Abstract
Chromochloris (Chlorella) zofingiensis (Dönz) Fucíková & Lewis is a carotenogenic chlorophyte producing valuable carotenoids such as astaxanthin and canthaxanthin. Due to a high culture robustness, it has a potential to become a major biotechnological source of natural carotenoid pigments in the nearest future. To the best of our knowledge, this is the first report on the comparative assessment of the biomass, carotenoid, and lipid productivity of C. zofingiensis monoculture under the condition of the two-stage batch cultivation with acetate supplementation. The results proved the feasibility of complete replacement of sodium nitrate by urea in the nutrient medium for C. zofingiensis. Carotenoids (0.3% of dry cell weight) and their composition, as well as the cell lipids and their fatty acid profile, were not affected significantly during the cultivation on urea-containing media as compared to the cultivation on standard media containing nitrate. The carotenoid profile of C. zofingiensis was dominated by the valuable ketocarotenoids astaxanthin, canthaxanthin, and adonixanthin. Furthermore, the use of urea as the sole N source increased the gross yield of lipids by 30.6% as compared to that achieved on the nitrate-based medium. The fatty acid composition of the resulting lipids was compatible with the current European biodiesel standard. The cost efficiency of C. zofingiensis cultivation can be increased by replacing nitrate with urea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromochloris zofingiensis (Dönz) Fucíková & Lewis (Chlorophyceae, Sphaeropleales), formerly Chlorella zofingiensis Dönz) (Fucikova and Lewis 2012), is a representative of a group of biotechnologically promising carotenogenic chlorophytes. These green algae are potential producers of the products constantly sought after at the market: valuable ketocarotenoids (KCs) from C40 family such as astaxanthin, canthaxanthin, and adonixanthin and lipids suitable for the production of biodiesel (Liu et al. 2010, 2014, 2016; Feng et al. 2012). The astaxanthin percentage of C. zofingiensis biomass is inferior to that of Haematococcus pluvialis Flotow, currently the main biotechnological source of natural astaxanthin (0.2–0.7% vs. 3–4%). However, this disadvantage is compensated by several advantages C. zofingiensis possesses from the standpoint of mass cultivation. These include a better tolerance to extreme temperature, salinity and shear stresses, a higher growth rate, increased lipid productivity (up to 250–300 mg L−1 day−1, up to 65% of dry biomass weight), and better extractability of the target compounds due to less tough cell wall in comparison with H. pluvialis.
Like in all carotenogenic microalgae, in C. zofingiensis, rapid cell division and intensive accumulation of KC and reserve lipids do not occur simultaneously. These processes take place at different stages of the life cycle and require dramatically different cultivation conditions (Feng et al. 2012; Chen and Wang 2013; Liu et al. 2014, 2016; Kim et al. 2016; Zhang et al. 2017; Huo et al. 2018; Mao et al. 2018). In accord with these features of C. zofingiensis biology, the two-stage cultivation separating biomass accumulation and KC generation is the best strategy to produce this microalga (Boussiba 2000; Fábregas et al. 2001; Zhang and Lee 2001; Benavente-Valdés et al. 2016; Minhas et al. 2016). Still, this approach is seldom used for C. zofingiensis cultivation and mostly under mixotrophic conditions (Chen and Wang 2013; Zhang et al. 2017) or nutrient deprivation (Mulders et al. 2015a, 2015b).
Confirmation of C. zofingiensis suitability for application in microalgal biotechnology under the laboratory conditions would be the first step to introduce this species in industrial production; the next essential step is achieving its profitable cultivation. It is accomplished, in particular, by (i) reducing the cost of the nutrient medium replacing expensive nitrates with cheaper nitrogen sources and (ii) shortening the duration of stage II (carotenogenic or “red cell” stage) by chemical stimulation of the KC formation and lipogenesis, also by increasing the C/N ratio in the nutrient medium.
An alternative source of nitrogen (N) for C. zofingiensis is urea (CO(NH2)2), the most N-enriched and the cheapest compound containing 46% of N and 20% of carbon. Urea-containing media are also well-established in the cultivation of microalgae from the genera Chlorella and Scenedesmus (Bekheet and Syrett 1979; Becker 1994; Hsieh and Wu 2009; Dhup et al. 2016). This is not the case for C. zofingiensis cultures where the effects of using urea as the sole N source are scarcely documented (Liu et al. 2013; Qin et al. 2016; Raven and Giordano 2016; Huo et al. 2018) although exceptions exist (Jiang et al. 2018). To the best of our knowledge, the literature lacks reports on the effects of urea as a sole N source on morphology, physiology, and productivity in two-stage batch culture in the presence of sodium acetate as a promoter of KC formation in C. zofingiensis monocultures.
The present study aims at bridging these gaps by evaluating the suitability of urea as N source in the nutrient medium for growing C. zofingiensis for KC and lipids in two-stage batch culture. An additional goal was testing the effect of sodium acetate addition on the secondary carotenogenesis and functional condition of the urea-fed cells of C. zofingiensis. Cell division rate, vegetative cell size, and stress tolerance as well as dry weight (DW), total carotenoid, and lipid productivities were monitored along with KC and total fatty acid DW percentages and composition.
Materials and methods
Strains and cultivation conditions
Chromochloris zofingiensis strain CALU-190 (CCAP-211/14 = UTEX 32 = SAG 211–14 = ATTC 30412) was obtained at 2006 from Saint-Petersburg University culture collection and preserved on Bold medium, BBM (Bold 1942) solidified with 1.5% agar at 15 °С and 35 μmol PAR quanta m−2 s−1 and subcultured each 45 days.
Precultures were obtained by re-suspending of the cells from the solidified medium in two-fold diluted liquid ВВМ and 7-day incubating in glass Erlenmeyer flasks under natural dim illumination at room temperature (20–22 °С) and bubbling with sterile air (0.3 L min−1 L−1). The cells were then harvested by centrifugation (5 min at 1000×g), re-suspended in a fresh ВВМ medium, and incubated for another 4 days under fluorescent tubes (Feron DL 20 W 6400, 120 μmol PAR quanta m−2 s−1) and 15/9 h light/dark photoperiod. The culture temperature and bubbling were as specified above. The resulting cultures comprised by young, vigorously dividing cells were used as the inoculum.
The effect of N source on potential productivity of C. zofingiensis was assessed in two-stage batch cultures. At stage I (the “green cell” stage), the microalga was cultivated in two modifications of ВВМ differing by the N source according to our pilot experiments with C. zofingiensis (Minyuk et al. 2019). The first modified BBM composition contained triple amount of NaNO3, so the final composition was as follows (g L−1 H2Odist.): NaNO3—0.75; MgSO4 · 7H2O—0.075; NaCl—0.03; K2HPO4 · 3H2O—0.04; KH2PO4—0.1; Na2-EDTA—0.05; KOH—0.03; Н3ВО3—0.011; ZnSO4 · 7H2O—0.0094; MnCl2 · 4H2O—0.0014; Na2MoO4 · 2H2O—0.0024; CuSO4 · 5H2O—0.0016; Co(NO3)2 · 6H2O—0.0005; FeSO4 · 7H2O—0.005; CaCl2—0.019; H2SO4conc.—1 μL. The second modification of BBM contained urea, CO(NH2)2, as N source in equimolar amount (8.83 mmol L−1 = 0.265 g L−1) while the rest of the components were the same as in the first modification of BBM. The composition of the medium was changed to avoid their depletion in the medium during the experiment basing on the results of our preliminary studies (Minyuk et al. 2019).
Other cultivation conditions were the same in both (nitrate- and urea-fed) variants of the experiment: the cultures were started in 0.65 L volume in 1-L Erlenmeyer flasks at the cell density (n) of 2.3–2.5 × 106 cells mL−1 and cell dry weight (DW) of 0.06–0.063 g L−1. At the green cell stage, the cultures were illuminated with Feron DL 20 W 6400 fluorescence tubes from one side (120 μmol PAR quanta m−2 s−1) and incubated for 17 days at 20 ± 1 °С and bubbling with sterile air (0.3 L min−1 L−1).
The biosynthesis of KC, including astaxanthin, was induced by depriving the cultures of macronutrients and increasing irradiance. At the end of the green cell stage, the culture replicas were pooled within each experimental variant. The pooled “green” cultures were diluted tenfold (to the cell density of 3.6 × 106 cells L−1) with the corresponding media tenfold depleted with regard to N and P, and the final concentrations of N and P were 0.88 and 0.16 mmol L−1, respectively. The pooled and diluted cultures were divided into two experimental variants: supplemented with 50 mmol L−1 sodium acetate and control without acetate supplementation (Fig. 1). The resulting cultures were continuously illuminated from both sides (240 μmol PAR quanta m−2 s−1) and incubated at 24–25 °С with increased bubbling velocity (0.6 L−1 min−1) for 15 days before the onset of cell number decline.
The experimental design for testing the effect of N source on two-stage batch cultures of Chromochloris zofingiensis (see “Materials and methods”)
Monitoring of the culture growth and cell size
Cells (mL−1) were counted with a hemocytometer. The DW content (g L−1) was determined gravimetrically on Whatman GF/C glass-fiber filters (1.2 μm pore size (Vonshak 1986).
Average productivity and specific growth rate were calculated for DW or cell density increase as follows (Wood et al. 2005):
where Р is an average productivity considering cell density increase (cell mL−1 day−1); n0—starting cell density (cell mL−1); n1—cell density at 17 days (cell mL−1); (t1 − t0)—duration of cultivation (d); Р1—average productivity considering DW increase (mg L−1 day−1); DW0—starting DW (mg L−1); DW1—final DW (mg L−1); μ—average specific rate of cell density increase (day−1); μ1—average specific rate of DW increase (day−1).
Cell length, L (μm), and width, W (μm), were measured using Leica DM-1000 microscope with the camera of the same manufacturer (Leica Microsystem AG, Germany) and ImageJ software (National Institute of Health, USA). Cell volume, V (μm3), was calculated using the formula for sphere or spheroid volume. To determine the size distribution of the cell population in the cultures, 100 cells were measured in each experimental variant.
Pigment analysis
Chlorophylls a and b (Chl а, Chl b) as well as total carotenoids (Car) were assayed in dimethyl sulfoxide extracts spectrophotometrically using a SF-2000 spectrophotometer (LOMO, Russia) and the following equations (Wellburn 1994):
where Dλ is optical density of the extract at wavelength λ; [Chl a], [Chl b], [Car]—concentrations of Chl a, Chl b, or total Car in the (diluted) extract (μg mL−1); V1—extract volume (mL); V2—volume of the extract aliquot taken from V1 for dilution (mL); V3—volume of the diluted aliquot V2 (mL); V4—volume of cell suspension taken for the extraction (mL).
Average culture productivity considering total Car was calculated according to Wood et al. (2005)):
where РCar—average total Car productivity (mg L−1 d−1); Car0—starting volumetric Car content in the culture (mg L−1); Car1—final volumetric Car content in the culture (mg L−1); (t1 − t0)—duration of cultivation (d).
Carotenoid composition was analyzed by HPLC Shimadzu LC20 (Shimadzu, Japan) equipped with an YМС 30 column (250 × 4.6 mm, 5 μm; YMC, Japan). For elution of Car, the methanol/tert-butyl methyl ester/water mixtures (v/v/v) were used as (A) 81/15/4 and (B) 6/90/4. A linear gradient was employed from 0 to 100% B over 60 min; flow rate—1.0 mL min−1. Car and Chl were monitored at 480 nm, Chl—at 650 nm. Carotenoids were identified by comparing their retention times to those of pure standards isolated from carotenogenic microalgae (Repeta and Bjørnland 1977) and comparison of absorbance spectra of the corresponding Car fractions recorded during HPLC run with those obtained during the analysis of authentic C. zofingiensis strains as well as those published in the literature (Peng et al. 2008; Chen et al. 2017; Patias et al. 2017) and the reference protocol from the column manufacturer (http://www.ymc.co.jp/en/columns/ymc_carotenoid/).
Total lipid determination and fatty acid profiling
Total cell lipids were extracted from the wet biomass according to Bligh and Dyer (1959)) and assayed gravimetrically. Average culture productivity considering lipids was calculated as follows (Wood et al. 2005):
where РL—average total lipid productivity (mg L−1 day−1); L0—starting volumetric lipid content in the culture (mg L−1); L1—final volumetric lipid content of the culture (mg L−1); (t1 − t0)—duration of cultivation.
Fatty acid (FA) composition of the total cell lipids was determined by GC-MS on Agilent 7890A GC with a 5975C MSD (Agilent Technologies, USA) fitted with a capillary column (DB23, Ser. no. US8897617H, 60 m × 0.25 mm) containing a grafted (50% cyanopropyl) methylpolysiloxane polar liquid phase as a 0.25-mm-thick film as described in Sidorov et al. (2012)). Briefly, the prepared FA methyl esters (FAME) were separated under the following conditions: carrier gas (helium) pressure in the injector, 191 kPa; operational gas pressure in the column at 1 mL min−1, 245 kPa; carrier gas flow linear velocity in the column, 18 cm s−1; sample volume, 1 μL (10 μg FAME); flow split ratio, 1: 20; evaporator temperature, 260 °C. The oven temperature program was as follows: from 130 to 170 °C at 6.5 °C min−1, to 215 °C at 2.75 °C min−1 (25 min hold at this temperature), to 240 °C at 40 °C min−1, and 50 min hold at 240 °C, operational temperature of the mass selective detector (5975C MSD), 240 °C. For identifying individual FAME species and calculating their concentrations in the mixture, a NIST08.L search library and an autointl.e integrator from the MSD Chem Station G1701EA E.02.00.493 were used. The unsaturation index (UI) of total FAs from each FAME fraction was calculated as described earlier (Ozerinina and Tsydendambaev 2011).
The potential properties of the biodiesel produced from the lipids of C. zofingiensis were estimated from the total lipids FA profile using the empirical equations from Karpagam et al. (2015)).
Statistical treatment
All measurements were done in triplicate (biological replications (n = 3) with three technical replications for each). Averages ± SD are presented in the text and the figures. Significance of the differences between averages was determined with Student’s two-sided t test at Р < 0.05.
Results
Growth and productivity at the “green cell” stage
Although the starting cultures were uniform regarding their N level and the cell density, the subsequent growth rate and cell size varied significantly between experimental variants differing by the N source in the medium. The urea-fed cultures showed a slower growth rate. Although the maximum growth rate, μmax, recorded during 2-to-3 days of cultivation in the urea-fed cultures was 57% higher than that in the nitrate-fed cultures, the average cell productivity (average growth rate, μ) calculated for the entire green cell stage was 25% higher in the nitrate-fed cultures than in urea-fed ones (Fig. 2а; Table 1).
Another prominent feature of the urea-fed cultures was their smaller cell size: at the end of the green cell stage, the average cell volume in this variant was 36.4% smaller than in the nitrate-fed cultures (Fig. 2с; Table 1). The urea-fed cultures were dominated by small cells (3–10 μm3; 56% of the cell population) whereas the nitrate-fed cultures were dominated by larger cells (15–20 μm3; 60% of the cell population). To take into account this difference, the key markers of the culture conditions (DW, lipid and pigment contents) were also calculated per unit cell volume (Table 1). This method of comparison showed that in the cultures grown in the presence of urea, the carbon-enriched substrate, DW accumulation by the cells increased by 42% at the end of the green cell stage. However, the urea-fed cultures lagged the nitrate-fed cultures by 32.6% in terms of average DW productivity because of lower cell density and smaller cell size of the former. At the same time, the lipid productivity of both cultures was similar (p > 0.05), and the DW percentage of lipids of the urea-fed culture was significantly higher than that of the nitrate-fed culture (Table 1). It is important to mention that pH of the urea-fed cultures at the green cell stage was relatively stable (pH 7.01–7.6) whereas the nitrate-fed cultures showed a trend for alkalization of the medium (from pH 6.7 to pH 9.1) suggesting actual uptake of organic carbon by the cells of the urea-fed cultures. At the same time, the average absorption rates of nitrate and amide nitrogen were similar (0.626 and 0.631 mmol L−1 day−1, respectively).
Carotenogenesis and productivity at the “red cell” stage
The cultures stressed without acetate supplementation, entered, after a 1-day acclimation lag, a short (1–3 days) period of vigorous cell division (μ = 0.39 day−1 or 0.52 day−1 in the nitrate- or urea-fed cultures, respectively). Later, the cell density remained practically unchanged until the end of the experiment. In the presence of acetate, the transient increase of cell division rate was not observed: the cells divided at a steadily low rate (μ = 0.114 day−1) until the end of the experiment (Fig. 3a, b). Lower rate of cell division in the presence of acetate correlated with an increase in cell volume (Fig. 4) and DW content (Fig. 3e–h). As a result, the average DW productivity at the red cell stage was higher in the acetate-supplemented cultures than in the control by 18–21%. In the 15-day red cell stage, the volumetric content of chlorophyll declined 4 and 5 times in the nitrate- and urea-fed cultures without acetate supplementation, respectively; acetate supplementation further promoted the decline in chlorophyll (Fig. 5a, b).
The effect of acetate on a, b the dynamics of cell number and c, d DW content per unit culture volume or e, f per cell or g, h cell volume at the “red cell” stage for the cells grown with a, c, e, g nitrate or b, d, d, h urea (see Fig. 1 and the “Material and methods”)
Changes in the chlorophyll and carotenoid contents in the cultures followed the opposite trends: the faster was chlorophyll decline, the higher was the rate of carotenoid accumulation. The maximal total carotenoid productivity (0.113 ± 0.006 mg L−1 day−1) was documented in the urea-fed culture without acetate (Fig. 6a), maximal yield (43.61 ± 1.05 mg per L of the green culture)—in the nitrate-fed culture without acetate (Fig. 6b). The DW percentage of carotenoids did not depend on the method of the induction of carotenogenesis comprising 0.3% (Fig. 6c).
Carotenoid productivity of the C. zofingiensis cultures grown with nitrate (1, 2) or urea (3, 4) in the absence (1, 3) or in presence (2, 4) of sodium acetate expressed as a total carotenoids at the “red cell” stage, b total carotenoid yield per liter culture for the entire cultivation period (32 days), and c final total carotenoid percentage of the dry weight
The composition of carotenoids from the red cells of C. zofingiensis did not depend on the N source used for growing the green cells. It was represented by a mixture of KC (88.5–91% of total carotenoids) with a minor (6–8.5%) fraction of primary (photosynthetic) carotenoids and unidentified carotenoids (≈ 3%) (Online resource 1, Fig. S1; Tables 2 and 3).
In all variants of the experiment, the major fractions were astaxanthin, canthaxanthin, and adonixanthin. Astaxanthin and adonixanthin were accumulated both in free form and in the form of mono- and diacyl esters differing in their FA composition (Online resource 1, Table S1). In both cases, the unsaturation of the FA in the xanthophyll monoesters was significantly higher than that of diesters and the FA of adonixanthin esters were more unsaturated in comparison with the esters of astaxanthin. The main FA esterifying both carotenoids was oleic acid (Δ9–18:1) which formed mostly monoesters with astaxanthin and diesters with adonixanthin. The second major FA in the esters of these KC was palmitic acid (16:0). The diesters of astaxanthin and adonixanthin were 2.3 and 1.7 times more enriched in palmitate than the corresponding monoesters.
Acetate supplementation had a noticeable effect on the ratio of free and esterified forms of the hydroxylated KC depending on the N source used at the “green cell” stage of cultivation. Thus, the supplementing of the nitrate-fed cultures with acetate led to an increase in the relative content of esterified carotenoids at the expense of their free forms (Online resource 1, Fig. S2a; Table 4). In the urea-fed cultures, the opposite effect was observed—in the presence of acetate, the proportion of free KC increased compared to the control, and the proportion of their esters decreased (Online resource 1, Fig. S2b; Table 4).
At the end of the red cell stage, the urea-fed cultures without acetate contained more lipids per unit culture volume and DW than the nitrate-fed cultures (by ca. 28% and 53%, respectively). The gross lipid yield from 1 L of the nitrate-fed initial culture (2 × 106 cells mL; calculated taking into account all dilutions and both cultivation stages) supplemented or not supplemented with acetate constituted 6.90 g or 9.14 g, respectively; in the corresponding figures for urea-fed cultures comprised 8.75 g and 11.68 g lipids.
At the same time, the FA composition of total cell lipids underwent significant changes, which led to a decline (ca. 1.3-fold) in their unsaturated index (UI). At the end of the “green cell” stage, the FA profiles of total cell lipids in both variants were dominated by palmitic (16:0), oleic (18:1), and α-linolenic (18:3) acids totaling to 63–67% of total FA. The onset of the red cell stage brought about nearly two-fold increase oleate, the main component of astaxanthin monoesters and adonixanthin diesters at the expense of the polyunsaturated FA (Table 4).
All the key quality parameters of the potential biodiesel from lipids obtained from the biomass harvested at the end of the red cell stage meet the requirements of the main European Standard for Biodiesel DIN EN 14214 (Table 5).
Discussion
To assess the possibility of replacing nitrate with urea in the nutrient medium for C. zofingiensis, we used a two-stage batch culture approach developed earlier for screening commercially promising astaxanthin producers (Minyuk et al. 2017, 2019). This approach presumes carrying out the green cell stage in the modified BBM nutrient medium with triple N content starting from the same initial cell density at a moderate irradiance. The first, green cell stage concludes with the decline of the bioavailable N and/or P in the nutrient medium below detection level. The onset of the second, red cell stage is promoted by increasing the irradiance ca. 20-fold (see “Materials and methods”). This approach along with supplementation of the medium with sodium acetate (50 mmol L−1), a compound promoting secondary carotenogenesis (see Peng et al. 2008; Minyuk et al. 2014; Patias et al. 2017) and the discussion below), shortens the red cell stage improving the profitability of the microalgae cultivation. In this work, we characterized the effect of N source on the C. zofingiensis culture at both green cell and red cell stages but acetate was added to the culture only at the red cell stage (see below).
The urea-fed cultures showed a slightly slower average growth rate, although the maximum growth rate in the urea-fed cultures was higher than that in the nitrate-fed cultures. Possible reason for this lag may be toxicity of ammonia formed during enzymatic hydrolysis of the excess of urea in the medium (Little and Mah 1970; Bekheet and Syrett 1979; Dhup et al. 2016). It is important to mention that pH of the urea-fed cultures at the green cell stage was relatively stable (pH 7.01–7.6) whereas the nitrate-fed cultures showed a trend for alkalization of the medium (from pH 6.7 to pH 9.1). At the same time, the average absorption rates of nitrate and amide nitrogen were similar (0.626 and 0.631 mmol L−1 day−1, respectively). Additional studies are needed to determine the optimal combination of the initial urea concentration, inoculum cell density, and the irradiance for C. zofingiensis to fully leverage the benefit of organic carbon supply in addition to N coming with urea.
At the red cell stage, C. zofingiensis displayed a high stress tolerance regardless of the N source used at the green cell stage and the method of the induction of carotenogenesis. None of the experimental variants showed a cessation cell division and/or high cell mortality often observed under similar conditions in other carotenogenic microalgae, e.g., H. pluvialis or Bracteacoccus minor (Minyuk et al. 2007, 2014). On the contrary, the cell density increased significantly by the end of the red cell stage in all the variants. At the same time, the kinetics of cell division depended on the presence of the chemical inductor of carotenogenesis (acetate) in the medium and the prehistory of the culture.
As in other carotenogenic microalgae, accumulation of DW in the stressed cells of C. zofingiensis preparing for transition to the red resting cells occurred along with the reduction of their photosynthetic apparatus and induction of the biosynthesis of KC protecting the cell against oxidative stress (Lemoine and Schoefs 2010; Solovchenko 2015). Indeed, for 15-day red cell stage, the volumetric content of chlorophyll declined 4 and 5 times in the nitrate- and urea-fed cultures without acetate supplementation, acetate supplementation further promoted the decline in chlorophyll (Fig. 5a, b). The net result of the boosting of secondary carotenogenesis with acetate is determined by two oppositely directed processes: increase in carotenoid per cell content due to accumulation of KC in the cells (Fig. 5e–h) and a decrease in productivity due to cell division slow-down (Fig. 3a, b). On one hand, the results do not support the using of acetate to increase the yield of KC in C. zofingiensis during the second (red cell) stage of two-stage cultivation. On the other hand, cultures supplemented with acetate are elucidating for gauging stress tolerance of carotenogenic microalgae and their potential regarding KC accumulation.
One of the first groups to use sodium acetate as an efficient promotor of secondary carotenogenesis in microalgae was that of Kobayashi (Kobayashi et al. 1993, 2001). Having convincingly demonstrated acceleration of astaxanthin biosynthesis by acetate in H. pluvialis, the authors did not mention its impact on cell viability, considering it insignificant. In fact, monad cell mortality in H. pluvialis during transition to the red cell stage supplemented with acetate can be as high as 45–80% (Dantsyuk 2010). Carotenogenic microalgae Bracteacoccus minor and B. giganteus also displayed a high sensitivity to acetate under similar conditions and the cell mortality of 30–40% (Chubchikova et al. 2011; Chelebieva et al. 2013a). At the same time, mature vegetative cells of Coelastrella rubescens (Minyuk et al. 2017), Scenedesmus rubescens (Chelebieva et al. 2013a), and Ettlia carotinosa (Chelebieva et al. 2013b), as well as C. zofingiensis studied in this work showed a relatively high tolerance to acetate manifested by retention of vigorous cell division and active accumulation of KC which was commensurate to that in non-supplemented cells regardless of N source (Fig. 6).
Kobayashi et al. (1993) suggested that acetate increases accumulation of ROS, likely due to over-reduction of electron carriers in photosynthetic electron transport chain. Another hypothesis relates with the hydrolysis of acetate with the formation of acetic acid disturbing the cell membranes (Zhao et al. 2016). Although the exact mechanism of potential toxicity of acetate is unclear, the using of acetate boost is warranted in screening of potential producers of astaxanthin and lipids, although doing so one needs to exercise caution and monitor all the relevant parameters of the culture condition.
The observed peculiarities of carotenoid composition including the major KC and their FA esters in C. zofingiensis might stem from the specifics of carotenoid biosynthesis in this microalga. Thus, Huang et al. (2006) suggested that the astaxanthin biosynthesis in C. zofingiensis might differ from that in H. pluvialis. Under heterotrophic conditions, they found a single bi-functional gene encoding β-carotene oxygenase (4,4′-β ionone ring oxygenase, CRTO) converting β-carotene to canthaxanthin via echinenone and zeaxanthin to astaxanthin via adonixanthin. At the same time, the stress-induced changes in carotenoid composition at the red cell phase depends on the condition of and nutrient availability in the source green culture (Wang and Chen 2008). Thus, 0.45 mmol acetate did not affect astaxanthin, canthaxanthin, or adonixanthin biosynthesis in 4-day-old autotrophic culture of C. zofingiensis; the latter two KCs were detected after glucose supplementation of the cultures in the dark. High light and salinity were more efficient in the induction of carotenogenesis, presumably mediated by ROS (Li et al. 2009).
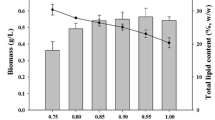
As in other carotenogenic microalgal species, accumulation of secondary KC in C. zofingiensis cells was closely related to the activation of biosynthesis of neutral lipids and their accumulation in cytoplasmic globules acting as carotenoid depots (Zhekisheva et al. 2002; Solovchenko 2012, 2015). Regardless of the cultivation stage, the urea-fed cultures surpassed the nitrate-fed cultures in lipid as well as average lipid productivity at the green stage and the overall productivity (Table 1; Fig. 7). Notably, at the red cell stage, these differences were much more pronounced than at the green cell stage. The average daily productivity of the urea-fed non-supplemented culture was superior to that of the nitrate-fed culture. The attempt to accelerate lipogenesis at the red cell stage by acetate supplementation did not give the expected results whereas growing the cultures with urea brought about a more significant effect (Fig. 7).
Generally, the changes of the FA composition in C. zofingiensis at the second (“red”) stage of cultivation were close to those recorded under similar cultivation conditions in another representative of the order Sphaeropleales, aerophilic microalga C. rubescens (Minyuk et al. 2017). This kind of FA profile is favorable for production of lipid-enriched biomass for conversion to biodiesel. This assessment was supported by the predicted characteristics of biodiesel (Islam et al. 2013) from lipids extracted from the biomass grown in our experiments (Table 5). Although the biomass samples obtained in this study met the requirements set by the European Union for biodiesel, it might be less attractive to use it directly for fuel production. Considering that a common shortcoming of microalgal biodiesels is their low oxidative stability (Stansell et al. 2012), the oil extracted from C. zofingiensis enriched with carotenoid antioxidants can be blended with diesel from other sources to improve their characteristics.
Judging from the comparison of previously published data with those obtained in the present study, one can conclude that the species accumulating almost pure astaxanthin, e.g., H. pluvialis, will likely remain in the niche of premium products and nutraceuticals given the cost and complexity of their industrial cultivation and the price of the highly purified astaxanthin. The species offering robust cultures and generating large amount of biomass enriched with a mixture of valuable carotenoids like C. zofingiensis are expected to be competitive in the sector of bulk products, including (aqua)feed additives. This biomass canthaxanthin can be also considered as a feedstock for production of an environment-friendly stabilizer additive for biodiesel ensuring its high oxidation stability and hence a long shelf life due to the presence of carotenoids.
Finally, this study confirmed the feasibility of substituting costly nitrates by relatively cheap urea in the nutrient medium for the chlorophyte C. zofingiensis, a potential producer of the two valuable carotenoids, astaxanthin and canthaxanthin. Under our experimental conditions, the acetate supplementation at the red cell stage was inefficient in enhancing the carotenoid accumulation in C. zofingiensis. These findings constitute a further step towards development of robust and cost-effective technologies for production of carotenoid-enriched microalgal biomass.
References
Becker E (1994) Microalgae: biotechnology and microbiology. Cambridge University Press, Melbourne
Bekheet I, Syrett P (1979) The uptake of urea by Chlorella. New Phytol 82:179–186
Benavente-Valdés JR, Aguilar C, Contreras-Esquivel JC, Méndez-Zavala A, Montañez J (2016) Strategies to enhance the production of photosynthetic pigments and lipids in Chlorophycae species. Biotechnol Rep 10:117–125
Bligh E, Dyer W (1959) A rapid method of total lipid extraction and purification. Can J Physiol Pharmacol 37:911–917
Bold H (1942) The cultivation of algae. Bot Rev 8:69–138
Boussiba S (2000) Carotenogenesis in the green alga Haematococcus pluvialis: cellular physiology and stress response. Physiol Plant 108:111–117
Chelebieva E, Minyuk G, Chubchikova I (2013a) Features of secondary carotenogenesis in green microalgae Scenedesmus rubescens (Dangeard) Kessler et al. in a two-stage batch culture. Sci Notes Taurian Natnl Univ 26:175–187
Chelebieva E, Minyuk G, Drobetskaya I, Chubchikova I (2013b) Physiological and biochemical characteristics of Ettlia carotinosa Komárek 1989 (Chlorophyceae) under experimental stress condition. Mors'kyi Ekolohichnyi Zhurnal (Mar Ecol) 12:78–87
Chen J-H, Liu L, Wei D (2017) Enhanced production of astaxanthin by Chromochloris zofingiensis in a microplate-based culture system under high light irradiation. Bioresour Technol 245:518–529
Chen T, Wang Y (2013) Optimized astaxanthin production in Chlorella zofingiensis under dark condition by response surface methodology. Food Sci Biotechnol 22:1–8
Chubchikova I, Drobetskaya I, Minyuk G, Dantsyuk N, Chelebieva E (2011) Screening of green microalgae as potential source of nature ketocarotenoids. 2. Features of growth and secondary carotenogenesis in the representatives of the genus Bracteacoccus (Chlorophyceae). Mar Ecol J 10:91–97
Dantsyuk N (2010) Effect of sodium acetate on intensity of secondary carotenogenesis of green microalgae Haematococcus pluvialis. Mar Ecol J 80:44
Dhup S, Kannan DC, Dhawan V (2016) Understanding urea assimilation and its effect on lipid production and fatty acid composition of Scenedesmus sp. SOJ Biochem. https://doi.org/10.15226/2376-4589/2/1/00108
Fábregas J, Otero A, Maseda A, Domínguez A (2001) Two-stage cultures for the production of astaxanthin from Haematococcus pluvialis. J Biotechnol 89:65–71
Feng P, Deng Z, Fan L, Hu Z (2012) Lipid accumulation and growth characteristics of Chlorella zofingiensis under different nitrate and phosphate concentrations. J Biosci Bioeng 114:405–410
Fucikova K, Lewis LA (2012) Intersection of Chlorella, Muriella and Bracteacoccus: resurrecting the genus Chromochloris Kol et Chodat (Chlorophyceae, Chlorophyta). Fottea 12:83–93
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specidications. Renw Sust Energy Rev 16:143–169
Hsieh C-H, Wu W-T (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 100:3921–3926
Huang J-C, Wang Y, Sandmann G, Chen F (2006) Isolation and characterization of a carotenoid oxygenase gene from Chlorella zofingiensis (Chlorophyta). Appl Microbiol Biotechnol 71:473–479
Huo S et al. (2018) Biomass accumulation of Chlorella zofingiensis G1 cultures grown outdoors in photobioreactors. Front Energy Res 6 doi:https://doi.org/10.3389/fenrg.2018.00049
Islam MA, Magnusson M, Brown RJ, Ayoko GA, Nabi MN, Heimann K (2013) Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies 6:5676–5702
Jiang X, Liu L, Chen J, Wei D (2018) Effects of Xanthophyllomyces dendrorhous on cell growth, lipid, and astaxanthin production of Chromochloris zofingiensis by mixed culture strategy. J Appl Phycol 30:3009–3015
Karpagam R, Raj KJ, Ashokkumar B, Varalakshmi P (2015) Characterization and fatty acid profiling in two fresh water microalgae for biodiesel production: lipid enhancement methods and media optimization using response surface methodology. Bioresour Technol 188:177–184
Kim D-Y, Vijayan D, Praveenkumar R, Han JI, Lee K, Park JY, Chang WS, Lee JS, Oh YK (2016) Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour Technol 199:300–310
Kobayashi M, Kakizono T, Nagai S (1993) Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl Environ Microbiol 59:867–873
Kobayashi M, Katsuragi T, Tani Y (2001) Enlarged and astaxanthin-accumulating cyst cells of the green alga Haematococcus pluvialis. J Biosci Bioeng 92:565–568
Lemoine Y, Schoefs B (2010) Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynth Res 106:155–177
Li Y, Huang J, Sandmann G, Chen F (2009) High-light and sodium chloride stress differentially regulate the biosynthesis of astaxanthin in Chlorella zofingiensis (Chlorophyceae). J Phycol 45:635–641
Little L, Mah R (1970) Ammonia production in urea-grown cultures of Clorella ellipsoidea. J Phycol 6:277–280
Liu J, Huang J, Fan KW, Jiang Y, Zhong Y, Sun Z, Chen F (2010) Production potential of Chlorella zofingienesis as a feedstock for biodiesel. Bioresour Technol 101:8658–8663
Liu J, Mao X, Zhou W, Guarnieri MT (2016) Simultaneous production of triacylglycerol and high-value carotenoids by the astaxanthin-producing oleaginous green microalga Chlorella zofingiensis. Bioresour Technol 214:319–327
Liu J, Sun Z, Gerken H, Liu Z, Jiang Y, Chen F (2014) Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: biology and industrial potential. Mar Drugs 12:3487–3515
Liu J, Sun Z, Zhong Y, Gerken H, Huang J, Chen F (2013) Utilization of cane molasses towards cost-saving astaxanthin production by a Chlorella zofingiensis mutant. J Appl Phycol 25:1447–1456
Mao X, Wu T, Sun D, Zhang Z, Chen F (2018) Differential responses of the green microalga Chlorella zofingiensis to the starvation of various nutrients for oil and astaxanthin production. Bioresour Technol 249:791–798
Minhas AK, Hodgson P, Barrow CJ, Adholeya A (2016) A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front Microbiol 7:546
Minyuk G, Chelebieva E, Chubchikova I (2014) Secondary carotenogenesis of the green microalga Bracteacoccus minor (Chodat) Petrova (Chlorophyta) in a two-stage culture. Int J Algae 16:354–368
Minyuk G et al (2017) Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids. Algae 32:245–259
Minyuk G, Dantsyuk N, Chelebieva E, Chubchikova I, Drobetskaya I, Solovchenko A (2019) The effect of diverse nitrogen sources in the nutrient medium on the growth of the green microalgae Chromochloris zofingiensis in the batch culture. Mar Biol J 4:41–52
Minyuk G, Terentyeva N, Drobetskaya I (2007) A comparison of characteristics of morphological-physiological and biochemical traits in three strains of Haematococcus pluvialis Flotow (Chlorophyta, Chlamydomonadales). Int J Algae 9:174–186
Mulders KJM, Weesepoel Y, Bodenes P, Lamers PP, Vincken J-P, Martens DE, Gruppen H, Wijffels RH (2015a) Nitrogen-depleted Chlorella zofingiensis produces astaxanthin, ketolutein and their fatty acid esters: a carotenoid metabolism study. J Appl Phycol 27:125–140
Mulders KJM, Lamers PP, Wijffels RH, Martens DE (2015b) Dynamics of biomass composition and growth during recovery of nitrogen-starved Chromochloris zofingiensis. Appl Microbiol Biotechnol 99:1873–1884
Ozerinina O, Tsydendambaev V (2011) Effect of pre-sowing γ-irradiation of sea buckthorn seeds on the content and fatty acid composition of total lipids in the seeds of the first plant generation. Russ J Plant Physiol 58:370–374
Patias LD, Fernandes AS, Petry FC, Mercadante AZ, Jacob-Lopes E, Zepka LQ (2017) Carotenoid profile of three microalgae/cyanobacteria species with peroxyl radical scavenger capacity. Food Res Int 100:260–266
Peng J, Xiang W, Tang Q, Sun N, Chen F, Yuan J (2008) Comparative analysis of astaxanthin and its esters in the mutant E1 of Haematococcus pluvialis and other green algae by HPLC with a C30 column. Sci China C 51:1108–1115
Qin L, Wang Z, Shu Q, Huo S, Zhu S, Xu J, Yuan Z (2016) Medium optimization for Chlorella zofingiensis biomass production using central composite design. Energy Sources A 38:769–776
Raven JA, Giordano M (2016) Combined nitrogen. In: Borowitzka MA, Beardall J, Raven J (eds) The physiology of microalgae. Springer, Cham, pp 143–154
Repeta D, Bjørnland T (1977) Preparation of carotenoids standards. In: Jeffry S, Mantoura R, Wright S (eds) Phytoplankton pigments in oceanography: guidelines to modern methods. UNESCO Publishing, Paris, pp 239–260
Sidorov R, Zhukov A, Vereshchagin A, Tsydendambaev V (2012) Occurrence of fatty acid lower-alkyl esters in Euonymus fruits. Russ J Plant Physiol 59:326–332
Solovchenko A (2012) Physiological role of neutral lipid accumulation in eukaryotic microalgae under stresses. Russ J Plant Physiol 59:167–176
Solovchenko AE (2015) Recent breakthroughs in the biology of astaxanthin accumulation by microalgal cell. Photosynth Res 125:437–449
Stansell GR, Gray VM, Sym SD (2012) Microalgal fatty acid composition: implications for biodiesel quality. J Appl Phycol 24:791–801
Vonshak A (1986) Laboratory techniques for cultivation of microalga. In: Richmond A (ed) Handbook of microalgal mass culture. CRC Press, Boca Raton, pp 117–145
Wang Y, Chen T (2008) The biosynthetic pathway of carotenoids in the astaxanthin-producing green alga Chlorella zofingiensis. World J Microbiol Biotechnol 24:2927–2932
Wellburn A (1994) The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Wood AM, Everroad R, Wingard L (2005) Measuring growth rates in microalgal cultures. In: Andersen RA (ed) Algal culturing techniques. Elsevier, Amsterdam, pp 269–285
Zhang DH, Lee YK (2001) Two-step process for ketocarotenoid production by a green alga Chlorococcum sp. strain MA-1. Appl Microbiol Biotechnol 55:537–540
Zhang Z, Huang JJ, Sun D, Lee Y, Chen F (2017) Two-step cultivation for production of astaxanthin in Chlorella zofingiensis using a patented energy-free rotating floating photobioreactor (RFP). Bioresour Technol 224:515–522
Zhao X, Jarboe L, Wen Z (2016) Utilization of pyrolytic substrate by microalga Chlamydomonas reinhardtii: cell membrane property change as a response of the substrate toxicity. Appl Microbiol Biotechnol 100:4241–4251
Zhekisheva M, Boussiba S, Khozin-Goldberg I, Zarka A, Cohen Z (2002) Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J Phycol 38:325–331
Funding
Financial support was provided by the Russian Ministry of Science and Higher Education (project 075-15-2019-1719/ RFMEFI60419X0213).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 222 kb)
Rights and permissions
About this article
Cite this article
Minyuk, G., Sidorov, R. & Solovchenko, A. Effect of nitrogen source on the growth, lipid, and valuable carotenoid production in the green microalga Chromochloris zofingiensis. J Appl Phycol 32, 923–935 (2020). https://doi.org/10.1007/s10811-020-02060-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02060-0