Abstract
Calorimetry, densimetry, 1H NMR and UV–vis spectroscopy were used to characterize inclusion complex formation of hydroxypropylated α- and β-cyclodextrins with meta- and para-aminobenzoic acids in aqueous solutions at 298.15 K. Formation of more stable inclusion complexes between para-aminobenzoic acid and cyclodextrins was observed. The binding of aminobenzoic acids with hydroxypropyl-α-cyclodextrin was found to be enthalpy-governed owing to the prevalence of van der Waals interactions and possible H-binding. Complex formation of hydroxypropyl-β-cyclodextrin with both acids is mainly entropy driven. The increased entropy contribution observed in this case is determined by dehydration of solutes occurring during the revealed deeper insertion of aminobenzoic acids into the cavity of hydroxypropyl-β-cyclodextrin. By comparing complex formation of aminobenzoic acids with native and substituted cyclodextrins it was found that the availability of hydroxypropyl groups slightly influenced the thermodynamic parameters and did not change the binding mode or driving forces of interaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Investigation and application of cyclodextrins (CDs) and their inclusion complexes in food, cosmetic and pharmaceutical industries as well as in separation technologies has greatly increased in the last decades [1–3]. CDs are cyclic oligosaccharides obtained by the degradation of starch and consisting of six (α-CD), seven (β-CD) and eight (γ-CD) glucose units. They are nontoxic and safe to use as encapsulating materials for drugs and biologically active compounds. Availability of a relatively non-polar internal cavity in the toroidal structure of CD brings about the inclusion complex formation of CDs with various organic molecules. The properties (solubility, stability, test, activity) of guest molecules placed inside the CD cavity can be significantly changed. Thus, the practical importance of CDs inclusion complexes is evident.
The possibility to use CDs for encapsulation of aminobenzoic acids (ABA) was discussed in our previous publications [4–6]. Aminobenzoic acids were chosen as guest molecules because of their properties; p-aminobenzoic acid (pABA) is an essential nutrient that is required for folic acid synthesis by many organisms. Moreover, pABA is often used in sunscreen formulations as a UV filter protecting the skin against ultra-violet radiation. m-Aminobenzoic acid (mABA) being the biologically inactive structural isomer of pABA is involved in the synthesis of dyes. Both acids are slightly soluble in water. Thus, inclusion complex formation with CDs can improve the physicochemical properties of ABAs.
Our previous investigations were concerned with complex formation of ABAs with native α- and β-cyclodextrins in solution [4–6]. These complexes were also intensively studied in literature [7–15]. The obtained results showed that the thermodynamic characteristics of complex formation in aqueous solution are more affected by the ABA structure than the CD cavity dimensions. In the solid state, the formation of the 1:1 inclusion complex between β-CD and pABA was confirmed by X-ray analysis [16]. pABA was found to penetrate deeply into β-CD cavity, with the amino group located on the wider side of cavity and the carboxyl at the narrow side. The binding mode of pABA with β-CD in the solid state is in agreement with those observed for the aqueous solution by Stalin et al. [14] and by us [4].
The complexation of ABAs with native CDs was studied in detail, whereas the binding with substituted CDs received relatively little consideration. To the best of our knowledge, only complex formation of hydroxypropyl-β-cyclodextrin with pABA was investigated and reported by Shuang et al. [7]. It should be noted that modified CDs have received much attention in recent years due to their higher solubility and improved physicochemical properties.
Thus, the aim of our work was to study complex formation of hydroxypropylated α- and β-CDs with pABA and mABA in water at 298.15 K using calorimetry, densimetry, 1H NMR and UV–vis spectroscopy. Specifically, it was planned to obtain the thermodynamic characteristics of complexation; to propose the binding mode and driving forces of interaction; to analyze the influence of reagent structure on the complex formation process; and to compare the binding affinity of native and hydroxypropylated CDs to ABAs.
Experimental
Materials
Hydroxypropyl-α-cyclodextrin (HP-α-CD) and hydroxypropyl-β-cyclodextrin (HP-β-CD) were purchased from Sigma–Aldrich. The average substitution degree was 0.6 per glucose unit. Water content in CDs was determined thermogravimetrically. pABA and mABA (from MP Biomedicals) were not further purified. All solutions were prepared by weight on the basis of double-distilled, degassed water. Deuterated water of 99.9% isotopic purity was used in 1H NMR measurements.
Methods
Calorimetry
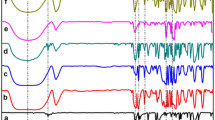
Calorimetric measurements were performed using a high-sensitivity MicroCal OMEGA isothermal titration microcalorimeter at 298.15 K. The calorimeter was equipped with a stainless steel titration vessel (1.3611 mL) filled with CD solution (0.001 mol kg−1). The 8 μL of ABA solution (0.01 mol kg−1) were injected stepwise using a syringe pump equipped with a 250 μL Hamilton syringe. The measured thermal effects were corrected for the dilution of CD and ABA solutions. The values of stability constants and enthalpy of complex formation were calculated using standard Microcal program.
UV–vis spectroscopy
Absorption spectra were recorded in the range of 200–400 nm on a UV-2401 PC UV–VIS Recording Spectrometer (Shimadzu, Japan) equipped with TCC-240 A temperature controlled cell holder.
The quantitative determination of the stability constants is based on the absorbance variation of ABA in the presence of different amounts of CD. In this case, the ABA concentration was constant (6.7 × 10−4 mol kg−1) and the CD concentration was changed from 0 to 4 × 10−2 mol kg−1. The CD solutions of corresponding concentration were used in the reference cuvette. The experiments were carried out using 1 cm quartz cuvettes at different temperatures (293.15, 298.15, 308.15 and 318.15 K).
1H NMR
1H NMR spectra were recorded on a Bruker AC-200 spectrometer operating at 200 MHz. Cyclohexane was used as reference substance. The chemical shift changes (Δδ) of ABA protons were determined at 298.15 ± 0.10 K. The concentration of ABA remained rigorously constant (0.005 mol kg−1), while CD concentrations were varied from 0 to 0.025 mol kg−1.
Densimetry
The densities of solutions were measured at 298.15 ± 0.01 K using a vibrating-tube digital densimeter (model DMA 4500, Anton Paar, Austria) with a precision of 1 × 10−5 g cm−3. The densimeter was daily calibrated with dry air and freshly prepared bidistilled water. The operation of the densimeter was checked by measuring the density of aqueous solutions of NaCl (Fluka, >99.99%). A good agreement with the literature data [17] was found.
The apparent molar volumes of CDs (V Ф) were calculated on the basis of the following relation:
where M is the CD molar mass; m is the molality; d 0 and d are the densities of solvent and solution, respectively. For binary systems (CD + H2O), water was the reference solvent with d 0 = 0.99704 g cm−3. For ternary systems (CD + ABA + H2O), the reference solvents were aqueous solutions of ABA. The V Ф were determined at constant CD concentration (0.005 mol kg−1) and varying ABA concentration (0–0.04 mol kg−1).
Results and discussion
In this work, thermodynamic study on complex formation of HP-α-CD and HP-β-CD with ABAs was carried out. The obtained thermodynamic parameters of complex formation reported in Tables 1, 2, 3, 4 were related to the process:
It was assumed that introduction of hydroxypropyl groups in CD molecule does not change the 1:1 stoichiometric ratio established early for complex formation of native α-CD and β-CD with ABAs [4–15]. In aqueous solution, ABAs can exist in three different states ranging from the fully protonated, through neutral, to anionic form. The pK values of the ionization are well known in the literature: pK 1 = 3.1 and pK 2 = 4.8 for mABA and pK 1 = 2.4 and pK 2 = 4.9 for pABA [18]. CDs dissociate in the alkaline medium (pK ~ 12.2) [19]. The predominance of each of the forms depends on pH. Measured pH of solutions of mABA and pABA was 3.9 and 3.7, respectively. These values are close to the isoelectric point of ABA at which the electrically neutral forms of all reagents are predominating. Thus, complex formation between CDs and the neutral species of ABAs takes place.
Table 1 reports the thermodynamic parameters of complex formation of hydroxypropylated α- and β-CDs with mABA and pABA evaluated from the calorimetric and UV–vis spectroscopic experiments. Thermodynamic parameters of complex formation of α-CD and β-CD with ABAs obtained in our previous work [6] are also included in Table 1. Calorimetric data are given in Fig. 1. Complexation of HP-β-CD with mABA was accompanied by thermal effects that were too small to calculate K and Δ c H 0. Therefore, the UV–vis spectroscopy was used. The UV–vis spectra of mABA were recorded in the presence of HP-β-CD and HP-α-CD at various temperatures. Spectroscopic study on complexation of HP-α-CD with mABA was also performed in order to compare the results obtained by calorimetry and UV–vis spectroscopy. It supported the validity of spectroscopic data.
Figure 2 shows the absorbance spectra of mABA in the presence of HP-α-CD and HP-β-CD. The addition of HP-α-CD and HP-β-CD increases the intensity and induces the red shift of absorption maxima at 306 nm in pure state of mABA. The absorbance spectra reflect the isosbestic points, suggesting the complex formation. Figure 3 illustrates the concentration dependences of the absorbance obtained for several temperatures. Stability constants of the complexes were estimated using the nonlinear fitting procedure described previously [5]. The values of K at different temperatures are given in Table 2. Subsequently, the enthalpy and entropy of complex formation were evaluated from the temperature dependence of the stability constant (Fig. 4) according to equation:
As it is seen from Table 2, stability constants depend on temperature only in the case of mABA/HP-α-CD complexation. The enthalpies of complex formation obtained from calorimetry and UV–vis spectroscopy data are in a good agreement (Table 1). The invariance of K with the temperature increase for binding of mABA with HP-β-CD denotes the approximately zero enthalpy change, which is consistent with the calorimetric results.
Table 1 shows that the complex formation of HP-α-CD with pABA and mABA is enthalpy driven. By contrast, the complexes of HP-β-CD with both acids are enthalpy–entropy stabilized. This indicates that the difference in the cavity dimensions is reflected in different driving forces of complex formation and binding modes, which will be proposed below. Here, we consider the Δ c H 0 and Δ c S 0 values (Table 1). The enthalpy and entropy of CD complex formation contain the contributions from the following processes: (1) dehydration of reagents upon binding; (2) release of “high-energy” water from the CD cavity [20]; (3) formation of the complex due to non-covalent interactions [20]; (4) hydration of the complex. The first three processes are particularly important and mainly determine the magnitudes of Δ c H 0 and Δ c S 0.
Complexation of pABA and mABA with HP-α-CD having a smaller cavity diameter is more exothermic than that with HP-β-CD. It is known from previous work [4] that ABAs are included into α-CD cavity. Thus, the tight fitting of both ABAs into HP-α-CD cavity takes place. Such binding should be accompanied by stronger van der Waals interactions between ABA and HP-α-CD, which results in the high exothermicity of complexation. This in turn leads to a substantial loss in the degrees of freedom of the reagents and, as a consequence, the entropy loss is observed (Δ c S 0 < 0) (Table 1).
The larger cavity of HP-β-CD can promote a deeper insertion of ABAs, with more water molecules substituted by the ABA molecule upon complex formation and released in the bulk aqueous solution. This results in the enthalpy gain [20]. However, this is not the case for HP-β-CD complexation. Data from Table 1 show that the enthalpy of HP-β-CD complex formation with both mABA and pABA is less exothermic in comparison with Δ c H 0 for HP-α-CD complexes. Probably, exothermic effects from van der Waals interactions and possible H-bonding as well as from the expulsion of cavity-bound water are overlapped by the endothermic effects from dehydration and hydrophobic interactions. Upon binding, the hydration shells of solutes are partially broken up and release of water molecules to the disordered bulk solvent takes place, producing the positive contribution to Δ c H 0 and Δ c S 0. Moreover, hydrophobic interactions of the ABA aromatic ring with relatively apolar cavity of CD, which are also accompanied by the positive enthalpy and entropy values, possibly result in the increase of Δ c H 0 and Δ c S 0.
As compared with mABA, the binding of pABA with HP-α-CD and HP-β-CD is more exothermic and results in formation of more stable complexes (Table 1). There are two possible explanations for this. First, the amino group in para-position of the aromatic ring can be structurally favorable for complex formation with CDs. Perhaps, insertion of pABA can be realized more easily due to the better shape-matching with the cavity. Second, the distinction in the solvation state of mABA and pABA molecules in aqueous solution can cause the different binding affinity. Evidence of predominant occurrence of mABA as zwitterions in water and in the solid state was published in [21]. In contrast to mABA, pABA exists in neutral molecular form [22], less hydrated and, consequently, exhibiting higher complexing ability. According to this, interaction of pABA with HP-α-CD and HP-β-CD is high-exothermic and results in formation of more stable complexes.
Hydroxypropylated CDs have some of the OH-groups substituted by hydroxypropyl groups. Hydroxypropyl groups in the CD exterior can result in a variety of complex formation processes owing to the changes in the cavity dimensions and hydrophobicity. Complexing properties of native and hydroxypropylated CDs can be compared from Table 1. It can be seen that hydroxypropyl groups practically do not affect the stability constants. Only a slight decrease of K is observed for complex formation of hydroxypropylated CDs with mABA (Table 1). However, the influence of hydroxypropyl-groups on Δ c H 0 and Δ c S 0 was more noticeable. The increase in the enthalpy and entropy changes can be caused by the positive contribution from dehydration of bulky substituents upon complex formation.
1H NMR technique can provide evidence for the inclusion complex formation of ABAs with hydroxypropylated CDs. Some concentration dependences of the chemical shift changes (Δδ) are shown in Fig. 5. 1H NMR spectra of CD protons were not examined because of the overlapping of some peaks. Stability constants and complexation-induced chemical shifts (Δ c δ) listed in Table 3 were calculated by non-linear regression analysis [4]. Comparison of K reported in Tables 1 and 3 shows a convergence of these values within the limits of experimental error.
The difference between Δ c δ observed for the investigated systems (see Table 2) shows that there are different binding modes involved. For complex formation of mABA with HP-α-CD, the measurable Δ c δ for H(4) and H(5) protons of the aromatic ring were observed. This means that there is a shallow penetration of only a part of the aromatic ring of mABA into the cavity of HP-α-CD. Probably, the NH2-group in the meta-position serves as a steric hindrance during inclusion complex formation. Binding mode is different for complex formation of mABA with HP-β-CD possessing a larger cavity diameter, when considerable Δ c δ values were detected for all protons of the aromatic ring. The Δ c δ is maximal for H(2) and H(6) protons which are close to the carboxylic group, indicating a deeper insertion of mABA and location of the carboxylic group inside the HP-β-CD cavity.
Complex formation of pABA with HP-α-CD induces a downfield shift of the signals of H(2) and H(6) protons. Probably, the amino group in para-position is favorable for the inclusion of aromatic ring with carboxylic group into the HP-α-CD cavity. Upon binding with HP-β-CD, the upfield shifts obtained for the signals of all pABA protons show again the deeper penetration of guest into the macrocyclic cavity.
Our results are in agreement with the previous data [13–15, 22] and show that the hydroxypropyl substituents do not dramatically change the binding mode of ABAs with CDs. It should also be noted that the revealed deeper insertion of both mABA and pABA in the cavity of HP-β-CD accompanied by the stronger dehydration and hydrophobic interactions is consistent with the less negative enthalpy and entropy changes observed for these processes (Table 1).
Dehydration plays important role during complex formation of HP-β-CD. To clarify the role of the solvent, we performed the volumetric measurements. The experimental dependence of the apparent molar volume of HP-β-CD from mABA concentration is shown in Fig. 6. From this dependence the stability constant and the apparent molar volume of fully complexed CD (V Ф,c ) can be obtained as previously described [23]. Calculated K and V Φ,c together with the experimental apparent molar volumes of free CD (V Φ,f ) are summarized in Table 4. The V Φ,f values are consistent with data obtained by Milioto et al. [24]. Trend of K variation remains in agreement with that revealed by calorimetry and 1H NMR.
Generally, the volume changes accompanying the complex formation are explained on the basis of the volumes of transfer (Δtr V):
The values of Δtr V are presented in Table 4. The magnitude and sign of Δtr V are determined by the ratio of two contributions from dehydration of CDs with ABAs occurring upon complex formation. One of them is the positive contribution caused by the volume increase due to the expulsion of cavity-bound water to the bulk solvent [25, 26]. The other is the contribution originating from destruction of solvation shells of the solutes and it is interpreted in terms of the cosphere overlap model [26, 27]. The magnitude of the second contribution depends on the nature of solute–solute interactions. According to this model, the overlap of cospheres of two hydrophilic solutes produces a volume increase, whereas the overlap of hydrophilic–hydrophobic and hydrophobic–hydrophobic groups results in volume decrease.
The Δtr V are negative for complex formation of HP-α-CD with pABA and mABA, and positive for the binding of HP-β-CD with both acids. The same tendency was found earlier for complexation of ABA with native α-CD and β-CD [6].
When ABAs are included into the CD cavity, water molecules are expulsed in the bulk solvent. Empty cavity of α-CD contains two water molecules [28] whereas the β-CD cavity contains from five to seven water molecules [25, 29]. Binding modes proposed on the basis of 1H NMR data argue in favor of the shallow penetration of ABAs into HP-α-CD cavity, with a partial release of cavity-bound water and, consequently, with reduced positive contribution from this process. Thus, the decrease in volume of HP-α-CD upon complex formation with ABAs is due to the prevalence of negative contribution from interaction of hydrophilic–hydrophobic and hydrophobic–hydrophobic groups. According to 1H NMR, HP-α-CD binds only a part of the aromatic moiety of mABA molecule (Table 3), decreasing the interaction in this system and the change of volume. On the other hand, the location of the carboxylic group of pABA inside the HP-α-CD cavity, which is not excluded according to 1H NMR, induces the hydrophilic–hydrophobic interactions and a considerable decrease of Δtr V.
The deeper inclusion of ABAs into HP-β-CD cavity, which was revealed by 1H NMR, is due to the release of more molecules of cavity-bound water, increasing the positive contribution to Δtr V. The volume change upon substitution of cavity-bound water by guest molecule is
where n is the number of ejected molecules of cavity-bound water; V(H2O) and V(g) are the molar volumes of water and included guest molecule (V(H2O) = 18.07 cm3 mol−1 [25]) [30]. The partial molar volumes of mABA (93.8 cm3 mol−1) and pABA (102.8 cm3 mol−1) [6] were taken for V(g) because the whole ABA molecule is placed inside, resulting in 6.5 and 6.7 water molecules expelled when mABA and pABA enter the HP-β-CD cavity. This result is in agreement with literature data [29–32]. In particular, González-Gaitano et al. [31] obtained n = 6.5 for complex formation of β-CD with decyltrimethylammonium bromide. The number of water molecules released from β-CD cavity upon binding with adenosine 5′-monophosphate and acetylsalicylic acid was found as n = 6 [32] and n = 5.5 [30], respectively.
The obtained results show that complexation of ABAs mainly with HP-β-CD involves the considerable solvent reorganization. This statement is supported by the less negative enthalpy and entropy values observed for HP-β-CD binding (Table 1). The following concordant orders of variation of thermodynamic properties of complex formation were found:
- Δtr V :
-
HP-β-CD + mABA > HP-β-CD + pABA > HP-α-CD + mABA > HP-α-CD + pABA
- Δ c H :
-
HP-β-CD + mABA > HP-β-CD + pABA > HP-α-CD + mABA > HP-α-CD + pABA
- Δ c S :
-
HP-β-CD + mABA > HP-β-CD + pABA > HP-α-CD + mABA > HP-α-CD + pABA.
The fact that more water molecules are released to the bulk solvent upon complexation, and also that lower exothermicity of binding and a higher degree of disorder in the system are observed is confirmed by the linear dependences Δ c H = f(Δtr V) and Δ c S = f(Δtr V) as well as by the enthalpy–entropy compensation effect, illustrated in Figs 7 and 8, respectively. The linear relationship between Δ c H and Δ c S (Fig. 8) means that enthalpy gain due to complex formation is compensated by the entropy loss.
Conclusions
Inclusion complex formation of HP-α-CD and HP-β-CD with isomeric aminobenzoic acids was studied by various experimental methods. Thermodynamic parameters of the 1:1 complex formation were calculated and analyzed in terms of the influence of reagents structure and solvent role. It was shown that CDs exhibit higher binding affinity to pABA than to mABA. Inclusion of ABAs into the cavity of HP-α-CD is enthalpy favorable owing to prevalence of van der Waals interactions and H-bonding as the main driving forces of binding. The observed deeper insertion of ABAs into the larger cavity of HP-β-CD is accompanied by considerable reorganization of solvent. Introduction of hydroxypropyl groups in CD molecules has no considerable influence on the complexation process.
References
Loftsson T, Duchêne D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329:1–11.
De Lisi R, Lazzara G. Aggregation in aqueous media of tri-block copolymers tuned by the molecular selectivity of cyclodextrins. Therm Anal Calorim. 2009;97:797–803.
Novăk Cs, Éhen Z, Fodor M, Jiesinszky L, Orgoványi J. Application of combined thermoanalytical techniques in the investigation of cyclodextrin inclusion complexes. J Therm Anal Calorim. 2006;84:693–701.
Terekhova IV, Kumeev RS, Alper GA. Inclusion complex formation of α- and β-cyclodextrins with aminobenzoic acids in aqueous solution studied by 1H NMR. J Incl Phenom Macrocycl Chem. 2007;59:301–6.
Terekhova IV, Obukhova NA. Study on inclusion complex formation of m-aminobenzoic acid with native and substituted β-cyclodextrins. J Solut Chem. 2007;36:1167–76.
Terekhova IV. Volumetric and calorimetric study on complex formation of cyclodextrins with aminobenzoic acids. Mend Commun. 2009;19:110–2.
Shuang S, Yang Y, Pan J. Study on molecular recognition of para-aminobenzoic acid species by α-, β- and hydroxypropyl-β-cyclodextrin. Anal Chim Acta. 2002;458:305–10.
Yang Y, Shuang S-M, Chao J-B, Zhang G-M, Ding H-Y, Dong C. Study on the molecular recognition interaction of β-cyclodextrin with aminobenzoic acid isomer. Acta Chim Sin. 2004;62:176–82.
Setniča V, Urbanová M, Král V, Volka K. Interactions of cyclodextrins with aromatic compounds studied by vibrational circular dichroism spectroscopy. Spectrochim Acta A. 2002;58:2983–9.
Harata K. Induced circular dichroism of cycloamylose complexes with meta- and para-disubstituted benzenes. Bioorg Chem. 1981;10:255–65.
Tanaka M, Kawaguchi Y, Nakae M, Mizobuchi Y, Shono T. Separation of disubstituted benzene isomers on chemically bonded cyclodextrin stationary phases. J Chromatogr A. 1982;246:207–14.
Lewis EA, Hansen LD. Thermodynamics of binding of guest molecules to α- and β-cyclodextrins. J Chem Soc Perkin Trans. 1973;2:2081–85.
Stalin T, Rajendiran N. Intramolecular charge transfer effects on 3-aminobenzoic acid. Chem Phys. 2006;322:311–22.
Stalin T, Shanthi B, Vasantha Rani P, Rajendiran N. Solvatochromism, prototropism and complexation of p-aminobenzoic acid. J Incl Phenom Macrocycl Chem. 2006;55:21–9.
Stalin T, Rajendiran N. Intramolecular charge transfer associated with hydrogen bonding effects on 2-aminobenzoic acid. J Photochem Photobiol A. 2006;182:137–50.
Zhang Y, Sh Yu, Bao F. Crystal structure of cyclomaltoheptaose (β-cyclodextrin) complexes with p-aminobenzoic acid and o-aminobenzoic acid. Carbohydr Res. 2008;343:2504–8.
Picker P, Tremblay E. A high-precision digital readout flow densimeter for liquids. J Solut Chem. 1974;3:377–84.
Christensen JJ, Wrathall DP, Izatt RM, Tolman DO. Thermodynamics of proton dissociation in dilute aqueous solution. IX. pK, ΔH 0, and ΔS 0 values for proton ionization from o-, m-, and p-aminobenzoic acids and their methyl esters at 25. J Phys Chem. 1967;71:3001–6.
Gelb RI, Schwartz LM, Bradshaw JJ, Laufer DA. Acid dissociation of cyclohexaamylose and cycloheptaamylose. Bioorg Chem. 1980;9:299–304.
Liu L, Guo Q-X. The driving forces in the inclusion complexation of cyclodextrins. J Incl Phenom Macrocycl Chem. 2002;42:1–14.
He Y, Wu C, Kong WJ. A theoretical and experimental study of water complexes of m-aminobenzoic acid mABA·(H2O) n (n = 1 and 2). J Phys Chem A. 2005;109:748–53.
Simova S, Schneider H.-J. NMR analyses of cyclodextrin complexes with substituted benzoic acids and benzoate anions. J Chem Soc Perkin Trans. 2000;2:1717–22.
Terekhova IV, De Lisi R, Lazzara G, Milioto S, Muratore N. Volume and heat capacity studies to evidence interactions between cyclodextrins and nicotinic acid in water. J Therm Anal Calorim. 2008;92:285–90.
De Lisi R, Milioto S, Pellerito A, Inglese A. Thermodynamic properties of sodium n-alkanecarboxylates in water and in water + cyclodextrin mixtures. Langmuir. 1998;14:6045–53.
Wilson LD, Verrall RE. A volumetric study of β-cyclodextrin/hydrocarbon and β-cyclodextrin/fluorocarbon surfactant inclusion complexes in aqueous solution. J Phys Chem. 1997;101:9270–7.
Spildo K, Høiland H. Complex formation between alkane-α,ω-diols and cyclodextrins studied by partial molar volume and compressibility measurements. J Solut Chem. 2002;31:149–64.
Friedman HL, Krishnan CV. In: Franks F, editor. Water, a comprehensive treatise, vol. 3, chap. 1. New York: Plenum Press; 1973.
Manor PC, Saenger W. Topography of cyclodextrin inclusion complexes. III Crystal and molecular structure of cyclohexaamylose hexahydrate, the water dimmer inclusion complex. J Am Chem Soc. 1974;96:3630–9.
Lindner K, Saenger W. β-Cyclodextrin-dodecahydrat: haufung von wassermolekulen in einer hydrophoben huhlund. Angew Chem. 1978;90:738–40.
Fukahori T, Kondo M, Nishikawa S. Dynamic study of interaction between β-cyclodextrin and aspirin by the ultrasonic relaxation method. J Phys Chem B. 2006;110:4487–91.
González-Gaitano G, Crespo A, Compostizo A, Tardajos G. Study at a molecular level of the transfer process of a cationic surfactant from water to β-cyclodextrin. J Phys Chem B. 1997;101:4413–21.
Kondo M, Nishikawa S. Inclusion kinetics of a nucleotide into a cyclodextrin cavity by means of ultrasonic relaxation. J Phys Chem B. 2007;111:13451–4.
Acknowledgements
We are grateful to M. Koźbiał and H. Szczogryn from the Institute of Physical Chemistry of PAS for their help with calorimetric measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zielenkiewicz, W., Terekhova, I.V., Wszelaka-Rylik, M. et al. Thermodynamics of inclusion complex formation of hydroxypropylated α- and β-cyclodextrins with aminobenzoic acids in water. J Therm Anal Calorim 101, 15–23 (2010). https://doi.org/10.1007/s10973-010-0797-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0797-6