Abstract
Nutrient and micropollutant removal, and bioactivity were studied in cultures of the green microalga Tetradesmus obliquus MACC-677 grown in centrate from municipal wastewater (WW). Two outdoor units, a thin-layer cascade (TLC) and a thin-layer raceway pond (TL-RWP), were tested for microalgal culturing in batch and semi-continuous regimes where their photosynthetic performance was monitored. The results revealed that the T. obliquus cultures grew well, showing a high specific growth rate µ of 0.31 day−1 and 0.25 day−1 when grown in WW in TLC and TL-RWP, respectively. The cultivation trials showed high nutrient removal efficiency for ammonium nitrogen (98.5%) as well as orthophosphate (89%), the most abundant forms of N and P occurring in municipal WW. The removal of selected pharmaceuticals and endocrine disruptors (e.g., ibuprofen, amitriptyline, bisphenol A, etc.) was also assessed. Ibuprofen was the most abundant micropollutant detected in the centrate, with concentrations up to 5000 ng L−1 and fast removal during the cultivation. The biomass produced in the centrate revealed antimicrobial activity against plant pathogens, including fungi, oomycota, and bacteria. These findings have shown that the culturing of T. obliquus can be considered a suitable way to contribute to a circular economy, to remove nutrients and micropollutants from municipal WW from which biomass extracts can be further used for plant protection in agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae have an ever wider range of applications in various areas such as dietary supplements, biopesticides, biofertilizers, wastewater (WW) treatment as well as potential production of cosmetics and biofuels. Many production systems have been developed for indoor and outdoor microalgal culturing on a scale from a few to millions of liters (Acién et al. 2017; Pereira et al. 2017). Large-scale cultivation plants on a hectare scale have been built by commercial companies (e.g., A4F, Aqualia, Biorizon Biotech, etc.) in Spain and Portugal recently. The major barrier that must be overcome is sustainable production cost which is still higher compared to agricultural biomasses (Costa and de Morais 2013; Baldev et al. 2021). To increase the cultivation sustainability and positive impact on the environment microalgae are cultured in WW using the necessary nutrients which might also reduce cost (Woertz et al. 2009; Li et al. 2011; Costa and de Morais 2013; Morillas-España et al 2021). Cultivation in WW as a cultivation growth medium is not suitable for all microalgal strains. Therefore, the most robust, fast-growing strains have been implemented to grow well under these conditions (Morales-Amaral et al. 2015a; Morales et al. 2019; Romero-Villegas et al. 2021).
The cultivation systems commonly used are rather simple as they are outdoors under natural conditions with a low control of environmental variables such as irradiance, temperature, rainfall, CO2 concentration, and mixing (Molina Grima et al. 2003) compared to indoor cultivation units (Dos Santos et al. 2013). The most frequently used cultivation systems for mass microalgal production are open ponds due to low construction costs, low power demand, and easy maintenance (Costa and de Morais 2013). A culture layer between 10–30 cm maintains insufficient cell irradiance and slow gas exchange that results in a low biomass density of about 1 g L−1 of dry weight (DW) in such units (Acién et al. 2017). Thin-layer (< 4 cm) cultivation systems are more efficient and productive (Masojídek et al. 2015). These outdoor cultivation units have been improved (better mixing, temperature control, and CO2 dosage) to achieve higher biomass production (Grivalský et al. 2019).
Biomass productivity and yield of valuable compounds are mostly affected by strain selection and a suitable type of photobioreactor (PBR) used for cultivation (Katarzyna et al. 2015). Due to ambiguous/unstable cultivation conditions, the number of species that have been successfully cultivated in open systems is relatively limited (Dos Santos et al. 2013). Microalgal production in open outdoor systems is considered to be inexpensive but allows for the growth of only a few selected microalgal strains. Worldwide, the fast-growing microalgae Arthrospira, Chlorella, Dunaliella, Scenedesmus, and Nannochloropsis are the most commonly cultivated strains in open units ( Bishop and Zubeck 2012; Borowitzka 2013; Costa and de Morais 2013). Selection and validation of robust microalgal strains for large-scale cultivation have become one of the key research topics for biotechnology production. Laboratory screening tests have relatively limited predictive power for the ability of strains to grow in outdoor ponds (Wen et al. 2016). Small-scale outdoor systems can be used as pilot devices to test microalgal growth in outdoor mass cultivation (Wen et al. 2016; Grivalský et al. 2019).
The quest for remediation, reduction of production costs, and sustainable growth of microalgae using municipal WW has been under investigation for more than half a century (Jensen 1957; Tam and Wong 1989; Lau et al. 1995; Woertz et al. 2009). Centrate, the liquid part of the activated sludge treatment process, is a rich nutrient source of ammonium and phosphorus, and other macroelements necessary for microalgal growth. Up to now, few studies have tested the suitability of microalgal cultivation in undiluted centrate (Li et al. 2011).
In this study we focused on the cultivation of the green microalga Tetradesmus obliquus MACC-677 in the undiluted centrate of municipal WW using two different open cultivation units – thin-layer cascade (TLC) and a thin-layer raceway pond (TL-RWP). Both thin-layer units are characterized by high biomass production, high average cell irradiance, and rapid gas exchange (O2 and CO2). For the present trials, the strain was selected due to its rapid growth (Ördög et al. 2013) and potential biostimulating and biopesticide activities (V. Ördög and F. Estrella, unpublished data). The growth and photosynthetic activity were correlated with the removal efficiency of nutrients and selected pharmaceuticals and endocrine disruptors as well as the production of bioactive compounds with biostimulating and antimicrobial activity.
Materials and methods
Strain and inoculum preparation
The green microalga Tetradesmus obliquus MACC-677 (previously known as Scenedesmus acutus) obtained from the Széchenyi István University, Mosonmagyaróvár, Hungary was used in these trials. In laboratory cultivations, the seed cultures were grown in BG-11 medium (Allen 1968) in 10-L Pyrex glass bottles in the laboratory at 28–30 °C, and at a light intensity of 200 µmol photons m−2 s−1. The cultures were mixed by bubbling with air and 1% CO2.
Laboratory cultivation
For laboratory growth experiments, flat-panel PBRs with a volume of 3 L and a short light path of 22 mm were used. The culture was grown at 28–30 °C under continuous illumination of 100 μmol photons m−2 s−1 using day-light fluorescent lamps (55 W, Dulux L, Osram, Germany). The mixing was provided by bubbling with air and 1% CO2 (v/v). The cultures were grown in two frequently used inorganic media BG-11 (Allen 1968) and BBM (Andersen et al. 2005; Bischoff and Bold 1963). The specific growth rate, cell numbers, and consumption of nitrate were assessed in a one-week trial to decide which cultivation media to use for further study.
Centrate preparation for outdoor trial
Activated sludge from the municipal wastewater treatment plant (WWTP) in Třeboň obtained after aerobic digestion, was collected and centrifuged at 3,000 × g for 5 min. Brownish-colored supernatant (further abbreviated as WW) was used as a cultivation medium without any dilution (see the composition in Table 1). The initial ratio of total nitrogen (TN) to total phosphorus (TP) was about 1.5:1. The content of TN in centrate (230–350 mg L−1) was similar to that of the BG-11 medium while the TP content (110–170 mg L−1) was more than 20-times higher, about 35:1.
Cultivation in outdoor units
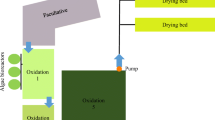
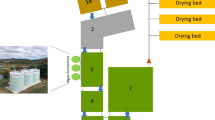
Two outdoor cultivation units – a thin-layer cascade (TLC) and a thin-layer raceway pond (TL-RWP) were used in the trials during summer (August 2019) at the Centre Algatech Třeboň (N 48°59', E 14°46', Czech Republic – temperate climate zone). They differ in the method of mixing – a pump for TLCs and a paddle wheel for TL-RWP – and the thickness of the culture layer (Grivalský et al. 2019). Circulation pumps can be selective for some strains of microalgae. Both cultivation units (each of 5 m2) were placed in separate polycarbonate greenhouses to protect the cultures from unfavourable environmental conditions and to reduce the risk of cross-contamination. A detailed description of the cultivation units has been reported earlier (Grivalský et al. 2019). The TL-RWP was operated continuously for 24 h at a working volume of about 100 L with a culture depth of 15–25 mm; culture flow velocity was set to 0.2 m s−1. The TLC was operated with a working volume of 70 L, the culture depth was kept between 8 and 10 mm and the flow speed was about 0.5 m s−1. The culture was circulated only during the day, while at night, it was stored in a retention tank and bubbled with air (light/dark regime about 12/12 h). The initial microalgal biomass density after inoculation was about 1 g DW L−1 in the cultivation in the BG-11 medium (Trial 1) and about 0.5 g DW L−1 in the cultivation in WW (Trial 2), as the dry matter content in WW itself was about 0.6 g DW L−1. It means that the density of the microalgal biomass was set lower compared to the cultivation in BG-11 in order to compensate for light absorption (optical density) by colored WW. The culture temperature in the morning was between 18–22 °C while at midday it ranged between 27 and 37 °C. The midday irradiance maxima in the greenhouse was between 100–1500 μmol photons m−2 s−1. The pH value was kept close to pH 8 ± 0.2 by automatic addition of pure CO2 (pH stat).
The cultures of T. obliquus were firstly grown for 6 days in a batch regime in inorganic BG-11 medium and then 5 days in a semi-continuous regime; then, the cultures were maintained for 5 days in the semi-continuous regime harvesting 20% of the culture volume daily which was replaced with fresh medium, i.e. dilution rate was 0.2 day−1 (Trial 1). In Trial 2 the culturing process was the same: the cultures were firstly grown for 6 days in the batch regime but in WW, and then for 5 days in the semi-continuous regime harvesting 25% of the culture volume daily which was replaced with WW. When microalgae were cultured in WW, the number of microorganisms measured in the culture was about 109 CFU L−1.
Dry weight and pigment analysis
The total biomass density (DW) was measured by filtering 5 mL of the culture on a pre-weighed glass filter (GC-50), washed twice with deionized water, dried in an oven at 105 °C for 8 h, and weighed (precision of ± 0.01 mg). The growth rate μ = (ln X2 – ln X1)/t2 – t1 (day−1) was calculated for the batch regime.
The pigment extraction was as follows: A 1 mL of the sample was centrifuged and the pellet was dissolved in methanol in a total volume of 2 mL. The cell wall was broken down by vortexing using a small amount of sand and measured by a high-resolution spectrophotometer (UV 2600 UV–VIS, Shimadzu, Japan) according to Ranglová et al. (2021). Total chlorophyll (Chl) and total carotenoid (Car) content was calculated (Wellburn 1994). The samples were taken daily at 09:00 h and analyzed in triplicates.
Chemical analysis of WW
The WW and culture samples were centrifuged (12,000 × g, 5 min) before syringe filtering (pore size of 0.45 µm) and kept in closed vials in a freezer for further analysis. All samples were analyzed at once after thawing. The samples for BOD (Biological Oxygen Demand) assay were transferred fresh to be analyzed 24 h after preparation. Analyses of BOD, COD (Chemical Oxygen Demand), TOC (Total Organic Carbon), ammonium nitrogen (NH4;-N), total nitrogen content (TN), nitrate concentration (NO3-N), nitrite concentration (NO2-N), orthophosphate-phosphorus (PO4-P) and total phosphorus (TP) were performed by a commercial company (Povodí Vltavy Ltd., České Budějovice) using certified methods for water management (Table 1).
Photosynthesis measurements
Photosynthetic activity of the cultures was measured ex-situ daily at 13:00 h. Before measurements, the culture samples were diluted to 0.2–0.3 g DW L−1 and dark-adapted for 10 min at cultivation temperature. Photosynthetic variables such as electron transport, photosynthetic efficiency, oxygen production, and respiration give a complete picture of the T. obliquus culture viability.
Rapid light-response curves
Rapid light-response curves (RLCs) of microalgal samples were measured in a light-protected measuring chamber with mixing (3 mL glass cuvette, light path of 10 mm) using a pulse-amplitude-modulation fluorimeter (PAM-2500, H. Walz, Germany). Analysis of RLCs was used to estimate changes in the relative electron transport rate (rETR) through photosystem II (PSII), calculated by multiplying the actual PSII photochemical yield YII [= (Fm’ – F’)/Fm’] with the respective PAR value (EPAR) (Hofstraat et al. 1994; Ralph and Gademann 2005; White et al. 2011). RLCs were fitted to the non-linear least-squares regression model using PamWin_3 software to determine the maximum electron transport rate (rETRmax) and the saturating irradiance EK (Eilers and Peeters 1988). The maximal PSII photochemical efficiency was calculated as the ratio of variable and maximal fluorescence, Fv/Fm = (Fm – Fo)/Fm (Strasser et al. 2004).
Photosynthetic oxygen evolution and respiration
The activity of photosynthetic oxygen evolution (POE) and respiration (RESP) was measured in an oxygen electrode chamber DW2/2 (Hansatech, UK) connected to a temperature-controlled water bath. The Oxylab + control unit (Hansatech, UK) and OxyTrace + Software were used in measurements as described previously (Ranglová et al. 2019). Light-response curves were recorded using 2-min light steps with stepwise increasing light intensities of 0, 200, 400, 600, 1200, and 1800 μmol photons m−2 s−1. The first step (no light) provided the information about RESP. Oxygen evolution was calculated in µmol O2 µg−1(Chl) h−1.
Bioactivity tests
At the end of the cultivation trials, the T. obliquus biomass was harvested taking 10 L of culture in the morning (08:00 h) and afternoon (13:00 h); where it was then centrifuged, washed, and lyophilized. Biomass samples of 10 mg DW mL−1 were re-suspended in distilled water and sonicated (Branson sonicator 150, amplitude 40%, 3 min) before carrying out assays of antimicrobial and biostimulant activity (Ranglová et al. 2020). The antimicrobial activities were evaluated using the antagonism bioassay. Two different tests were performed to determine the biostimulant activity: cress seed germination and mung bean rooting. All bioassays were performed in triplicate.
Antagonism Bioassays by Dual Culture
The antimicrobial activity of biomass samples was tested for phytopathogenic fungi, bacteria, and oomycetes using the dual culture bioassay, following the published protocols (Sánchez et al. 2008; Suárez-Estrella et al. 2014). The activity of the biomass extracts was tested against two fungi—Fusarium oxysporum f.sp. melonis CECT 20,474, Rhizoctonia solani CECT 2824, two oomycetes—Pythium ultimum CECT 2365 and Phytophthora capsici CECT 20,433, and four bacterial strains—Xanthomonas campestris CECT 95, Pectobacterium carotovorum subsp. carotovorum CECT 225, Pseudomonas syringae pv. tomato CECT 4393 and Clavibacter michiganensis subsp. michiganensis CECT 790. All strains were provided by the Spanish Bank of Algae (BEA) located in Gran Canaria (Spain).
Antagonism Bioassays against phytopathogenic fungi using the Dual Culture
Antagonist effect was demonstrated using modified techniques of Landa et al. (1997). First, 2% water agar (WA) plates were prepared. Once the agar solidified, four 8 mm-diameter steel cylinders were placed equidistantly from the edge. In the case of phytopathogenic fungi, a second layer of PDA was added to the WA plates. Once the cylinders were removed, the wells were filled with 80 μL of the cyanobacteria extract at a stock concentration of 10 mg mL−1. Then, a plug of 5-day-old phytopathogen agent culture, removed from a PDA plate, was placed in the center of the assay plate. Four replicates were prepared for each combination extract-phytopathogen. In vitro growth inhibition of fungi was measured at 25 ± 1 °C under dark conditions after 5 days. The inhibition index was calculated according the following formula: I = [(C—T) / C] × 100, where I is the Inhibition Index in percentage (%), C is the diameter of pathogenic culture in the absence of microalgal extract (mm) and T is the diameter of pathogen in the presence of algal extract (mm). In all cases, distilled water was used as a control.
Antagonism Bioassays against phytopathogenic bacteria by Dual Culture
In the case of phytopathogenic bacteria, a second layer of NA was added on the WA plates instead of PDA. A 48 h old bacterial culture prepared in 5 mL of Nutrient Broth (NB, CM0001 Oxoid Ltd. UK) and incubated at 30 ± 1 °C under dark conditions, was spread over the surface of the double layer of culture medium with a sterile swab previously soaked in the bacterial culture. The wells were filled with 80 μL of the different extracts. Four replicates were done for each extract-combination. A Petri dish inoculated only with the phytopathogenic bacteria and with distilled water inside the wells was considered as a negative control of the assay. The whole experiment was repeated twice.
Finally, inhibition of in vitro growth of phytopathgoenic bacteria was measured after incubating at 30 ± 1 °C under dark conditions for 48 h. The inhibition index (I) was expressed as the percentage of growth inhibition in the presence of the algal extract using the following formula: I = 100-[G-(D-8) / 90] × 100, where I is the Inhibition Index (%), G is the growth of the phytopathogenic agent in absence of the antagonistic extract (in this case it would be 90 mm, since the bioassays were carried out in 90 mm Petri dishes), and D is the diameter of growth inhibition around the wells in presence of antagonistic extracts (mm). Distilled water was used as a control.
Germination Index Bioassays
The effect of microalgal biomass on the seed growth was tested on 100 cress seeds (Lepidium sativum) using two extract concentrations, 0.5 and 2 mg DW mL−1, following the protocol described previously (Zucconi et al. 1981). The percentage of seed germination and the radicle elongation were calculated to assess the germination index (GI), based on the following formula: GI [%] = (GS x LS) / (GW x LW), GS is the percentage of germinated seeds in the presence of the sample, GW is the percentage of germinated seeds in the presence of distilled water, LS is the mean of radicle elongation [mm] in the presence of the sample and LW is the mean of radicle elongation [mm] in the presence of distilled water.
Mung bean bioassay
The bioassay was performed according to Hess (1961) using mung beans (Vigna radiata L. Wilczek). The biomass extracts of 0.5, 1.0, 2.0, and 3.0 g DW L−1 were used to determine the biostimulant activity via mung bean rooting. The number of roots longer than 1 mm was counted after 8 days of growth. To assess the auxin-like activity of microalgal extracts, a standard curve was prepared using different concentrations of 0, 0.3, 0.5, 0.7, and 1 mg DW L−1 of indol-3-butyric acid (IBA). The results are given in IBA equivalent concentrations.
The described design of bioactivity tests was based on the results of various sets of preliminary experiments that were carried out with lower and higher IBA concentrations and lower and higher biomass concentrations.
Analysis of micropollutants
Nine pharmaceuticals and endocrine disruptors (EDs) were analyzed only during the batch mode cultivation in Trial 2 to study compound removal; in semi-continuous mode, this assay would be distorted by the addition of fresh media. The 1-L samples taken from both cultivation units were immediately stabilized by the addition of EDTA (0.5 g L−1) and pH was adjusted to 2.5 to avoid chelation and/or biodegradation of the analytes. The samples were centrifuged and filtered (pores of 5–10 µm) to separate the aqueous (supernatant) and solid (biomass) fractions.
The aqueous samples were processed by solid-phase extraction using the hydrophilic-lipophilic-balanced (HLB) sorbent at pH = 7.5 (adjusted with NH4OH before extraction). The sorbent was conditioned with 3 mL of methanol (MeOH, > 99.9%) and 3 mL of milli-Q (MQ) water (purification system Barnstead Smart2Pure, Thermo Fisher, USA). After sample loading (6 mL min−1), sorbent rinsing (4 mL of MQ water), and a subsequent drying step (15 min), the analytes were eluted with 6 mL of MeOH. The solid samples were lyophilized and extracted by MeOH in three cycles at 80 °C and a pressure of 10 MPa using the Accelerated Solvent Extractor 200 (Dionex, USA). Aliquots of both extracts were taken for analysis by liquid chromatography with tandem mass spectrometry detection (LC–MS/MS).
Two analytical methods were used for the determination of micropollutants. The mobile phase consisted of (A) MeOH and (B) 0.5 mM NH4F (Honeywell, Fluka, Germany) in MQ for endocrine disruptors. For the analysis of pharmaceuticals, the water phase further contained 0.01% formic acid (LC–MS quality, Labicom, Czech Republic). Gradient separation was achieved using the 1260 Infinity II LC system (Agilent, USA) coupled to triple quadrupole 6470 (Agilent, USA) and equipped with a security guard and the Poroshell 120 EC-C18 chromatographic column (2.7 µm, 3.0 × 100 mm; Agilent, USA). Two multiple reaction monitoring (MRM) transitions were recorded for each analyte (quantifier and qualifier). External calibration in particular matrix (sample extract) was used for quantification. MassHunter Quantitative Analysis 10.0 was used for data handling. The concentration in the sample is expressed as the total analyte mass per liter of suspension.
Statistical analysis
All measurements and tests were carried out in triplicates unless stated otherwise. Sigma Plot 11.0 was used to determine significant differences between treatments. One-way ANOVA, Holm-Sidak test, and Friedman's non-parametric test were conducted for every binary combination of data. P-values lower than 0.05 were considered significantly different.
Results
Laboratory cultivation
In these trials the growth of T. obliquus was initially compared in two inorganic media, BG-11 and BBM medium (Andersen et al. 2005) using 3-L flat panel photobioreactors (PBRs). In a 7-day trial the specific growth rate of the culture in BG-11 was slightly lower (µ = 0.28 day−1) as compared to that in BBM medium (µ = 0.35 day−1). However, the total number of cells (size in the range of 8–12 µm) at the end of the experiment was more than 3 times higher in the BG-11 medium as compared to that found in the BBM medium. Nevertheless, BG-11 contains a much higher amount of nitrate (almost 6 times) when compared to BBM; therefore, the concentration of NO3− after 7 days of cultivation was still 8 mM, about half of the initial. This implied the greater suitability of the BG-11 medium for mass cultivation under high outdoor irradiance in thin-layer cultivation units. Thus, BG-11 was used for further outdoor cultivations.
Outdoor trials
The cultures of T. obliquus were grown in two different cultivation units – TLC and TL-RWP in batch mode (1st week) and subsequently in semi-continuous mode (2nd week) using the BG-11 medium (Trial 1) and the centrate from municipal WW (Trial 2) (Fig. 1). In each trial, both units were compared using the same source of nutrients either BG-11 or WW. Both cultures grew well; the higher biomass density was achieved in the BG-11 medium in both units (3.5 and 2.7 g L−1, for TLC and TL-RWP, respectively). In WW the biomass density was higher due to the presence of solid particles and reached 2.4 and 1.7 g L−1 in TLC and TL-RWP, respectively. Thus, when calculated between days 3 and 6, the growth rate values were in WW, 0.25 and 0.31 day−1 for TL-RWP and TLC, respectively, and in BG-11 0.21 and 0.25 day−1, respectively. It has to be reminded that the initial biomass density after inoculation was higher in BG-11 (1 g DW L−1) compared to WW (0.5 g DW L−1). The growth in TLC was faster by about 20–25% than that in TL-RWP. During the batch mode measurements (days 1–6) of nutrient removal, photosynthesis, bioactivity, and remediation were analyzed more rigorously, whereas the semi-continuous regime with daily dilutions was primarily intended to show the cultivation strategy which is considered to be more advantageous in terms of long-term biomass extraction.
Growth measured as the change of biomass density (DW) in T. obliquus MACC 677 cultured in batch and semi-continuous mode: A in BG 11 medium using thin-layer cascade (TLC) and thin-layer raceway pond (TL-RWP) and B in the centrate of municipal WW in TLC and TL-RWP. Several outdoor trials were carried out; exemplary ones are shown in the figure. Bars represent standard deviation as DW was measured in triplicate
Starting from day 6, the batch regime was altered to a semi-continuous regime, harvesting 25% (dilution rate DR = 0.25 day−1) of the culture volume daily and replaced by either BG-11 or undiluted WW.
Nutrient removal
Removal efficiency of NH4-N and PO4-P, the most abundant forms of nitrogen and phosphorus in WW, as well as TN and TP by T. obliquus was evaluated during the batch regime of Trial 2 (Table 2). The removal efficiency of NH4-N and PO4-P (calculated in %) was higher in TLC than that in TL-RWP as almost all N in the form of NH4-N (98.5%) was utilized in the former (Fig. 2). However, the removal efficiency of NH4-N, PO4-P, as well as total nitrogen (calculated in mg m−2 per day) was higher in TL-RWP (524; 352; 640 mg m−2 day−1) compared to TLC (441; 300; 549 mg m−2 day−1, respectively) due to the greater culture depth in TL-RWP. The daily supplementation in a semi-continuous regime showed to be efficient to maintain the growth for regular harvesting of biomass.
The concentrations of TN and TP found in TLC at the end of the batch regime were 94 mg L−1 and 68 mg L−1, respectively which was lower compared to TL-RWP where the concentration for TN and TP was 130 mg L−1 and 110 mg L−1, respectively. The lower uptake ability of the TL-RWP was probably caused by lower light availability (thicker culture layer) and thus a lower growth rate of the culture in TL-RWP (Fig. 1).
Photosynthetic performance
The changes in photosynthetic activity expressed as the maximum PSII photochemical yield (Fv/Fm) were monitored daily at 13:00 h (Fig. 3) An initial one-day lag phase (photoacclimation) was observed in the cultures grown in both units during Trial 1 and Trial 2 which corresponded to the lag phase found in the growth curve (Fig. 1). Compared to the 1-g cultures grown in BG-11 the initial Fv/Fm values were lower (0.55–0.6) in the more diluted cultures (0.5 g L−1) grown in WW as these were exposed to high outdoor irradiance. Moreover, the lower values of Fv/Fm during the first days can also be attributed to ammonium inhibition (Li et al. 2019; Wang et al. 2019). After the initial acclimation, from the second day, both cultures quickly recovered as Fv/Fm ranged between 0.69 and 0.77 showing that the cultures were in very good physiological condition. Small changes in the Fv/Fm values could be caused by weather fluctuation (irradiance, temperature) during the trials (Supplementary Figs. S1 and S2). During the first day of both trials, the POE activity was low (Fig. 4). The maximum POE on day 1 was about 200 µmol O2 µg−1(Chl) h−1. This could be affected by photo-stress due to acclimation of the laboratory grown culture to outdoor conditions in accord with the Fv/Fm measurements. During the growth in batch mode, the maxima of POE activity measured in BG-11 medium were higher in TLC reaching almost up to 400 µmol O2 µg−1(Chl) h−1 as compared to the TL-RWP with the POE maxima of about 280 µmol O2 µg−1(Chl) h−1. When grown in semi-continuous mode, the POE was about 200 µmol O2 µg−1(Chl) h−1, almost comparable in both cultures. It means that POE during the semi-continuous regime decreased as the denser culture obtained less light. The RESP rate in TL-RWP was increasing as the cultures were becoming more dense and low-light adapted due to the thicker culture layer as compared to the thinner ones in the TLC. When the cultures started to be diluted in semi-continuous mode after 6 days of cultivation, the RESP rate decreased again. During the batch regime of the cultivation performed in WW, the POE was mostly higher in TLC, keeping the activity between 360 and 750 µmol O2 µg−1(Chl) h−1 as compared to the lower activity found in TL-RWP, where the POE was between 230 and 670 µmol O2 µg−1(Chl) h−1. In general, higher POE was observed in cultures grown in WW probably caused by better weather conditions (higher irradiance and temperature) during the batch mode in WW (as compared to BG-11) while the activity of RESP was variable (Supplementary Figs. S1 and S2).
Changes in the maximum quantum yield of PSII (Fv/Fm) in the culture of T. obliquus cultivated in thin-layer cascade (TLC; black bars) and thin-layer raceway pond (TL-RWP; grey bars) in batch and semi-continuous (25% dilution rate; 0.25 day−1) regime using two different nutrient sources: A BG-11 medium (Trial 1), B centrate from municipal WW (Trial 2). Error bars represent analytical standard deviation as the measurements were performed in triplicate. Values with the same symbol did not differ significantly from each other (P > 0.05) when evaluated by one-way ANOVA and Holm-Sidak test
Changes in photosynthetic oxygen evolution (POE) and respiration (RESP) during the cultivation trials Tetradesmus obliquus in thin-layer cascade (TLC) and thin-layer raceway pond (TL-RWP) when BG-11 and the centrate from municipal WW were used as cultivation media. The maxima of A POE and B RESP were measured after 10 min dark adaptation in culture samples taken at 13:00 h
Bioactivity determination
In this work the antimicrobial activity of the biomass extracts sampled at the end of both trials was assessed against 8 plant pathogens. In all trials the samples for assays were taken on two times, as the production of different concentrations of substances responsible for required bioactivity was expected. This consideration was based on the results published previously as the biopesticide activity of Chlorella biomass was usually higher when the biomass was harvested in the afternoon (Ranglová et al. 2021).
No antimicrobial activity was found in the biomass extract from Trial 1 (BG-11). On the contrary, the highest antibacterial activity against C. michiganensis was observed in the biomass extract of the culture grown in WW in the TLC unit which was harvested in the morning (08:00 h) (Table 3). The same cultivation also revealed high antifungal activity against F. oxysporum and R. solani, with an inhibition index ranging from 27 to 35% and from 39 to 45%, respectively. All biomass extracts from the cultures grown in WW also showed high antimicrobial activity with inhibition indexes ranging from 24 to 49% against oomycete P. capsici. No antibacterial activities against P. ultimum, P. carotovorum, P syringae, and X. campestris were found.
No biostimulant activity of the biomass harvested at the end of Trial 1 (BG-11 medium) was observed when assayed the germination of cress seeds. In the case of Trial 2 (WW), we can say in general, that, no significant change in biostimulant activity was found in the samples harvested from TLC (97–101%) and a partial increase of that might be seen in the samples harvested from TL-RWP (90–100%) (not shown here).
The mung bean bioassay showed the highest auxin-like activity, equivalent to 0.7 mg DW L−1 of IBA, in the extracts of 2 and 3 g DW L−1 when the samples were harvested from both units in the morning. When the cultures were harvested at midday, a similar auxin-like activity was observed only at the highest biomass concentration (Table 4). No effect was found on mung bean rooting of the biomass grown in WW.
Remediation of WW
In these experiments, the centrate was taken from the WW treatment plant after aerobic digestion, the pharmaceuticals and endocrine disruptors occur in the efflux even after complete processing. Relatively short (4-day) trials were carried out to minimize the overlap of microalgal impact and spontaneous decay of these xenobiotics. The assessment of nine compounds – amitriptyline (antidepressant), diclofenac (nonsteroidal anti-inflammatory drug), carbamazepine (anticonvulsant), bisphenol A (plastics additive), gabapentin (anticonvulsant), cetirizine (antihistamine), hydrochlorothiazide (diuretic for high blood pressure treatment), ibuprofen (nonsteroidal anti-inflammatory drug) and tetracycline (antibiotic) showed that the remediation in the cultures grown in both TLC and TL-RWP units was similar. Almost all compounds apart from diclofenac and gabapentin were successfully removed from the supernatant (p < 0.05). However, diclofenac, carbamazepine, gabapentin, cetrizine and tetracycline remained in the pellet after 4-days of cultivation (Fig. 5). Three effects of xenobiotics occurrence can be described: (i) First, amitriptyline, bisphenol A, and hydrochlorothiazide were not accumulated in the biomass and their content in the medium decreased. Here, we can speculate about the effect on the environment. (ii) The second group of xenobiotics, namely carbamazepine and cetirizine accumulated exclusively in the biomass. (iii) The third group of analyzed compounds – diclofenac, gabapentin, tetracycline, and ibuprofen were accumulated in the biomass but were also partly found in the medium As concerns Bisphenol A and ibuprofen, these were present in high initial concentrations, about 100 and 4000—5000 ng L−1, respectively, but their removal in the cultivation trial of T. obliquus was efficient. Moreover, the complete removal of bisphenol A and amitriptyline within two days was observed in TLC as well as in TL-RWP. The concentration of diclofenac slightly increased during the cultivation in both, supernatant (aqueous phase) as well as in biomass (solid phase). Tetracycline and gabapentin were present in the supernatant as well as in the biomass and their concentration decreased by 10–30% at the end of the trials.
Assessment of micropollutants in biomass (solid phase, open columns) and supernatant (aqueous phase, dashed columns) in the samples harvested from the T. obliquus cultures grown in the centrate from municipal WW in the 4-day trials (Trial 2) using A thin-layer cascade (TLC) and B thin-layer raceway pond (TL RWP). Results are shown as a mean ± standard deviation (n = 3). The degradability of individual pharmaceuticals was accessed by the Friedman's non-parametric test. Data with P-values lower than 0.05 were considered significantly different
Discussion
In this study the fast-growing green microalga Tetradesmus obliquus was grown in the centrate collected from a local wastewater treatment plant in Třeboň. The aim was to assess nutrient and micropollutant removal capacity as well as potential biopesticidal and biostimulating activity of produced biomass. Instead of traditional deep culture raceway ponds, the thin-layer cultivation systems are suitable due to light utilization for microalgal growth in the dark-colored WW (Acién et al. 2017). Previously, the culturing of Scenedesmus sp. in undiluted centrate after anaerobic digestion as a source of nutrients was reported (Morales-Amaral et al. 2015a, b).
This study also includes the selection of a suitable inorganic medium which could serve as a control medium for outdoor cultivation in organic wastewater. Inorganic BG-11 medium and BBM medium were compared in this experiment. Significantly lower cell number (after 7 days of growth) measured in the culture grown in BBM medium was caused by the consumption of all nitrogen, which is accompanied by the cessation of cell division and the accumulation of nutrients in the cells (Danesh et al. 2017). The specific growth rate was not affected which was expressed as the increase in the dry weight.
During outdoor cultivation both cultures (in TLC and TL-RWP) grew well in WW except day 1 when a short lag phase was observed; then the growth accelerated. The higher biomass density achieved in BG-11 was probably affected by the slightly higher initial microalgal biomass density when compared to WW. Both inocula were diluted enough to build up a typical microbial growth curve.
Some studies have reported that microalgae prefer to utilize NH4+ if both NH4+ and N-NO3 are available (Xin et al. 2010; Manser et al. 2016; Schulze et al. 2017). The optimal inorganic N/P ratio for freshwater microalgal growth was found in the range of 6.8–10 (Wang et al. 2010). The BG-11 medium used for outdoor cultivation (Trial 1) contains only N-NO3 (250 mg L−1) while in WW (Trial 2) the TN content was 290 mg L−1 and the most abundant nitrogen form represented NH4+ (160 mg L−1). The content of phosphate (PO43−) in the WW was 120 mg L−1 (TP 160 mg L−1) giving the N:P ratio of about 2:1. The N consumption was similar as in BG-11 while the P removal was about twice slower in batch mode although the P content was 22 times higher than in BG-11. Utilization of 83% N as NH4+ and 90% P as PO43− was reported by the chlorophyte Chlorella sp. from municipal WW (Wang et al. 2010) showing that the nutrient removal rates were independent of the optimal N/P ratio. This finding corresponds to our results for T. obliquus as 98.5 and 82% utilization of N as NH4+ and 89 and 73% of P as PO43− were measured after a 6-day batch cultivation trial in TLC and TL-RWP, respectively. Comparing both units, the TL-RWP showed to be more effective in the removal of NH4-N and PO4-P due to the greater depth and total volume of this unit. Here, it has to be considered that some ammonia may have been lost due to release in gaseous form (Álvarez and Otero 2020). This assumption was observed mainly during the first two days of cultivation when the ammonium dropped by 76% in TLC and 63% in TL-RWP respectively, while the dry weight of the culture remained almost unchanged. The specific phenomenon referred to as “luxury uptake” was observed at the same time for P-PO4 which also decreased dramatically during the first two days. According to Solovchenko et al. (2019a, b) and Brown and Shilton (2014) this could be due to the high content of phosphorus in wastewater when microalgae uptake more phosphorus than required for growth. Interesting is also that the removed PO4-P content, 300 mg m−2 day−1 for TLC and 352 mg m−2 day−1 for TL-RWP was higher than the total removed phosphorus content of 202 mg m−2 day−1, 120 mg m−2 day−1, respectively. This could have been a result of cell rupture, releasing the intercellular phosphate content into the medium, a phenomenon that has been described by Martinez et al. (2000).
Our data suggests that a longer cultivation period in the batch regime could provide the necessary output to reach the admissible values for WW discharge to the environment in the range of 10–15 mg TN L−1 and 1–2 mg TP L−1 (directive 91/271/EEC 1991). The settings of the experiment when the batch mode was selected for only 6 days is based on 2 previous publications (Ranglová et al. 2021, Carneiro et al. 2021) where Chlorella MACC-1 and co-culture of MACC-1 and T. obliquus MACC-677 (used in this work) were cultivated in the same cultivation units. During the cultivation of Chlorella, the content of ammonia was completely consumed after 2 days, the amount of nitrate was consumed in TLC after 3 days and in TL-RWP after 7 days. During the co-cultivation when MACC-1 and MACC-677 (used in this work as a monoculture), the ammonia level was completely consumed after 3 days from the original 150 mg L−1 and the nitrate content was consumed after 7 days. Based on these findings, when the weekly experiment provided a sufficient amount of biomass and at the same time almost absolute exhaustion of nutrients, 6 days of cultivation in the batch mode were chosen as sufficient for this experiment as well. The strain would probably be able to remove a higher amount of nutrients in a longer batch cultivation period but these trials aimed to study the growth sustainability and biomass production of T. obliquus in WW using the semi-continuous regime to obtain applicable data for cultivation in large volumes of several hectares (Fig. 2).
The productivity of microalgal culture in any photobioreactor predominantly depends on the rate of photosynthesis where the most crucial substrate, light (Zijffers et al. 2008), is directed by the ratio of the exposed surface to total volume (S/V) ratio (Costa and de Morais 2013). In this respect, the thin-layer culturing systems are most suitable.
Photosynthetic measurements – Chl fluorescence quenching and oxygen production have been used as reliable and sensitive techniques to monitor the photosynthetic activity of microalgal cultures as they reflect the performance of photosynthetic processes, and consequently the physiological status and growth of the culture. These methods have often been combined to examine the effects of adverse environmental conditions – high irradiance, temperature extremes, nutrient deficiency, and their synergism on microalgal growth (Torzillo et al. 1998; Masojídek et al. 2011; Figueroa et al. 2013). Photosynthesis production measurements can cover a wider range of metabolic pathways as compared to Chl fluorescence data. On the other hand, Chl fluorescence techniques – compared with measurements of O2 production – can generate analogous, but complementary information, but these techniques are considerably faster, more sensitive, and can provide data on energy distribution between the photochemical and non-photochemical (heat dissipation) processes (for a review see Baker 2008; Masojídek et al. 2011; Malapascua et al. 2014). Chl fluorescence measurements record changes in the redox status of the PSII complex which depends on the rate of photosynthetic electron transport while oxygen production measurements cover a wider range of metabolic pathways as compared to Chl fluorescence. Practically, good photosynthetic performance (measured as Fv/Fm) and oxygen evolution resulting in fast growth and high biomass density was observed in thin-layer units. Moreover, the T. obliquus culture grew well in WW (Trial 2). Thus, based on our measurements, the photosynthetic variables and oxygen evolution can be used to monitor and estimate the growth (and productivity) of the microalgal cultures. One of the major hydrodynamic and photosynthetic advantages of both thin-layer systems used in this study, is efficient mixing inducing fast light–dark cycles (matching the turnover of the photosynthetic apparatus), due to a cell to cell shading effect and the efficient gas exchange (Richmond 2003; Masojídek et al. 2011) which results in high productivity even at high biomass density. Moreover, the ambient irradiance in shallow cultures positively affected the growth of T. obliquus in brownish-colored WW compared to widely used deep culture units (Acién et al. 2017; Carneiro et al. 2021).
Concerning the production of biomass and bioactive compounds, microalgae have higher photosynthetic efficiency (carbon fixation) compared to terrestrial plants due to short life cycles and lower energy requirements for competitive metabolic processes, and thus give high biomass yield (Packer 2009; Lakatos et al. 2021).
The bioactivity of the biomass was not affected by slightly different initial biomass in BG-11 and WW because samples for activity determination were taken in the log phase and the activity was calculated per mg of biomass. The ability of microalgae to grow in WW predispose them toreuse of industrial and municipal waste and biofixation of CO2 (Acién et al. 2016). In addition, nutrients fixed in the biomass can be released by bacterial activity when it is applied to the soil, making them available to plants as biofertilizers (Mulbry et al. 2005). The potential use of microalgal biomass obtained from the cultivation in WW as a bio-fertilizer for plants was successfully demonstrated by several studies (Mulbry et al. 2005, 2007; Bird et al. 2012; Das et al. 2019).
Here, we may speculate that WW contains substances and microorganisms which can stimulate the production of bioactive compounds.
Microalgae can synthesize various compounds such as polyphenols, tocopherols, carbohydrates, proteins, saponins, or signal molecules with potential biopesticide activities (Crouch and van Staden 1993; Costa et al. 2019). These cultures can recycle nutrients and produce some compounds responsible for antimicrobial activity and other plant growth-promoting substances such as amino acids, vitamins, various enzymes, and polyamines. In addition, microbial communities present in WW and cultivation units contribute to this phenomenon (Tate et al. 2013; Grivalský et al. 2016; Mógor et al. 2018; Plaza et al. 2018; Costa et al. 2019). Thus, the important part of our study was to show that T. obliquus can grow in the centrate of urban WW as a low-cost nutrient source and the subsequential biomass use for agricultural purposes as a biopesticide and biofertilizer. These trials revealed the possibility to prevent the growth of plant pathogens. The harvested biomass was active against selected strains of pathogenic fungi (R. solani, F. oxysporum), oomycetes (P. ultimum, P. capsici), and bacteria (C. michiganensis). However, it is difficult to find products similar to our sonicated algae extracts we have experimented with other types of products based on algal biomass. The results derived from the dual culture bioassays using a commercial brand of algal biomass (ARIES UMweltprodukte GmbH & Co.KG) was significantly worse in comparison with those obtained from our sonicated T. obliquus extracts (Table 3).
The effect of microalgal biomass on the seed growth tested at a concentration of 0.5 and 2 mg DW mL−1 showed no phytotoxicity problems. Although there are no significant differences between both concentrations in relation to the germination rate, other similar studies have revealed that concentrations of 2 mg DW mL−1 are more phytotoxic than 0.5 mg DW mL−1 (Toribio et al. 2020, 2021). Therefore, the results derived from this work were very positive, since the applied cultivation protocols did not generate phytotoxic products. Similarly, other commercial brands prepared from algal biomass (ARIES UMweltprodukte GmbH & Co.KG), showed a very discrete promotion of root development (around 100–107% germination rate) after application on cress seeds (unpublished data).
The lack of biostimulant activity in these bioassays indicated the presence of some inhibitors in the freeze-dried samples of WW (Garcia et al. 1995) which could also contribute to higher antimicrobial activity.
One of the significant benefits of microalgal cultivation in WW is the ability to facilitate the removal of contaminants as many of them are stated as toxic and endocrine disruptors (Kümmerer 2009). Tetradesmus obliquus can be cultivated in different types of WW containing different source of nutrients and toxic compounds (Oliveira et al. 2021).
Various mechanisms, e.g., biodegradation, photodegradation, sorption, bioaccumulation are involved in the removal of compounds during the microalgae cultivation (Hena et al. 2021). Although the aim of the this study was not to identify the removal mechanisms and spontaneous decay which can not be distinguished from the microalgal remediation, at least the contribution of sorption and bioaccumulation could be partly assessed from the data of concentration in biomass. Generally, the sorption is probably species-specific (Hena et al. 2021).
The distribution of chemicals between aqueous (supernatant) and solid (biomass) phase is described using partition coefficient (Kow). The correlation of Kow and bioaccumulation by microalgae (Chlorella fusca) was observed for 41 organic chemicals in laboratory experiments (Geyer et al. 1984). However, no relationship was found between Kow and concentration in biomass and supernatant in this study (for example, compare log Kow of bisphenol A = 3.32 and log Kow of cetirizine = 1.7 and their concentration in biomass and supernatant). It could be due to many factors, but most likely because of differing initial concentrations of studied chemicals. In our study, ibuprofen was identified as the most widespread drug in watercourses in the Czech Republic, with a maximum concentration of 3210 ng L−1 (Marsik et al. 2017). However, five chemicals accumulated in the biomass which should be considered before the further biomass use as the compounds were not transformed or inactivated.
Escapa et al. (2016) determined a removal of diclofenac up to 79% from culture medium by T. obliquus while two algal strains performed lower remediation. In our study, diclofenac was not totally removed from supernatant and sorption to biomass was observed. Moreover, an increase in the supernatant concentration was measured what is in accordance to study of Zhou et al. (2014). The increase could be result of transformation of metabolites of diclofenac. On the other hand, the increase was not observed in this study for ibuprofen as it was described by Zhou et al. (2014). Biotransformation of ibuprofen to hydroxylated products by algae and bacteria was proved in the study of Larsen et al. (2019).
Poor removal for carbamazepine was observed in lab scale cultivation of different algal strains including T. obliquus (Tolboom et al. 2019). No transformation was observed in a bioreactor with Scenedesmus obliquus (Larsen et al. 2019). In our study, only sorption was recorded for carbamazepine. Similarly, the sorption to Scenedesmus almeriensis was an important removal mechanism for antibiotics e.g. tetracycline (Zambrano et al. 2021).
Total removal of bisphenol A after 7 days in a lab scale experiment when using WW was shown for four algal strains including T. obliquus (Zhou et al. 2014) and our data supports the high degradation potential of bisphenol A potentially even on a larger scale. Similarly, amitriptyline was completly removed in few days by S. obliquus in a lab scale experiment in growth medium (Gojkovic et al. 2019). It is in accordance to this study.
Overall, various removal rates (0 – 100%) achieved for different algal strains were summarized for several contaminats such as bisphenol A, diclofenac carbamazepine, and ibuprofen (Sousa et al. 2022). The removal of other studied compounds could not be discussed as there is a lack of literature.
Conclusions
Both batch and semi-continuous cultivation of Tetradesmus obliquus in outdoor pilot-scale units (up to 200 L) using WW were feasible with no limitations. The culture performance and growth were comparable with that of inorganic media. Photosynthesis monitoring (POE, Fv/Fm, ETR, etc.) proved to be a rapid and reliable indicator of culture viability. Nitrogen and phosphorus stripping from WW were effective. Moreover, the biomass of T. obliquus grown in WW revealed antimicrobial activity against plant pathogens. The cultures also showed the ability to decompose or accumulate some pharmaceuticals and endocrine disruptors that are found in communal WW.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Acién FG, Gómez-Serrano C, Morales-Amaral MM, Fernández-Sevilla JM, Molina-Grima E (2016) Wastewater treatment using microalgae: how realistic a contribution might it be to significant urban wastewater treatment? Appl Microbiol Biotechnol 100:9013–9022
Acién FG, Molina E, Reis A, Torzillo G, Zittelli GC, Sepulveda C, Msojidek J (2017) Photobioreactors for the production of microalgae. In: Gonzalez-Fernandez C, Muñoz R (eds) Microalgae-Based Biofuels and Bioproducts. From Feedstock Cultivation to End-Products. Woodhead Publishing, Duxford pp 1–44
Allen MM (1968) Simple conditions for growth of unicellular blue-green algae on plates. J Phycol 4:1–4
Álvarez X, Otero A (2020) Nutrient removal from the centrate of anaerobic digestion of high ammonium industrial wastewater by a semi-continuous culture of Arthrospira sp. and Nostoc sp. PCC 7413. J Appl Phycol 32:2785–2794
Andersen RA, Berges JA, Harrison PJ, Watanabe MM (2005) Recipes for Freshwater and Seawater Media (Appendix). In: Andersen A (ed) Algal Culturing Techniques. Elsevier, Amsterdam, p 578
Baker NR (2008) Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Baldev E, Ali DM, Pugazhendhi A, Thajuddin N (2021) Wastewater as an economical and ecofriendly green medium for microalgal biofuel production. Fuel 294:120484
Bird MI, Wurster CM, de Paula Silva PH, Paul NA, de Nys R (2012) Algal biochar: Effects and applications. GCB Bioenergy 4:61–69
Bischoff HW, Bold HC (1963) Some soil algae from Enchanted Rock and related algal species. Phycological Studies, University of Texas IV, pp 1–95
Bishop MW, Zubeck MH (2012) Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J Nutr Food Sci 2:147
Borowitzka MA (2013) High-value products from microalgae-their development and commercialisation. J Appl Phycol 25:743–756
Brown N, Shilton A (2014) Luxury uptake of phosphorus by microalgae in waste stabilisation ponds: current understanding and future direction. Rev Environ Sci Biotechnol 13:321–328
Carneiro M, Ranglová K, Lakatos GE, Manoel JAC, Grivalský T, Kozhan DM, Toribio A, Moreno J, Otero A, Varela J, Malcata FA, Suárez-Estrella F, Acién JG, Molnár Z, Ördög V, Masojídek J (2021) Growth and bioactivity of two chlorophyte (Chlorella and Scenedesmus) strains co-cultured outdoors in two different thin-layer units using municipal wastewater as a nutrient source. Algal Res 56:102299
Costa JAV, de Morais MG (2013) An open pond system for microalgal cultivation. In: Pandey A, Lee D-J, Chisti Y, Soccol CR (eds) Biofuels from Algae. Elsevier, Amsterdam pp 1–22
Costa JAV, Freitas BCB, Cruz CG, Silveira J, Morais MG (2019) Potential of microalgae as biopesticides to contribute to sustainable agriculture and environmental development. J Environ Sci Health B 54:366–375
Crouch IJ, van Staden J (1993) Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul 13:21–29
Danesh AF, Ebrahimi S, Salehi A, Parsa A (2017) Impact of nutrient starvation on intracellular biochemicals and calorific value of mixed microalgae. Bioch Eng J 15:56–64
Das P, Quadir MA, Thaher MI, Alghasal GSHS, Aljanbri HMSJ (2019) Microalgal nutrients recycling from the primary effluent of municipal wastewater and use of the produced biomass as bio-fertilizer. Int J Environ Sci Technol 16:3355–3364
Directive 91/271/EEC C (1991) Council Directive of 21 May 1991 concerning urban wastewater treatment. Off J Eur Communities L135:40–52
Dos Santos MDO, Martins MA, Coimbra JSdR, Gates RS, Corredo LdP (2013) Rheological behavior of Chlorella sp. and Scenedesmus sp. cultures in different biomass concentrations. Eng Agric 33:1063–1071
Eilers PHC, Peeters JCH (1988) A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol Modell 42:199–215
Escapa C, Coimbra RN, Paniagua S, García AI, Otero M (2016) Comparative assessment of diclofenac removal from water by different microalgae strains. Algal Res 18:127–134
Figueroa FL, Jerez CG, Korbee N (2013) Use of in vivo chlorophyll fluorescence to estimate photosynthetic activity and biomass productivity in microalgae grown in different culture systems. Lat Am J Aquat Res 41:801–819
Garcia C, Ceccanti B, Masciandaro G, Hernandez T (1995) Fractionation and characterization of humic substance fractions with different molecular weights, obtained from animal wastes. Soil Sci Plant Nutr 41:649–658
Geyer H, Politzki G, Freitag D (1984) Prediction of ecotoxicological behaviour of chemicals: Relationship between n-octanol/water partition coefficient and bioaccumulation of organic chemicals by alga Chlorella Chemosphere 13:269–284
Gojkovic Z, Lindberg RH, Tysklind M, Funk C (2019) Northern green algae have the capacity to remove active pharmaceutical ingredients. Ecotoxicol Environ Saf 170:644–656
Grivalský T, Bučková M, Puškárová A, Kraková L, Pangallo D (2016) Water-related environments : a multistep procedure to assess the diversity and enzymatic properties of cultivable bacteria. World J Microbiol Biotechnol 32:42
Grivalský T, Ranglová K, da Câmara Manoel JA, Lakatos GE, Lhotský R, Masojídek J (2019) Development of thin-layer cascades for microalgae cultivation: milestones (review). Folia Microbiol (praha) 64:603–614
Hena S, Gutierrez L, Croué JP (2021) Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J Hazard Mater 403:124041
Hess CEC (1961) Mung bean bioassay for the detection of root promoting substances. Plant Physiol 36:21
Hofstraat JW, Peeters JCHH, Snel JFHH, Geel C (1994) Simple determination of photosynthetic efficiency and photoinhibition of Dunaliella tertiolecta by saturating pulse fluorescence measurements. Mar Ecol Prog Ser 103:187–196
Jensen HL (1957) Biological transformation of thiourea. Arch Mikrobiol 28:145–152
Katarzyna L, Sai G, Avijeet Singh O (2015) Non-enclosure methods for non-suspended microalgae cultivation: Literature review and research needs. Renew Sustain Energy Rev 42:1418–1427
Kümmerer K (2009) Antibiotics in the aquatic environment - A review - Part I. Chemosphere 75:417–434
Lakatos GE, Ranglová K, Manoel JC, Grivalský T, Masojídek J (2021) Photosynthetic monitoring techniques indicate maximum glycogen accumulation in nitrogen-limited Synechocystis sp. PCC 6803 culture. Algal Res 55:102271
Landa BB, Hervás A, Bettiol W, Jiménez-Díaz RM (1997) Antagonistic activity of bacteria from the chickpea rhizosphere against Fusrium oxysporum f. sp. ciceris. Phytoparasitica 25:305–318
Larsen C, Yu ZH, Flick R, Passeport E (2019) Mechanisms of pharmaceutical and personal care product removal in algae-based wastewater treatment systems. Sci Total Environ 695:133772
Lau PS, Tam NFY, Wong YS (1995) Effect of algal density on nutrient removal from primary settled wastewater. Environ Pollut 89:59–66
Li X, Li W, Zhai J, Wei H, Wang Q (2019) Effect of ammonium nitrogen on microalgal growth, biochemical composition and photosynthetic performance in mixotrophic cultivation. Bioresour Technol 273:368–376
Li Y, Chen YF, Chen P, Min M, Zhou W, Martinez B, Zhu J, Ruan R (2011) Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour Technol 102:5138–5144
Malapascua JRF, Jerez CG, Sergejevová M, Figueroa FL, Masojídek J (2014) Photosynthesis monitoring to optimize growth of microalgal mass cultures: application of chlorophyll fluorescence techniques. Aquat Biol 22:123–140
Manser ND, Wang M, Ergas SJ, Mihelcic JR, Mulder A, van de Vossenberg J, van Lier JB, van der Steen P (2016) Biological nitrogen removal in a photosequencing batch reactor with an algal-nitrifying bacterial consortium and anammox granules. Environ Sci Technol Lett 3:175–179
Marsik P, Rezek J, Židková M, Kramulová B, Tauchen J, Vaněk T (2017) Non-steroidal anti-inflammatory drugs in the watercourses of Elbe basin in Czech Republic. Chemosphere 171:97–105
Martınez ME, Sánchez S, Jimenez JM, El Yousfi F, Munoz L (2000) Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour Technol 73:263–272
Masojídek J, Kopecký J, Giannelli L, Torzillo G (2011) Productivity correlated to photobiochemical performance of Chlorella mass cultures grown outdoors in thin-layer cascades. J Ind Microbiol Biotechnol 38:307–317
Masojídek J, Sergejevová M, Malapascua JR, Kopecký J (2015) Thin-layer systems for mass cultivation of microalgae: flat panels and sloping cascades. In: Prokop A, Bajpai RK, Zappi ME (eds) Algal Biorefineries, vol 2. Cham, pp 237–261
Mógor ÁF, Ördög V, Lima GPP, Molnar Z, Mógor G (2018) Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J Appl Phycol 30:453–460
Molina Grima E, Belarbi EH, Acién Fernández FG, Robles Medina A, Chisti Y (2003) Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol Adv 20:491–515
Morales-Amaral MdM, Gómez-Serrano C, Acién FG, Fernandez-Sevilla JM, Molina-Grima E (2015) Production of microalgae using centrate from anaerobic digestion as the nutrient source. Algal Res 9:297–305
Morales-Amaral MdM, Gómez-Serrano C, Acién FG, Fernández-Sevilla JM, Molina-Grima E (2015b) Outdoor production of Scenedesmus sp. in thin-layer and raceway reactors using centrate from anaerobic digestion as the sole nutrient source. Algal Res 12:99–108
Morales M, Collet P, Lardon L, Helias A, Steyer J-P, Bernard O (2019) Life-cycle assessment of microalgal-based biofuel. In: Pandey A, Chang JS, Soccol CR, Lee DJ, Chist Y (eds) Biofuels from Algae. Elsevier, Amsterdam, pp 507–550
Morillas-España A, Lafarga T, Acién-Fernández FG, Gómez-Serrano C, González-López CV (2021) Annual production of microalgae in wastewater using pilot-scale thin-layer cascade photobioreactors. J Appl Phycol 33:3861–3871
Mulbry W, Kondrad S, Pizarro C (2007) Biofertilizers from algal treatment of dairy and swine manure effluents: Characterization of algal biomass as a slow release fertilizer. J Veg Sci 12:107–125
Mulbry W, Westhead EK, Pizarro C, Sikora L (2005) Recycling of manure nutrients: Use of algal biomass from dairy manure treatment as a slow release fertilizer. Bioresour Technol 96:451–458
Oliveira CYB, Oliveira CDL, Prasad R, Ong HC, Araujo ES, Shabnam N, Gálvez AO (2021) A multidisciplinary review of Tetradesmus obliquus: a microalga suitable for large-scale biomass production and emerging environmental applications. Rev Aquac 13:1594–1618
Ördög V, Stirk WA, Bálint P, Lovász C, Pulz O, van Staden J (2013) Lipid productivity and fatty acid composition in Chlorella and Scenedesmus strains grown in nitrogen-stressed conditions. J Appl Phycol 25:233–243
Packer M (2009) Algal capture of carbon dioxide; biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energy Policy 37:3428–3437
Pereira EG, Martins MA, Mendes MDSA, Mendes LBB, Nesi AN (2017) Outdoor cultivation of Scenedesmus obliquus BR003 in stirred tanks by airlift. Eng Agric 37:1041–1055
Plaza BM, Gómez-Serrano C, Acién-Fernández FG, Jimenez-Becker S (2018) Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus sp.) on Petunia x hybrida growth. J Appl Phycol 30:2359–2365
Ralph PJ, Gademann R (2005) Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Ranglová K, Lakatos GE, Câmara Manoel JA, Grivalský T, Suárez Estrella F, Acién Fernández FG, Molnár Z, Ördög V, Masojídek J (2021) Growth, biostimulant and biopesticide activity of the MACC-1 Chlorella strain cultivated outdoors in inorganic medium and wastewater. Algal Res 53:102136
Ranglová K, Lakatos GE, Manoel JAC, Grivalský T, Masojídek J (2019) Rapid screening test to estimate temperature optima for microalgae growth using photosynthesis activity measurements. Folia Microbiol (Praha) 64:615–625
Richmond A (2003) Growth characteristics of ultrahigh-density microalgal cultures. Biotechnol Bioprocess Eng 8:349–353
Romero-Villegas GI, Burboa-Charis VA, Navarro-López E, Cerón-García MC, Acién-Fernandez FG, Estrada-Alvarado MI, Rout NP, Cira-Chávez LA (2021) Biomass production and urban centrate nutrient removal using native microalgae tolerant to high nitrogen concentration and temperature. J Appl Phycol 33:2921–2931
Sánchez JF, Fernández JM, Acién FG, Rueda A, Perez-Parra J, Molina E (2008) Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis Process Biochem 43:398–405
Schulze PS, Carvalho CF, Pereira H, Gangadhar KN, Schüler LM, Santos TF, Varela JC, Barreira L (2017) Urban wastewater treatment by Tetraselmis sp. CTP4 (Chlorophyta). Bioresour Technol 1:175–183
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: A signature of photosynthesis. Springer, Dordrecht, pp 321–362
Solovchenko A, Khozin-Goldberg I, Selyakh I, Semenova L, Ismagulova T, Lukyanov A, Mamedov I, Vinogradova E, Karpova O, Konyukhov I, Vasilieva S, Mojzes P, Dijkema C, Vecherskaya M, Zvyagin I, Nedbal L, Gorelova O (2019a) Phosphorus starvation and luxury uptake in green microalgae revisited. Algal Res 43:101651
Solovchenko AE, Ismagulova TT, Lukyanov AA, Vasilieva SG, Konyukhov IV, Pogosyan SI, Lobakova ES, Gorelova OA (2019b) Luxury phosphorus uptake in microalgae. J Appl Phycol 31:2755–2770
Sousa H, Sousa CA, Simões LC, Simões M (2022) Microalgal-based removal of contaminants of emerging concern. J Hazard Mater 423:127153
Suárez-Estrella F, Ros M, Vargas-García MC, Lopez MJ, Moreano J (2014) Control of Xanthomonas campestris pv. vesicatoria using agroindustrial waste-based compost. J Plant Pathol 96:243–248
Tam NFY, Wong YS (1989) Wastewater nutrient removal by Chlorella pyrenoidosa and Scenedesmus sp. Environ Pollut 58:19–34
Tate JJ, Gutierrez-Wing MT, Rusch KA, Benton MG (2013) The effects of plant growth substances and mixed cultures on growth and metabolite production of green algae Chlorella sp.: A Review. J Plant Growth Regul 32:417–428
Tolboom SN, Carrillo-Nieves D, de Jesús R-A, de la Cruz QR, Barceló D, Iqbal HM, Parra-Saldivar R (2019) Algal-based removal strategies for hazardous contaminants from the environment–a review. Sci Total Environ 665:358–366
Toribio AJ, Jurado MM, Suárez-Estrella F, López-González JA, Martínez-Gallardo MR, López MJ (2021) Application of sonicated extracts of cyanobacteria and microalgae for the mitigation of bacterial canker in tomato seedlings. J Appl Phycol 33:3817–3829
Toribio AJ, Suárez-Estrella F, Jurado MM, López MJ, López-González JA, Moreno J (2020) Prospection of cyanobacteria producing bioactive substances and their application as potential phytostimulating agents. Biotechnol Rep 26:e00449
Torzillo G, Bernardini P, Masojídek J (1998) On-line monitoring of chlorophyll fluorescence to assess the extent of photoinhibition of photosynthesis induced by high oxygen concentration and low temperature and its effect on the productivity of outdoor cultures of Spirulina platensis (Cyanobacteria). J Phycol 34:504–510
Wang J, Zhou W, Chen H, Zhan J, He C, Wang Q (2019) Ammonium nitrogen tolerant Chlorella strain screening and its damaging effects on photosynthesis. Front Microbiol 9:3250
Wang L, Min M, Li Y, Chen P, Chen Y, Liu Y, Wang Y, Ruan R (2010) Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol 162:1174–1186
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Wen X, Du K, Wang Z, Peng X, Luo L, Tao H, Xu Y, Zhang D, Geng Y, Li Y (2016) Effective cultivation of microalgae for biofuel production: A pilot-scale evaluation of a novel oleaginous microalga Graesiella sp. WBG-1. Biotechnol Biofuels 9:123
White S, Anandraj A, Bux F (2011) PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids. Bioresour Technol 102:1675–1682.
Woertz I, Feffer A, Lundquist T, Nelson Y (2009) Algae grown on dairy and municipal wastewater for simultaneous nutrient removal and lipid production for biofuel feedstock. J Environ Eng 135:1115–1122
Xin L, Hong-ying H, Ke G, Ying-xue S (2010) Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour Technol 101:5494–5500
Zambrano J, García-Encina PA, Hernández F, Botero-Coy AM, Jiménez JJ, Irusta-Mata R (2021) Removal of a mixture of veterinary medicinal products by adsorption onto a Scenedesmus almeriensis microalgae-bacteria consortium. J Water Process Eng 43:102226
Zhou GJ, Ying GG, Liu S, Zhou LJ, Chen ZF, Peng FQ (2014) Simultaneous removal of inorganic and organic compounds in wastewater by freshwater green microalgae. Environ Sci Process Impacts 16:2018–2027
Zijffers J-WF, Salim S, Janssen M, Tramper J, Wijffels RH (2008) Capturing sunlight into a photobioreactor: Ray tracing simulations of the propagation of light from capture to distribution into the reactor. Chem Eng J 145:316–327
Zucconi F., Pera A., Forte M, de Bertoldi M (1981) Evaluating toxicity in immature compost. Biocycle 22:54–57
Acknowledgements
The authors thank Ms. Soňa Pekařová, Mr. Michal Bureš, Mr. Petr Novotný and Mr. Daniyar Malikuly Kozhan for technical assistance during cultivation and Mr. Miroslav Kajan for consultation on wastewater use.
Funding
This work was supported by the EU program Horizon 2020 [project SABANA, grant No.727874] and partly supported by the Program INTERREG V-A Austria – Czech Republic 2014 – 2020 [project Plastocyan ATCZ260], and the ESF project International mobility of researchers—MSCA-IF III (Institute of Microbiology of the CAS, v.v.i.), [reg. nr. CZ.02.2.69/0.0/0.0/19_074/0014484].
Author information
Authors and Affiliations
Contributions
Tomáš Grivalský, Jiří Masojídek, Vince Ördög conceived research. Tomáš Grivalský, Karolína Ranglová, Gergely Ernö Lakatos, João Artur Câmara Manoel, Tereza Černá, Marta Barceló-Villalobos, Francisca Suárez Estrella, analysed data. Tomáš Grivalský prepared the text of the manuscript. Tomáš Grivalský, Karolína Ranglová, Gergely Ernö Lakatos and Tereza Černá prepared the figures of the manuscript. Jiří Masojídek, Vince Ördög revised and edited the manuscript. Tomáš Grivalský finalized the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(PDF 410 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grivalský, T., Ranglová, K., Lakatos, G.E. et al. Bioactivity assessment, micropollutant and nutrient removal ability of Tetradesmus obliquus cultivated outdoors in centrate from urban wastewater. J Appl Phycol 34, 2955–2970 (2022). https://doi.org/10.1007/s10811-022-02828-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02828-6