Abstract
This study investigated the mixotrophic cultivation of the green microalga Chromochloris zofingiensis on glycerol, acetate, and vinasse, as low-cost substrates. The alga was cultivated on different concentrations of glycerol (0.5–8 g L−1), sodium acetate (0.01–1 g L−1), and vinasse (0.12–3.6 g L−1) (the green stages) in the presence of either air (0.04% CO2) or enriched air (2% CO2). Subsequently, the cells were subjected to stress (simultaneous high light intensity and nitrogen starvation, red stage). The final biomass concentrations and composition achieved at the end of green stages as well as the biochemical composition of biomass, the total carotenoid, astaxanthin, and canthaxanthin contents at the end of red stages were elucidated. Glycerol and acetate concentrations higher than 2 and 0.1 g L−1, respectively, did not enhance the final biomass concentration and vinasse concentrations higher than 1.2 g L−1 led to a reduction in final biomass concentration. Hence, the optimum concentrations of glycerol, acetate, and vinasse were found to be 2, 0.1, and 1.2 g L−1, respectively. Moreover, the application of stress on cells resulted in higher lipid and carbohydrate contents and increased dry weight. However, there was no significant difference in carotenoid, astaxanthin, and canthaxanthin contents of biomass among all cultivations. Therefore, cultivation on 1.2 g L−1 vinasse is proposed as a potential method for the production of carotenoid-rich cells of C. zofingiensis due to the ease of medium preparation (the medium contained only vinasse) and lower cost medium compared to Bold’s Basal Medium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are a diverse group of photosynthetic microorganisms that can convert sunlight to various high-value components like carotenoids (such as astaxanthin and canthaxanthin), lipids, proteins, and polysaccharides (Rammuni et al. 2019; Tang et al. 2020). Carotenoids have various applications in the pharmaceutical, nutraceutical, cosmetic, aquaculture feed, and functional food industries (Chen et al. 2020). As the world’s population grows, the requirements for food, pharmaceutical, and cosmetic products, and consequently carotenoids, increase. As a result of utilizing synthetic carotenoids in these products, health issues such as allergic and hyperactivity reactions are at an emerging rate; however, the utilization of natural carotenoids may prevent these issues (Tang et al. 2020). Hence, there has been increased interest in the production of carotenoids from natural resources. Microalgae have been considered a potential source of carotenoids because they grow fast and do not require fertile soil (Sajjadi et al. 2018).
Several algal species, such as Haematococcus pluvialis, Chromochloris zofingiensis, Chlorella vulgaris, Chlamydomonas nivalis, and Botryococcus braunii, have been proposed for their ability to produce various carotenoids, especially astaxanthin. Of these, C. zofingiensis (previous name Chlorella zofingiensis) is well known for its ability to accumulate both astaxanthin and canthaxanthin under stress conditions (Mulders et al 2015; Zhang et al. 2017a). Accordingly, C. zofingiensis has undergone many investigations to enhance biomass and carotenoid productivity and content (Mao et al. 2018; Zhang et al. 2018).
Mixotrophic cultivation of microalgae (utilization of light and organic carbon as the energy source, utilization of inorganic (CO2), and organic carbon as the carbon source) is proposed as a cost-effective strategy to enhance the growth rate and accumulation of biochemicals (lipids and carbohydrates) of algae (Meng et al. 2020). Glucose is a widely used organic carbon source for the mixotrophic cultivation of different species, such as C. zofingiensis (Chen et al. 2017; Zhang et al. 2017b). This carbon source has been reported to be sufficient for biomass and lipid productivity enhancement; however, it is expensive and makes the cultures prone to contamination (Rai et al. 2013). In this regard, the utilization of other inexpensive carbon sources may reduce the overall cost of algal cultivation. Glycerol, a by-product of biodiesel production (Xu et al. 2019), and acetate, a C2 carbon from fermentative hydrogen production (Turon et al. 2016), and vinasse, the by-product of the bioethanol production process, are inexpensive and could be utilized as suitable organic carbon sources for microalgae cultivation (Leite et al. 2015; Smith et al. 2020). For instance, glycerol enhanced the accumulation of lipids and carbohydrates in Scenedesmus obliquus by 21.7 and 16.4%, respectively (Xu et al. 2019). Similarly, mixotrophic cultivation of Chlorella sorokiniana and C. vulgaris on glycerol enhanced the final biomass concentration by more than 100% (Paranjape et al. 2016b). Besides glycerol, mixotrophic cultivation of Chlorella pyrenoidosa on sodium acetate caused ca. 20% increase in biomass (Liu et al. 2018). Notably, the mixotrophic cultivation of C. sorokiniana on acetate derived from the oxidized wine waste lees resulted in ca. 100% increase in biomass (León-Vaz et al. 2019). Similarly, efficient cultivation of Arthrospira (Spirulina) maxima on sugarcane vinasse has been reported (dos Santos et al. 2016; Saejung and Puensungnern 2020) with low concentrations of vinasse causing significant biomass production and high protein accumulation.
To our knowledge, there is no report on the mixotrophic cultivation of C. zofingiensis on glycerol, acetate, and vinasse. Nevertheless, the cultivation of this microalga on wastewaters and other organic carbon sources has been studied (Sun et al. 2008; Zhou et al. 2018). Hence, in this study, for the first time, the mixotrophic growth of C. zofingiensis on glycerol, acetate, and vinasse was investigated. Then, the cells were exposed to stress. Subsequently, the lipid, protein, carbohydrate, and biomass dry weight at the end of green and red stages and total carotenoids, astaxanthin, and canthaxanthin contents of the biomass after stress were elucidated. The data obtained in this work provided a basis for further comparison of different cultivations of C. zofingiensis in terms of economic criteria.
Materials and methods
Microalgae cultivation and induction
Chromochloris zofingiensis was purchased from the Culture Collection of Algae at Göttingen University (SAG211-14, Germany) and maintained at 4 °C on agar plates of Bold’s Basal Medium (BBM). In order to prepare medium, the cells were transferred to the liquid BBM and cultivated axenically using Erlenmeyer flasks under continuous illumination of 80 µmol photons m−2 s−1 at 25 ± 1 °C (Mao et al. 2018). The flasks were aerated with sterile air. Cells in the log phase (optical density at 680 nm = 1) were used as the inoculum for further experiments (medium to inoculum volume ratio = 9:1).
In order to investigate the mixotrophic cultivation of C. zofingiensis, different concentrations of glycerol (0.5–8 g L−1) and sodium acetate (0.01–1 g L−1) were added to BBM as presented in Table 1. The cultures were aerated with either air (Exp. Nr. 1 to 10, Table 1) or enriched air (Exp. Nr. 17 to 26, Table 1).
The cultivations on vinasse were performed using concentrated sugarcane vinasse purchased from Sepahan Bio-product Company, Isfahan, Iran. It was obtained by evaporating the stillage to remove ca. 80% of water. The amount of purchased vinasse was enough to perform all experiments of this study. Therefore, the composition of the vinasse, which was used in this study, was the same in all experiments. The medium containing vinasse was characterized in terms of chemical oxygen demand (COD), total organic carbon (TOC), total Kjeldahl nitrogen content, and the concentration of nutrients such as phosphorus, iron, sulfur, magnesium, and potassium. They were all analyzed according to the Standard methods for the examination of water and wastewater (APHA 2012).
Concentrated vinasse was centrifuged at 5000 × g for 10 min to remove suspended solids and debris and then diluted with distilled water to achieve the concentrations presented in Table 1 (Exp. Nr. 11–15 and 27–31, Table 1). Subsequently, the pH of the medium was adjusted to 7 using 1 M KOH. The medium was autoclaved for 20 min at 121 °C before inoculation. The cultivations on vinasse were aerated with either air (Exp. Nr. 11–15, Table 1) or enriched air (Exp. Nr. 27–31, Table 1). The cultivations on BBM were taken as the controls (Exp. Nr. 16 and 32, Table 1).
All cultivations were conducted at 25 ± 1 °C, 80 µmol photons m−2 s−1 using 1 L Erlenmeyer flasks containing 800 mL working volume. The flasks were aerated using either air (0.04% CO2) or enriched air (2% CO2) at 1.25 vvm (volume per volume per minute) to investigate the effect of CO2 on the growth and biochemical characteristics of microalgae.
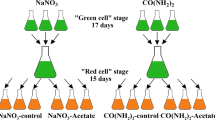
The two-stage method was used for the cultivation of carotenoid-rich microalgae, i.e., at the end of the logarithmic phase of growth (green stage), the cells were harvested by decantation, washed two times with distilled water, and then transferred to nitrogen-free BBM (red stage). The decantation was performed in a circular glass dish (ca. 1200 mL capacity, 230 mm inner diameter, 30 mm height) at 4 °C for 2 h in the dark. The optical density of the supernatant was measured at 680 nm in order to check if the supernatant is free of cells. Then, the supernatant was removed by a peristaltic pump and the remaining concentrate was transferred to centrifuge tubes and mixed with distilled water. Then, they were centrifuged at 5000 × g for 5 min. This was done two times. At the end of washing, the pellets were mixed with nitrogen-free BBM in order to perform the red stage.
The green and red stages lasted 7 and 14 days, respectively. The duration of the red stage was chosen according to the carotenoid profile obtained during the red stages in preliminary experiments. The results revealed that after 14 days, there will not be a significant increase in the concentration of carotenoids when the red stage is executed at the conditions of this study (continuous illumination at 250 µmol photons m−2 s−1, the temperature of 25 ± 1 °C). Therefore, it was assumed that 14 days is long enough to synthesize the maximum possible carotenoids. Interestingly, this was in accordance with previous reports (Mulders et al. 2014). In the red stages, the cultures were aerated at 1.25 vvm using filtered air. Finally, after 14 days of cultivation, carotenoid-rich cells were harvested using decantation, washed twice with distilled water (as described above), and freeze dried. Then, the dried biomass was powdered using a mortar and pestle and kept for subsequent carotenoid extraction.
In order to elucidate more information about the growth of the microalgae on the investigated substrates, the growth curves were also provided by daily measurement of the optical density of the cultures at 680 nm.
Biochemical composition and dry weight of biomass
The dry cell weight was measured at the end of the green and red stages. In order to determine the biomass dry weight, a specific volume of the culture (50 mL) was collected, centrifuged at 5000 × g for 5 min, and the sedimented cells were washed twice using distilled water. Subsequently, the pellets were dried at 95 °C for 48 hand weighed.
The dried biomass obtained from the green stage was used for the determination of total lipid, protein, and carbohydrate contents and the carotenoid content was also determined in the biomass collected at the end of the red.
The total lipid content was determined according to the method of Bligh and Dyer (1959) using the mixture of chloroform:methanol:distilled water (2:2:1.8, v/v).
The protein content of the biomass was measured based on the micro Kjeldahl method (Sáez-Plaza et al. 2013). The carbohydrate content was elucidated according to the phenol–sulfuric acid method (Xu et al. 2019). The biomass used in this method was pretreated beforehand as follows: an amount of 10 mg of the freeze-dried biomass was mixed with 5 mL of 1 N NaOH and then kept in a boiling water bath for 2 h.
The total carotenoid content was determined using methanol as the solvent. Briefly, 5 mL of methanol was added to 10 mg of freeze-dried biomass and the mixture was heated for 5 min at 64 °C. Then, the mixture was centrifuged at 10,000 × g for 10 min and the supernatant was collected. Afterwards, again 5 mL methanol was added to the residual biomass and heated for the same period at the same temperature as the previous step. Then, again the mixture was centrifuged, and the supernatant was collected. This was repeated until the cells became colorless. The absorbance (A) of the collected supernatants was measured at 665.2, 652.4, 470, and the chlorophyll a (Ca), chlorophyll b (Cb), and total carotenoids (Ccar) were calculated according to the following equations (Lichtenthaler and Buschmann 2001).
In order to determine the astaxanthin and canthaxanthin contents of the biomass obtained at the end of the red stages, the biomass was first ultrasonicated to disrupt the cells using an ultrasonic homogenizer device (Topsonics, UP400A, Iran). Briefly, 30 mL acetone was added to a 100 mL glass bottle containing 1 g microalgal biomass. The glass bottle was placed in an ice-water container and the device probe was put in the glass bottle, and the mixture was sonicated for 10 min. The probe provided a constant frequency of 20 kHz and power of 400 W. Afterwards, the mixture was centrifuged at 5000 × g for 5 min. Then, the supernatant was collected and the residual biomass was resuspended in 30 mL acetone. This procedure was repeated until the supernatant became colorless. The collected supernatants were used for astaxanthin measurements by a high-performance liquid chromatography (HPLC) system (Agilent 1260 infinity, USA) equipped with a UV/VIS detector (Jasco International Co., Japan). A Venusil MP C18 column (Bonna-Agela, China) kept at 25 °C was used for the analysis. The mobile phase was a mixture of acetonitrile and methanol (90:10, v/v) at a flow rate of 0.8 mL min−1 (Hosseini et al. 2020).
In order to measure the canthaxanthin concentration in the collected supernatant, it was necessary to have the canthaxanthin in n-hexane. Therefore, the collected supernatants were first dried by nitrogen gas and then the dried pigments were dissolved in n-hexane. Afterwards, the canthaxanthin was measured spectrophotometrically according to the procedure of Mulders et al. (2015).
All measurements were executed in triplicate and all experiments were performed in duplicate with the data reported as mean ± SD (standard deviation).
Measurement of the glycerol and acetate concentrations in the medium
The concentration of glycerol and acetate in the medium was measured at the end of green stages using an HPLC system equipped with an RI (Jasco International Co., Japan) detector and an Aminex HPX-87H column (Bio-Rad, USA) at 60 °C using 0.6 mL min−1 of 0.005 M H2SO4. The sample was prepared beforehand as follows: 1 mL of the culture media was collected at the end of the cultivation and centrifuged at 12,000 × g for 5 min. The obtained supernatant (20 µL) was injected to the column.
Statistical analysis
The comparison of the biomass concentrations and biochemical composition of the biomass was performed by the analysis of variance (ANOVA) or two-sample t-test where appropriate. The statistical analyses were performed using Excel 2013. The level of significance was taken as 0.05 in all tests.
Results
Effects of glycerol, sodium acetate, and vinasse concentrations on growth
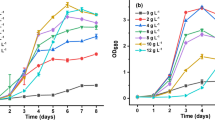
The biomass concentrations obtained at the end of the green stages of different cultivations (Exp. Nr. 1 to 32) are shown in Fig. 1 a, b, and c. The biomass concentrations achieved in mixotrophic cultivation on glycerol, sodium acetate, and vinasse range from 0.3 to 1.2 g L−1. The highest value was obtained from the cultivation on 2 g L−1 glycerol aerated with enriched air (1.2 g L−1 biomass) and the maximum biomass concentrations obtained from cultivation on sodium acetate and vinasse were 0.9 and 1.0 g L−1, respectively. The biomass concentrations obtained from the cultivations aerated with enriched air were significantly (p < 0.05) higher than those aerated with air (Fig. 1). In addition, all mixotrophic cultivations on glycerol and sodium acetate resulted in higher biomass concentrations compared to the controls. For example, the biomass concentrations obtained from cultivations on glycerol (sodium acetate) aerated with enriched air were in the range of 0.7 to 1.1 (0.6 to 0.9) g L−1 and that of the control was 0.4 (0.3) g L−1. Furthermore, Fig. 1c clearly shows that the biomass concentrations achieved in mixotrophic cultivations on vinasse (Exp. Nr. 11–15 and 27–31) were higher than the control (Exp. Nr. 16 and 32). In other words, the biomass concentrations obtained from Exp. Nr. 11 to 15 were in the range of 0.5 to 0.6 g L−1 while that of the control was 0.3 g L−1. Nevertheless, the biomass concentration obtained from Exp. Nr. 11 was not significantly different (p > 0.05) from the control (Exp. Nr. 16) (ca. 0.3 g L−1 in both Exp. Nr. 11 and 16).
The biomass concentration increased when the glycerol content in the cultures increased up to 2 g L−1 (Fig. 1a) but the increase in glycerol concentration more than 2 g L−1 did not significantly affect the biomass concentration. Further increase in glycerol concentration led to no further increase in biomass concentration since the biomass concentrations achieved in Exp. Nr. 3–5 (19–21) were not significantly different from each other (one-way ANOVA (p > 0.05)). Like glycerol, sodium acetate in the medium up to 0.1 g L−1 increased the maximum biomass concentration from 0.6 to 0.9 g L−1 (Exp. Nr. 22–24, Fig. 1b). However, concentrations higher than 0.1 g L−1 of sodium acetate did not result in a significant biomass concentration enhancement. For instance, the maximum biomass concentrations achieved in Exp. Nr. 24–26 were not significantly different from each other (one-way ANOVA, p > 0.05).
Figure 1c shows an increasing trend in biomass concentration followed by a decreasing trend. For example, the biomass concentration increased from 0.3 to 1.0 g L−1 and then decreased from 1.0 to 0.6 g L−1 (Exp. Nr. 27 to 31). In other words, when vinasse concentration was below 1.2 g L−1, the effect of vinasse concentration was positive, but when vinasse increased higher than 1.2 g L−1, the impact was negative. Table 2 shows the effect of microalga cultivation on the vinasse composition. The COD of the medium reduced from 700 mg L−1 to 40 and 46 mg L−1 in Exp. Nr. 13 and 29, respectively (aerated air and enriched air). Similar to COD, TOC, total Kjeldahl nitrogen, sulfur, potassium, magnesium, and iron concentrations were also reduced significantly (Table 2c).
As depicted in Fig. 1, among the cultivations on glycerol, 2 g L−1 glycerol provided the maximum biomass concentration (Exp. Nr. 3 and 19). Similarly, among different sodium acetate and vinasse concentrations tested, 0.1 g L−1 sodium acetate (Exp. Nr. 8 and 24) and 1.2 g L−1 vinasse (Exp. Nr. 13 and 29) resulted in higher biomass concentrations. Hence, the growth curves of these best-performing cultivations and the controls (Exp. Nr. 16 and 32) are shown in Fig. 2. There were no significant differences between the optical densities measured on day 6 and day 7 and the final optical densities measured in controls (Exp. Nr. 16 and 32) were below the final optical densities measured in the mixotrophic cultivations.
Biochemical composition of biomass at the end of green stages
Table 3 presents the protein, carbohydrate, and lipid contents of the biomass measured at the end of the green stages. The lipid contents obtained in all mixotrophic cultivations conducted in this study ranged from 20.0 to 29.8%. Implementation of enriched air resulted in an increment in lipid and carbohydrate contents despite a reduction in protein contents (Table 3). This effect was observed in all experiments. For example, lipid, protein, and carbohydrate contents obtained from Exp. Nr. 3 (2 g L−1 glycerol aerated with air) were 29.8, 38.4, and 24%, while those of Exp. Nr. 19 (2 g L−1 glycerol, aerated with enriched air) were 30.7, 34.2, and 28.6%.
The highest protein content, obtained from the cultivations on vinasse aerated with air (Exp. Nr. 11–15, Table 3), was about 40%. Hence, it seems that the cultivation of C. zofingiensis on 1.2 g L−1 vinasse aerated with air could be a potential method for producing microalgae-based proteins.
Chemical composition of microalgal biomass at the end of the red stages
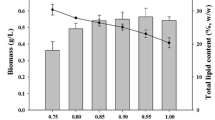
Two kinds of stresses (high-light intensity and nitrogen starvation) were simultaneously imposed on the green cells obtained from the green stages of eight cultivations. The cultivations include the controls (Exp. Nr. 16 and 32) and cultivations on 2 g L−1 glycerol (Exp. Nr. 3 and 19), 0.1 g L−1 sodium acetate (Exp. Nr. 8 and 24), and 1.2 g L−1 vinasse (Exp. Nr. 13 and 29). The biochemical composition of the microalgal biomass at the end of red stages is presented in Table 4. The carbohydrate content was remarkably increased when the stresses were imposed on cells. For example, the carbohydrate content of biomass increased from 30.2 to 50.3% (Exp. Nr. 29) and the highest carbohydrate content was 50.3% (Exp. Nr. 29) at the end of the red stage.
The comparison of the protein contents before (Table 3) and after (Table 4) the red stage revealed the fact that the stresses caused a significant reduction in protein content (two-sample t-test, p < 0.05). For instance, the protein content reduced from 33.5 to 12.7% in Exp. Nr. 29 (cultivation on 1.2 g L−1 vinasse aerated with enriched air). Although the protein contents declined noticeably after imposing the stresses on cells, the lipid contents increased considerably. For example, the lipid contents of biomass obtained from green stages of Exp. Nr. 13 and 29 were 20.7 and 23.4%, while the lipid contents at the end of red stages were 28.7 to 30.3%, respectively (two-sample t-test, p-value < 0.05). Comparing the biochemical composition of biomass obtained from the cultivations on vinasse with the respective controls shows no significant differences. For example, the lipid, protein, and carbohydrate contents of the biomass in Exp. Nr. 13 were 28.7, 14, and 46.7%, while those of Exp. Nr. 16 were 28.9, 14.8, and 46.7%, respectively.
In addition to the variations in chemical composition, the variations in dry biomass weight were also observed when the cells were under stress. Interestingly, the dry biomass weights obtained at the end of red stages were more than two times higher than those obtained at the end of green stages (Table 4). The significant difference in dry biomass weights between green and red stages was also confirmed by the two-sample t-test (p-value < 0.05).
Carotenoid induction under stress conditions
Table 5 presents total carotenoid, astaxanthin, and canthaxanthin contents of the biomass obtained at the end of red stages. The sum of astaxanthin and canthaxanthin contents account for more than 75% of the total carotenoids and astaxanthin accounted for more than 65% of the total carotenoid contents, while canthaxanthin constitutes about 10% of total carotenoids. For example, the total carotenoid content of the biomass was 9.9 mg g−1, while the astaxanthin and canthaxanthin contents were 6.7 and 1.0 mg g−1, respectively (Table 5, Exp. Nr. 13).
Discussion
The biomass concentrations achieved in this study (Fig. 1) were similar to other reported studies. For example, the mixotrophic cultivation of C. vulgaris on 4 g L−1 glucose resulted in a 1.4 g L−1 biomass concentration (Heredia-Arroyo et al. 2011). Additionally, the highest biomass concentration of 0.38 g L−1 was reported in mixotrophic cultivation of Chlorella minutissima on 10 g L−1 glucose (Bhatnagar et al. 2010). The higher biomass concentrations in the experiments cultivated with enriched air might be due to the provision of the higher amount of carbon source. A similar effect has also been reported in the cultivation of Scenedesmus almeriensis with enriched air (Molino et al. 2019). Further, the higher biomass concentrations obtained from mixotrophic cultivations compared to the autotrophic cultivations (controls) were due to the higher amounts of carbon sources (both CO2 and organic carbon) available to the cells in mixotrophic cultures. This effect has also been reported previously in the cultivation of C. sorokiniana (Zhang et al. 2011).
It has been reported that the high concentration of glycerol may not influence growth because the cells may be incapable of assimilating the glycerol beyond a specific rate (Paranjape et al. 2016a). This study shows that concentrations of glycerol higher than 2 g L−1 cannot improve the final biomass concentration. This was also confirmed by measuring the residual concentration of the substrate in the medium (Table 2). Notably, there was no residual glycerol in the case of Exp. Nr. 1, 17 and 2, 18 in which the initial concentration of the glycerol were 0.5 and 1 g L−1, respectively. Moreover, the residual concentrations of glycerol in Exp. Nr. 3 and 19 (2 g L−1 glycerol) were close to zero (0.19 and 0.15 g L−1, respectively). However, the concentration of the glycerol remained in the medium in the case of Exp. Nr. 4, 20 and 5, 21 (initial glycerol concentration of 4 and 8 g L−1) were ca. 2 and 5 g L−1, respectively. This reflected that the concentrations beyond 2 g L−1 did not lead to complete the assimilation of glycerol. Consequently, according to the results of this study, the optimum concentration of glycerol for mixotrophic cultivation of C. zofingiensis was introduced as 2 g L−1.
Similar results were observed for cultivations using sodium acetate. It can be concluded that the microalgae were not able to assimilate sodium acetate completely when its concentration was higher than 0.1 g L−1 in the medium. This was also confirmed by the residual concentrations of sodium acetate (Table 2). The residual concentration of sodium acetate in the cultivations on 0.01, 0.05, and 0.1 g L−1 were below the detection limit (Exp. Nr. 22–24, Table 2). Nevertheless, about 50 and 80 percent of the initial sodium acetate were remained intact when 0.5 and 1 g L−1 sodium acetate were used (Exp. Nr. 25–26, Table 2). Hence, 0.1 g L−1 was introduced as the optimum concentration of sodium acetate in the mixotrophic cultivation of C. zofingiensis. Although the effect of sodium acetate on biomass concentration of C. zofingiensis has not been reported until now, it has been reported that the concentration of sodium acetate, higher than 9 and 0.5 g L−1, respectively, does not influence the growth of C. sorokiniana and Tetraselmis suecica positively (Azma et al. 2011; Chen et al. 2017).
Regarding the effect of vinasse concentration on growth (Fig. 1c), the low concentration of biomass when microalgae cultivated using 0.12 g L−1 vinasse might be due to lack of nutrients. As increasing vinasse concentration up to 1.2 g L−1 positively affected the biomass concentration, it can be concluded that more nutrients were provided when vinasse concentrations increased. It should be noted that the composition of the vinasse at the end of cultivation on 1.2 g L−1 vinasse revealed that there was almost no residual nutrient in the medium at the end of cultivation (Table 2c ). Although higher concentrations of vinasse (more than 1.2 g L−1) in the medium provide high amounts of sugars and organic carbon, it causes low light penetration (dos Santos et al. 2016; Engin et al. 2018), results in a lower concentration of biomass. Additionally, the effect of a high concentration of vinasse on the inhibition of microalgal growth has been reported previously (Barrocal et al. 2010; Engin et al. 2018). Therefore, it seems that the optimum concentration of vinasse in the medium was 1.2 g L−1. However, concentrations higher than 1.2 g L−1 of vinasse in the medium resulted in lower biomass concentration.
The biomass concentration of 1.0 g L−1 obtained from the cultivation on 1.2 g L−1 vinasse (Exp. Nr. 29, Fig. 1), was higher than the respective control (Exp. Nr. 32). This revealed that vinasse provided necessary nutrients for microalgal growth, and no other component was needed to be added to the medium. Additionally, the microalga C. zofingiensis was able to utilize nutrients and organic compounds available in the vinasse (Table 2c).
One may ask whether the biomass concentrations presented in Fig. 1 were the highest concentrations that can be achieved. In other words, the higher concentrations could probably be attained if the culture would last more than 7 days. In order to answer this question, the growth curves were investigated (Fig. 2). The growth curves presented in Fig. 2 illustrate that the cultures were in the stationary phase after 6 days of cultivation. Therefore, the continuation of the cultures for more than 7 days would not result in higher biomass concentrations.
The final optical densities presented in Fig. 2 are in accordance with the biomass concentrations reported in Fig. 1. For example, the highest biomass concentration and optical density were obtained in the cultivation on 2 g L−1 glycerol. Additionally, the final optical densities measured in cultivations aerated with air were lower than those aerated with enriched air.
As illustrated in Fig. 1, the biomass concentrations obtained from mixotrophic cultivations of C. zofingiensis on vinasse, glycerol, and sodium acetate were in the range of 0.9 to 1.2 g L−1. Since the medium used for cultivation on vinasse contained only vinasse as the source of nutrients, this medium was much more inexpensive than BBM supplemented with glycerol and sodium acetate. Moreover, the preparation of vinasse containing medium is more straightforward than BBM because it includes only one component while BBM contains 15 components. Therefore, if commercialization of the cultivation of C. zofingiensis is the goal, vinasse containing medium is proposed as a potential candidate because of the lower cost and ease of preparation.
The higher carbohydrate and lipid contents in biomass, obtained from cultures aerated with enriched air (Table 3), might be due to the utilization of CO2 and organic carbon by C. zofingiensis (Meng et al. 2020). Lipid contents obtained at the end of green stages (Table 3) were in accordance with the value of 27.3% reported earlier (Feng et al. 2011).
The results of this study on carbohydrate increment at the end of red stages (Table 4) are in line with previously reported studies. The increase in carbohydrate content of three different microalgae (S. obliquus, Phaeodactylum tricornutum, and C. zofingiensis) from 30 to 50–60% due to nitrogen starvation has been reported previously (Breuer et al. 2012). Additionally, the accumulation of carbohydrates (up to 66.9%) due to nitrogen starvation of C. zofingiensis has been reported earlier (Zhu et al. 2014). Also, the decrease in the protein content at the end of the red stage was probably because of the absence of nitrogen source in the medium (Yamamoto et al. 2020) and the presence of high light intensity (Benavente-Valdés et al. 2016).
In the case of lipid contents, the measured values of this study were consistent with the lipid contents reported earlier when nitrogen starvation was imposed on the microalga C. zofingiensis (Zhu et al. 2015). Moreover, the comparison of the chemical composition of the biomass at the end of green and red stages reflected that the protein and lipid contents were inversely related. The higher the protein content, the lower the lipid content and vice versa. This effect was also reported earlier (Jeyakumar et al. 2020).
As described in the previous section, the biochemical composition of the controls and that of cultivated using 1.2 g L−1 vinasse were approximately similar. However, the biomass concentration obtained at the end of the green stage was more than two times higher than that of the controls (Fig. 1c). This means that the cultivation of C. zofingiensis on low-cost vinasse increases the biomass productivity in comparison with the cultivation on BBM, while the biochemical composition remained unchanged.
The increase in biomass composition after stress conditions was also observed when the microalga C. zofingiensis was subjected to nitrogen starvation for 14 days (Breuer et al. 2012). The increase in dry biomass weight might be due to the increase in carbohydrate and lipid contents of the cells (Table 4) (Hosseini et al. 2020). Moreover, the difference in the size of the cells was also noticeable in microscopic images taken from the cells at the end of green (average cell size of 6.5 µm, Fig. 3a) and red stages (average cell size of 9.5 µm, Fig. 3b). This is evidence for the increase in biomass dry weight during the red stages (Table 4). It should be noted that according to the our observations, the morphology and average cell size were similar among all performed experiments. These similarities were in addition to the similarities in total carotenoid content of the cells (“Carotenoid induction under stress conditions” section, Table 5). Therefore, the cell images of the best-performing media set (Exp. Nr. 19) were illustrated to represent the morphology and cell size of the microalga C. zofingiensis attained in this study.
As previously reported by Chen et al., (2017) more than 75% of the total carotenoids in the microalga C. zofingiensis belongs to the astaxanthin and canthaxanthin. This is in accordance with the finding of the present study. Furthermore, the astaxanthin and canthaxanthin contents were in the same order of magnitude as the values reported in other studies. For instance, the astaxanthin content of 6.3 mg g−1 was observed after imposing high light intensity and nitrogen starvation on the microalga C. zofingiensis (Orosa et al. 2001). Similarly, astaxanthin and canthaxanthin contents of 2.4 and 1.3 mg g−1 have been achieved when the microalga C. zofingiensis was under nitrogen starvation (Mulders et al. 2014). The higher content of astaxanthin compared to the canthaxanthin was in accordance with previous studies, indicates that C. zofingiensis naturally synthesizes more astaxanthin (Mulders et al. 2015; Chen et al. 2020).
Noticeably, the total carotenoid, astaxanthin, and canthaxanthin contents, observed in all cultivations presented in Table 5 were similar, and there were no significant differences among the cultivations. This was also confirmed by ANOVA (p-value > 0.05). Hence, it can be concluded that the type of substrate used in this study did not influence the carotenoid content of the biomass. However, the biomass concentrations obtained at the end of red stages (Table 5) were different, which is related to the different growth they have during the green stages (Fig. 1). Therefore, if the production of carotenoids, especially astaxanthin, is desirable, mixotrophic cultivation on vinasse using enriched air is proposed because of the low-cost medium and ease of medium preparation (the medium contains only vinasse).
Conclusions
This study showed that C. zofingiensis was able to grow mixotrophically on glycerol, acetate, and vinasse. The biochemical composition of the biomass at the end of green stages revealed that the cultivation on vinasse resulted in a protein content of 40%. Additionally, cultivation on 1.2 g L−1 vinasse resulted in almost complete consumption of COD, TOC, total Kjeldahl nitrogen, potassium, magnesium, phosphorous, and sulfur contents of the medium. Moreover, the carotenoid content of biomass obtained from cultivations on glycerol, acetate, and vinasse was similar (ca. 10 mg g−1 total carotenoid content, there were no significant differences). Although the maximum biomass concentration achieved in cultivations on vinasse was close to those of cultivations on glycerol, the mixotrophic cultivation of C. zofingiensis on 1.2 g L−1 vinasse is proposed as a potential method for production of carotenoid-rich microalgae because of lower medium cost and ease of medium preparation.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, Washington, DC, USA
Azma M, Mohamed MS, Mohamad R, Rahim RA, Ariff AB (2011) Improvement of medium composition for heterotrophic cultivation of green microalgae, Tetraselmis suecica, using response surface methodology. Biochem Eng J 53:187–195
Barrocal VM, García-Cubero MT, González-Benito G, Coca M (2010) Production of biomass by Spirulina maxima using sugar beet vinasse in growth media. New Biotechnol 27:851–856
Benavente-Valdés JR, Aguilar C, Contreras-Esquivel JC, Méndez-Zavala A, Montañez J (2016) Strategies to enhance the production of photosynthetic pigments and lipids in chlorophycae species. Biotechnol Rep 10:117–125
Bhatnagar A, Bhatnagar M, Chinnasamy S, Das KC (2010) Chlorella minutissima—a promising fuel alga for cultivation in municipal wastewaters. Appl Biochem Biotechnol 161:523–536
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour Technol 124:217–226
Chen J-h, Liu L, Wei D (2017) Enhanced production of astaxanthin by Chromochloris zofingiensis in a microplate-based culture system under high light irradiation. Bioresour Technol 245:518–529
Chen J-h, Wei D, Lim P-E (2020) Enhanced coproduction of astaxanthin and lipids by the green microalga Chromochloris zofingiensis: Selected phytohormones as positive stimulators. Bioresour Technol 295:122242
dos Santos RR, Araújo OdQF, de Medeiros JL, Chaloub RM (2016) Cultivation of Spirulina maxima in medium supplemented with sugarcane vinasse. Bioresour Technol 204:38–48
Engin IK, Cekmecelioglu D, Yücel AM, Oktem HA (2018) Evaluation of heterotrophic and mixotrophic cultivation of novel Micractinium sp. ME05 on vinasse and its scale up for biodiesel production. Bioresour Technol 251:128–134
Feng P, Deng Z, Hu Z, Fan L (2011) Lipid accumulation and growth of Chlorella zofingiensis in flat plate photobioreactors outdoors. Bioresour Technol 102:10577–10584
Heredia-Arroyo T, Wei W, Ruan R, Hu B (2011) Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 35:2245–2253
Hosseini A, Jazini M, Mahdieh M, Karimi K (2020) Efficient superantioxidant and biofuel production from microalga Haematococcus pluvialis via a biorefinery approach. Bioresour Technol 306:123100
Jeyakumar B, Asha D, Varalakshmi P, Kathiresan S (2020) Nitrogen repletion favors cellular metabolism and improves eicosapentaenoic acid production in the marine microalga Isochrysis sp. CASA CC 101. Algal Res 47:101877
Leite GB, Paranjape K, Abdelaziz AE, Hallenbeck PC (2015) Utilization of biodiesel-derived glycerol or xylose for increased growth and lipid production by indigenous microalgae. Bioresour Technol 184:123–130
León-Vaz A, León R, Díaz-Santos E, Vigara J, Raposo S (2019) Using agro-industrial wastes for mixotrophic growth and lipids production by the green microalga Chlorella sorokiniana. New Biotechnol 51:31–38
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV‐VIS spectroscopy. Curr Protoc Food Analytic Chem 1(1):F4. 3.1-F4. 3.8
Liu L, Zhao Y, Jiang X, Wang X, Liang W (2018) Lipid accumulation of Chlorella pyrenoidosa under mixotrophic cultivation using acetate and ammonium. Bioresour Technol 262:342–346
Mao X, Wu T, Sun D, Zhang Z, Chen F (2018) Differential responses of the green microalga Chlorella zofingiensis to the starvation of various nutrients for oil and astaxanthin production. Bioresour Technol 249:791–798
Meng TK, Kassim MA, Cheirsilp B (2020) Mixotrophic cultivation: biomass and biochemical biosynthesis for biofuel production. In: Yousuf A (ed) Microalgae cultivation for biofuels production. Elsevier, London, pp 51–67
Molino A, Mehariya S, Karatza D, Chianese S, Iovine A, Casella P, Marino T, Musmarra D (2019) Bench-scale cultivation of microalgae Scenedesmus almeriensis for CO2 capture and lutein production. Energies 14:2806–2820
Mulders KJ, Janssen JH, Martens DE, Wijffels RH, Lamers PP (2014) Effect of biomass concentration on secondary carotenoids and triacylglycerol (TAG) accumulation in nitrogen-depleted Chlorella zofingiensis. Algal Res 6:8–16
Mulders KJ, Weesepoel Y, Bodenes P, Lamers PP, Vincken J-P, Martens DE, Gruppen H, Wijffels RH (2015) Nitrogen-depleted Chlorella zofingiensis produces astaxanthin, ketolutein and their fatty acid esters: a carotenoid metabolism study. J Appl Phycol 27:125–140
Orosa M, Valero JF, Herrero C, Abalde J (2001) Comparison of the accumulation of astaxanthin in Haematococcus pluvialis and other green microalgae under N-starvation and high light conditions. Biotechnol Lett 23:1079–1085
Paranjape K, Leite GB, Hallenbeck P (2016) Effect of nitrogen regime on microalgal lipid production during mixotrophic growth with glycerol. Bioresour Technol 214:778–786
Paranjape K, Leite GB, Hallenbeck PC (2016) Strain variation in microalgal lipid production during mixotrophic growth with glycerol. Bioresour Technol 204:80–88
Rai MP, Nigam S, Sharma R, bioenergy (2013) Response of growth and fatty acid compositions of Chlorella pyrenoidosa under mixotrophic cultivation with acetate and glycerol for bioenergy application. Biomass 58:251–257
Rammuni M, Ariyadasa TU, Nimarshana P, Attalage R (2019) Comparative assessment on the extraction of carotenoids from microalgal sources: astaxanthin from H. pluvialis and β-carotene from D. salina. Food Chem 277:128–134
Saejung C, Puensungnern L (2020) Evaluation of molasses-based medium as a low cost medium for carotenoids and fatty acid production by photosynthetic bacteria. Waste Biomass Valoriz 11:143–152
Sáez-Plaza P, Michałowski T, Navas MJ, Asuero AG, Wybraniec S (2013) An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, chemistry of the procedure, and titrimetric finish. Crit Rev Anal Chem 43:178–223
Sajjadi B, Chen WY, Raman AAA, Ibrahim S (2018) Microalgae lipid and biomass for biofuel production: a comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew Sust Energ Rev 97:200–232
Smith JP, Hughes A, McEvoy L, Day J (2020) Tailoring of the biochemical profiles of microalgae by employing mixotrophic cultivation. Bioresour Technol Rep 9:100321
Sun N, Wang Y, Li YT, Huang JC, Chen F (2008) Sugar-based growth, astaxanthin accumulation and carotenogenic transcription of heterotrophic Chlorella zofingiensis (Chlorophyta). Process Biochem 43:1288–1292
Tang DDY, Khoo KS, Chew KW, Tao Y, Ho SH, Show PL (2020) Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour Technol 304:122997
Turon V, Trably E, Fouilland E, Steyer J-P (2016) Potentialities of dark fermentation effluents as substrates for microalgae growth: a review. Process Biochem 51:1843–1854
Xu S, Elsayed M, Ismail GA, Li C, Wang S, Abomohra AEF (2019) Evaluation of bioethanol and biodiesel production from Scenedesmus obliquus grown in biodiesel waste glycerol: a sequential integrated route for enhanced energy recovery. Energy Convers Manag 197:111907
Yamamoto S, Bossier P, Yoshimatsu T (2020) Biochemical characterization of Rhodomonas sp. Hf-1 strain (cryptophyte) under nitrogen starvation. Aquaculture 516:734648
Zhang H, Wang W, Li Y, Yang W, Shen G (2011) Mixotrophic cultivation of Botryococcus braunii. Biomass Bioenergy 35:1710–1715
Zhang Z, Huang JJ, Sun D, Lee Y, Chen F (2017) Two-step cultivation for production of astaxanthin in Chlorella zofingiensis using a patented energy-free rotating floating photobioreactor (RFP). Bioresour Technol 224:515–522
Zhang Z, Sun D, Cheng K-W, Chen F (2018) Inhibition of autophagy modulates astaxanthin and total fatty acid biosynthesis in Chlorella zofingiensis under nitrogen starvation. Bioresour Technol 247:610–615
Zhang Z, Sun D, Wu T, Li Y, Lee Y, Liu J, Chen F (2017) The synergistic energy and carbon metabolism under mixotrophic cultivation reveals the coordination between photosynthesis and aerobic respiration in Chlorella zofingiensis. Algal Res 25:109–116
Zhou W, Wang Z, Xu J, Ma L (2018) Cultivation of microalgae Chlorella zofingiensis on municipal wastewater and biogas slurry towards bioenergy. J Biosci Bioeng 126:644-648
Zhu S, Wang Y, Huang W, Xu J, Wang Z, Xu J, Yuan Z (2014) Enhanced accumulation of carbohydrate and starch in Chlorella zofingiensis induced by nitrogen starvation. Appl Biochem Biotechnol 174:2435–2445
Zhu S, Wang Y, Shang C, Wang Z, Xu J, Yuan Z (2015) Characterization of lipid and fatty acids composition of Chlorella zofingiensis in response to nitrogen starvation. J Biosci Bioeng 120:205–209
Acknowledgements
The financial support provided for this project by the Isfahan University of Technology is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Conceptualization: Mohammadhadi Jazini; supervision: Mohammadhadi Jazini; investigation: Mohammadhosein Rahimi; writing–original draft: Mohammadhadi Jazini, Mohammadhosein Rahimi; writing–review and editing: Mohammadhadi Jazini, Mohammadhosein Rahimi; funding acquisition: Mohammadhadi Jazini; project administration: Mohammadhadi Jazini.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahimi, M., Jazini, M. Mixotrophic cultivation of Chromochloris zofingiensis on glycerol, acetate, and vinasse. J Appl Phycol 33, 3579–3590 (2021). https://doi.org/10.1007/s10811-021-02568-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02568-z