551–554 (2008)
Kazakevitch, Y.V., Eltekov, Y.A.: Thermodynamic interpretation of the capacity factor. Chromatographia 25(11), 965–968 (1988)
Swadesh, J.: HPLC: Practical and Industrial Applications, Second Edition ed. ACS publication, UK (2000)
Alhaique, F., Giacchetti, D., Marchetti, M., Riccieri, F.M.: Effect of a second solubilizate on the partition coefficient of drugs in micellar solution and their permeation rate across an artificial membrane. J. Pharm. Pharmacol. 29(7), 401–406 (1977)
Cross, S.E., Magnusson, B.M., Winckle, G., Anissimov, Y., Roberts, M.S.: Determination of the effect of lipophilicity on the in vitro permeability and tissue reservoir characteristics of topically applied solutes in human skin layers. J. Invest. Dermatol. 120(5), 759–764 (2003)
Kretsos, K., Kasting, G.B., Nitsche, J.M.: Distributed diffusion-clearance model for transient drug distribution within the skin. J. Pharm. Sci. 93(11), 2820–2835 (2004)
Cripe, C.R., Walker, W.W., Pritchard, P.H., Bourquin, A.W.: A shake-flask test for estimation of biodegradability of toxic organic substances in the aquatic environment. Ecotoxicol. Environ. Saf. 14(3), 239–251 (1987)
Unger, S.H., Cook, J.R., Hollenberg, J.S.: Simple procedure for determining octanol–aqueous partition, distribution, and ionization coefficients by reversed-phase high-pressure liquid chromatography. J. Pharm. Sci. 67(10), 1364–1367 (1978)
Ayouni, L., Cazorla, G., Chaillou, D., Herbreteau, B., Rudaz, S., Lantéri, P., Carrupt, P.-A.: Fast determination of lipophilicity by HPLC. Chromatographia 62(5–6), 251–255 (2005)
Musilek, K., Jampilek, J., Dohnal, J., Jun, D., Gunn-Moore, F., Dolezal, M., Kuca, K.: RP-HPLC determination of the lipophilicity of bispyridinium reactivators of acetylcholinesterase bearing a but-2-ene connecting linker. Anal. Bioanal. Chem. 391(1), 367–372 (2008)
Liu, X., Tanaka, H., Yamauchi, A., Testa, B., Chuman, H.: Determination of lipophilicity by reversed-phase high-performance liquid chromatography. Influence of 1-octanol in the mobile phase. J. Chromatogr. A 1091(1–2), 51–59 (2005)
Horvath, C., Melander, W., Molnar, I.: Solvophobic interactions in liquid chromatography with nonpolar stationary phases. J. Chromatogr. 125, 129–156 (1976)
Yuan, C., Jin, Z., Lia, X.: Evaluation of complex forming ability of hydroxypropyl-β-cyclodextrins. Food Chem. 106(1), 50–55 (2008)
Buvari-Barcza, A., Kajtar, J., Szente, L., Barcza, L.: Hydroxypropyl-β-cyclodextrins: induced circular dichroism spectra of included phenolphthalein as a function of the degree of substitution. J. Chem. Soc. Perkin Trans. 2, 489–491 (1996)
Suzuki, M., Ohmori, H., Kajtar, M., Szejtli, J., Vikmon, M.: The association of inclusion complexes of cyclodextrins with azo dyes. J. Incl Phenom. Macrocycl Chem. 18(3), 255–264 (1994)
Sueishii, Y., Kasahara, M., Inoue, M., Matsueda, K.: Effects of substituent and solvent on inclusion complexation of β-cyclodextrins with azobenzene derivatives. J. Incl Phenom. Macrocycl Chem. 46(1–2), 71–75 (2003)
Saenger, W.: Cyclodextrin inclusion compounds in research and industry. Angew. Chem. IntEd. Engl. 19(5), 344–362 (1980)
Inoue, Y., Hakushi, T., Liu, Y., Tong, L., Shen, B.: Thermodynamics of molecular recognition by cyclodextrins. 1. Calorimetric titration of inclusion complexation of naphthalenesulfonates with alpha-cyclodextrin, beta-cyclodextrin, and gamma-cyclodextrin—enthalphy entropy compensation. J. Am. Chem. Soc. 115(2), 475–481 (1993)
Inoue, Y., Liu, Y., Tong, L.-H., Shen, B.-J., Jin, D.-S.: Calorimetric titration of inclusion complexation with modified β-cyclodextrins. Enthalpy–entropy compensation in host–guest complexation: from ionophore to cyclodextrin and cyclophane. J. Am. Chem. Soc. 115(23), 10637–10644 (1993)
Connors, K.A.: The stability of cyclodextrin complexes in solution. Chem. Rev. 97(5), 1325–1357 (1997)
Lemieux, R.U., Delbaere, L.T.J., Beierbeck, H., Spohr, U.: Involvement of water in host–guest interactions. Ciba Found Symp. 158, 231–245 (2007). discussion: 245–248
Luscher-Mattli, M.: Thermodynamic functions of biopolymer hydration. 11. Enthalpy-entropy compensation in hydrophilic hydration processes. Biopolymers 21, 419–429 (1982)
Liu, L., Guo, Q.-X.: The driving forces in the inclusion complexation of cyclodextrins. J. Incl Phenom. Macrocycl Chem 42, 1–14 (2002)
Acknowledgments
The research described was supported by Grant Number NIH U19AI051661 from the National Institute of Allergy and Infectious Diseases (NIAID). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID. We gratefully acknowledge CONRAD for providing UC781 for these studies. We thank Dr. Rama Mallipeddi and Dr. Lindsay Ferguson for their assistance in reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, H., Parniak, M.A., Hillier, S.L. et al. A thermodynamic study of the cyclodextrin-UC781 inclusion complex using a HPLC method. J Incl Phenom Macrocycl Chem 72, 459–465 (2012). https://doi.org/10.1007/s10847-011-0019-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-011-0019-2