Abstract
Though less attention has been paid to microalgae as a feedstock for bioethanol production, many microalgae seem to have this potential since they contain no lignin, minor hemicellulose, and abundant carbohydrate. The objective of this study was to investigate the effect of nitrogen starvation on carbohydrate and starch accumulation in green microalga Chlorella zofingiensis and assess the feasibility of using this microalga as a bioethanol feedstock. The results showed that the specific growth rate under nitrogen starvation (0.48 day−1) was much lower than that under nitrogen repletion (1.02 day−1). However, nitrogen starvation quickly induced the accumulation of carbohydrate, especially starch. After merely 1 day of nitrogen starvation, carbohydrate and starch increased 37 % and 4.7-fold, respectively. The highest carbohydrate content reached 66.9 % of dry weight (DW), and 66.7 % of this was starch. In order to obtain enough carbohydrate productivities for bioethanol production, two-stage cultivation strategy was implemented and found to be effective for enhancing biomass, carbohydrate, and starch simultaneously. The optimal biomass, carbohydrate, and starch productivities of C. zofingiensis were obtained after 5 days of cultivation, and their values were 699, 407, and 268 mg L−1 day−1, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Depleting fossil resources and rapid increasing energy demand have led to soaring petroleum prices over the past few years. Moreover, the environmental problems associated with overconsumption of fossil fuels have been widely concerned around the world. Hence, there is an urgent need for sustainable and affordable alternative energy [1]. Bioethanol has been recognized as one of the most widely used biofuels [2]. To date, bioethanol has been mostly produced from sugar-based (sugar beets, sugarcane) and starch-based (corn, wheat, barley) feedstocks. However, these feedstocks raise the issues of food competition and arable land usage. Although lignocellulosic biomass such as agricultural and forest residues is abundantly available without competition for food supply and arable land, the cost of ethanol production from these materials is still relatively high [3]. Furthermore, there are still problems arising from the biodegradative recalcitrance of lignin and inefficient fermentation of pentose from the hemicellulose component [4].
Recently, microalgae have emerged as a promising feedstock for biofuel production because of several advantages, such as high photosynthetic efficiency, high growth rate, and high energy yields. Many microalgae are capable of harnessing sunlight and CO2 to produce energy-rich compounds such as lipids and/or carbohydrate, which can be converted into biofuels [5, 6]. In addition, microalgae seem to be superior to the lignocellulosic materials as feedstock for bioethanol production since microalgae contain no lignin and low hemicellulose content rendering their saccharification much easier and the problems associated with pentose fermentation essentially eliminated [4, 7]. Today, there has been considerable interest in using microalgae as a feedstock for biodiesel production, while less attention has been paid to bioethanol production. In fact, many microalgae accumulate large amounts of carbohydrate in certain environmental conditions (Table 1), endowing them with great potential for bioethanol production.
Carbohydrate accumulation in microalgae usually occurs under environmental stress, typically nutrient deficiency. In addition, green algae are prone to accumulate starch as the primary carbon and energy storage product [2, 8]. Thus, in this study, the effect of nitrogen starvation on carbohydrate and starch accumulation in green microalga Chlorella zofingiensis was investigated. Strategy to enhance carbohydrate and starch production was further explored to evaluate the feasibility of this algal strain as a potential feedstock for bioethanol production.
Materials and Methods
Microalga and Growth Medium
The microalga C. zofingiensis was maintained in flasks containing BG11 culture medium at 25 °C. BG11 medium consisted of (g L−1) the following: NaNO3, 1.5; K2HPO4·3H2O, 0.04; MgSO4·7H2O, 0.075; CaCl2·2H2O, 0.036; Na2CO3, 0.02; Na2EDTA·2H2O, 0.001; FeCl3·6H2O, 0.00315; citric acid, 0.006, and 1 mL of microelements stock solution. The microelements stock solution contained (g L−1) the following: H3BO3, 2.86; MnCl2·4H2O, 1.81; ZnSO4·7H2O, 0.222; Na2MoO4·2H2O, 0.391; CuSO4·5H2O, 0.079; and Co(NO3)2·6H2O, 0.05. The algal cells were grown under a light intensity of approximately 60 μmol m−2 s−1. The light intensity was measured by a photosynthetically active radiation (PAR) detector.
Operation of Photobioreactors
The photobioreactor (PBR) was a 1-L glass-made air bubble column of 60 cm height and 5.0 cm external diameters. Light was continuously supplied by cool white fluorescent lamps at the single side of the PBR with an average irradiance of 150 μmol m−2 s−1 at 25 °C. Aeration and mixing were achieved by the sparging air enriched with 1 % CO2 from the bottom of the reactor. Cells were initially grown to the late logarithmic phase (4 days old). These pre-cultured cells were collected by centrifugation (4,000 rpm × 5 min) and inoculated into regular BG11 or nitrogen-depleted BG11-N medium with an inoculum size of approximately 2 × 107 cells per milliliter. No nitrogen was detected in BG11-N medium, indicating that nitrogen starvation condition was realized (data not shown). Samples for analysis were taken immediately after resuspension (0 day) and at regular intervals. Each experiment was performed in triplicate.
Determination of Growth Kinetic Parameters
Samples were taken at the indicated times, and the growth parameters were measured. Dry weight (DW) was measured by filtering 10 mL of sample through pre-weighed Whatman GF/C filters. Then the filter paper was dried at 80 °C in an oven until constant weight and cooled down to room temperature in a desiccator before weighting. Finally, filters were weighed to determine algal biomass. Cell numbers were counted using a hemocytometer after appropriate dilution.
The specific growth rate (μ) at the exponential phase was calculated according to the equation, μ = (ln Nt1 − ln Nt0)/(t 1 − t 0), where Nt1 is the number of cells at sampling time and Nt0 is the number of cells at the beginning of the experiment [9].
The productivity of biomass, carbohydrate, or starch (P i , mg L−1 day−1) was calculated by the following: P i = ΔX i /Δt, where ΔX i is the variation of the concentration of biomass, carbohydrate, or starch (mg L−1) within cultivation time of Δt (days).
Determination of Carbohydrate and Starch Content
Aliquots of lyophilized algal biomass were disintegrated by vortexing with 0.5 mL of glass beads (200 μm diameter) for 4 min (2,700 rpm) in 0.25 mL of distilled water. The concentration of total carbohydrate was analyzed by the phenol-sulfuric method [10], using glucose as the standard.
Starch was determined by the method described by Branyikova et al. [11], based on the total hydrolysis of starch by 30 % perchloric acid and quantification of liberated glucose by colorimetry.
Statistics
Unless otherwise indicated, tables and figures show means and standard deviations of three independent experiments.
Results and Discussion
Effect of Nitrogen Starvation on Microalgal Biomass and Carbohydrate Accumulation
Nitrogen is an essential nutrient for microalgal growth, as it participates in the formation of vital compounds such as DNA, proteins, pigments, etc. If nitrogen is removed from the cultivation medium, the microalgae change their metabolic flux, leading to the alteration of their biomass composition [8]. In nitrogen-rich medium, the biomass increased rapidly from 0.2 to 2.3 g L−1 within 4 days and reached 3.1 g L−1 by day 10, while the biomass exposed to stress increased mildly from 0.27 to 0.56 g L−1 within the first 2 days and reached 0.7 g L−1 by day 10 (Fig. 1a). The maximum specific growth rate and biomass productivity under nitrogen repletion were 1.02 day−1 and 519.2 mg L−1 day−1 respectively, whereas under nitrogen depletion, the maximum specific growth rate, 0.48 day−1, and maximum biomass productivity, 195 mg L−1 day−1, were much lower than under normal condition (Table 2). These suggested that microalgal growth was severely inhibited upon nitrogen starvation.
Under nitrogen-rich condition, carbohydrate content was reduced within the first 2 days from 40.5 to 24.1 % of DW, then increased to 45.9 % of DW by day 4, and finally decreased to 24.2 % of DW at the end of the experiment (Fig. 1b). The drop of carbohydrate content during the first 2 days of cultivation might be attributed to the rapid cell division in favorable growth condition. While in response to nitrogen starvation, carbohydrate content increased rapidly within the first day, from 48.8 % to a peak 66.9 % of DW, which decreased at the following day to 54.0 % of DW and then decreased with a slower rate to 47.2 % of DW by day 10 (Fig. 1b). Nitrogen starvation quickly induced carbohydrate accumulation, increasing 37 % within merely 1 day. However, it was worthy to mention that carbohydrate transiently accumulated and then reduced to the initial level at the end of the experiment. This was in accordance to the report of Recht et al. [12] that carbohydrate accumulated up to 63 % of DW of Haematococcus pluvialis by day 1 and partially degraded thereafter. In Chlorella vulgaris, carbohydrate content increased by nearly 6-fold after nitrogen exhaustion without significant decrease afterward [13]. Wang et al. deemed that carbohydrate as the major product of photosynthesis was abundant in most algae species under normal or mild stress culture conditions [14]. However, some species such as Nannochloropsis sp. did not accumulate carbohydrate during nitrogen starvation [12]. These findings indicated that the response of microalgal carbohydrate to nitrogen stress might be species-dependent.
Though nitrogen starvation triggered carbohydrate accumulation in C. zofingiensis cells, the carbohydrate yield was apparently not satisfactory (Fig. 1c). Since nitrogen-rich cells of C. zofingiensis possessed moderate carbohydrate content per se and propagated rapidly, carbohydrate yield and productivity could reach 1.04 and 240.8 mg L−1 day−1, respectively, during the 4-day cultivation (Fig. 1c and Table 2). Contrarily, nitrogen starvation inhibited cell division severely, resulting in the maximum carbohydrate yield and productivity of only 0.348 and 180.3 mg L−1 day−1, respectively (Fig. 1c and Table 2).
Effect of Nitrogen Starvation on Microalgal Starch Accumulation
To our knowledge, the reserved carbohydrate in microalgae is species-specific. For example, green algae tend to accumulate starch while cyanobacteria synthesize glycogen [15], and diatoms often store chrysolaminarin [16]. These major storage polysaccharides exhibit the potential as feedstocks for bioethanol conversion because not all sugar forms are easily digested. Thus, it is crucial to determine the predominant types of carbohydrate for fermentation to produce ethanol. Figure 2 shows the time courses of starch production under nitrogen repletion and nitrogen starvation, respectively. Under nitrogen repletion condition, starch content of C. zofingiensis was slightly fluctuated within the range of 4.5~9.7 % of DW, suggesting that C. zofingiensis did not accumulate a significant amount of starch. While in response to nitrogen starvation, starch content increased about 6-fold, yielding the highest starch content (43.4 % of DW) after only 1 day and was significantly higher compared to that under nitrogen repletion. In accordance with our observation, Yao et al. [17] found a marine green microalga Tetraselmis subcordiformis peaked starch content immediately following the day that nitrogen was exhausted. The results indicated that nitrogen depletion was a trigger for microalgal starch accumulation. Though specific growth rate and biomass productivity under nitrogen starvation were much lower than those under nitrogen repletion, the starch yields under these two conditions were comparable, 220.7 mg L−1 under nitrogen repletion by day 4 versus 206.3 mg L−1 under nitrogen starvation by day 1 (Fig. 2b). Starch productivity under nitrogen starvation (185.1 mg L−1 day−1) even far surpassed over that under nitrogen repletion (51.3 mg L−1 day−1) (Table 2), implying that nitrogen stress was a good way for rapid and massive production of starch. It is noted that nitrogen assimilation and metabolism constitutes a significant sink for carbon in growing cells, and nitrogen starvation increases carbon availability, causing carbon flux shifted to storage compounds [18]. In this case, C. zofingiensis cells tended to rapidly accumulate starch rather than lipid [19], probably because starch synthesis requires less energy [20].
Figure 3 shows the ratios of starch to carbohydrate of C. zofingiensis under different conditions. The ratio of starch to carbohydrate was basically constant (~20 %) under nitrogen-rich condition, while rose sharply to 66.7 % after 1 day of cultivation under nitrogen starvation, and then gradually reduced to 39.4 % after 10 days. This demonstrated that starch accumulation occurred promptly after nitrogen starvation and caused the increase of carbohydrate content. In other words, most stored carbohydrate in C. zofingiensis cells was starch. The content of the rest of carbohydrate in C. zofingiensis kept relatively constant during nitrogen starvation in the range of 21~28 % of DW, which might be mainly cell wall sugars such as cellulose and pectin as structural components [8]. That is why the patterns of the accumulation of starch and carbohydrate during the progression of nitrogen starvation were similar. Some other microalgae, like diatoms, accumulate chrysolaminarin instead of starch as the principal energy storage carbohydrate, which generally comprises 10~20 % of the total cellular carbon in fast growing cells but can accumulate 80 % of the total carbohydrate in cells under nitrogen depletion conditions [21]. In this study, nitrogen starvation might cause excess photosynthetic carbon within the cell, thus generating an accumulation of metabolite 3-phosphoglycerate, the primary product of CO2 fixation [22]. This metabolite can activate ADP-glucose pyrophosphorylase, a regulatory enzyme for starch synthesis [23]. The increase in starch may relate to an increase in the activity of ADP-glucose pyrophosphorylase and is yet to be explored.
As mentioned above, nitrogen starvation enhanced the content of carbohydrate, mainly starch, in C. zofingiensis. Starch-rich microalgal cells seem to be superior to conventional lignocellulosic biomass. Although both starch and cellulose can be converted to fermentable sugars, starch has more advantages over cellulose in saccharification since amylase is more cheaply and easily available in comparison to cellulase. Furthermore, algal strains have no lignin and very low hemicellulose [4], making them more easily hydrolyzed. On the other hand, we found that starch synthesis in C. zofingiensis strain took place much faster than lipid accumulation and starch content was also much higher than the lipid content under the same cultivation conditions [19]. Therefore, the microalga C. zofingiensis is feasible and advantageous as a feedstock for bioethanol production, especially under nitrogen starvation condition.
Strategy to Enhance Carbohydrate and Starch Production
The microalga C. zofingiensis would be ideal for producing bioethanol if both the carbohydrate/starch content and biomass could attain to a satisfactory level simultaneously. Unfortunately, the culture conditions for maximum growth and maximum secondary metabolite content are usually mutually contradictory. Our results suggested that while nitrogen starvation led to an increase in the carbohydrate content, it also significantly lowered the biomass (Table 2), thereby resulting in lower carbohydrate productivity. Two-stage cultivation seems to be a good strategy to solve this problem. In this strategy, a nutrient-rich medium is used in the first stage to promote algal cell growth and then the culture is subjected to a stress condition in the second stage to trigger the target product accumulation. This strategy has been used for the production of lipids, astaxanthin, starch, and so on [24–27]. For C. zofingiensis, the required nitrogen starvation time is short for carbohydrate accumulation, which is very suitable for two-stage cultivation mode, as the risks of contamination during cultivation would be diminished. When cultivated under prolonged stress, microalgae normally become frail and vulnerable to invaders and predators.
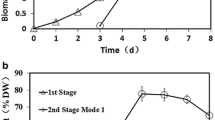
In this work, the cells of C. zofingiensis were cultivated under nitrogen-rich condition for approximately 3 days to reach the middle log phase, and then switched to nitrogen-free medium for rapid accumulation of carbohydrate and starch. As shown in Fig. 4, the production of biomass, carbohydrate, and starch under two-stage cultivation mode was improved drastically compared to that obtained merely under nitrogen-rich or nitrogen-free condition. The biomass concentration of C. zofingiensis rapidly rose to 3 g L−1 after 4 days of cultivation (1 day after nitrogen starvation) (Fig. 4a). By day 7 of total cultivation (4 days after nitrogen starvation), the biomass reached 4.29 g L−1, increasing 1.4-fold compared to normal growth by day 10. During two-stage cultivation, the maximum contents of carbohydrate and starch (57.2 and 37.1 % of DW, respectively) occurred after 2 days of starvation period (5 days of total cultivation), which were a little lower than those under simply nitrogen starvation condition. This phenomenon might be because reduced irradiation by virtue of dense culture weakened photosynthetic activity involved in carbohydrate biosynthesis.
The concentrations of carbohydrate and starch increased rapidly until day 5 for total cultivation (day 2 for nitrogen starvation), which were 2.1 and 1.4 g L−1, respectively. After that, carbohydrate slowly increased while starch gradually decreased (Fig. 4b). From the perspective of engineering applications, target product productivity of a microalgal strain is a vital indicator for evaluating its potential of commercialization [28, 29]. Our results showed that carbohydrate and starch productivities both reached their highest levels by day 5 (day 2 under nitrogen stress), which were 407 and 268 mg L−1 day−1, respectively (Fig. 4c). The results are superior to most of the previous reports [4, 30]. Taken together, in order to obtain algal biomass for bioethanol production, harvesting of C. zofingiensis cells should be implemented on day 5 of total cultivation, at which point the highest algal carbohydrate and starch productivities were achieved. Our results also indicated that the two-stage cultivation strategy was very effective in enhancing microalgal carbohydrate/starch production.
Conclusions
Nitrogen starvation caused a severe inhibition of cell growth and a quick increase of the contents of carbohydrate and starch in C. zofingiensis. Massive accumulation of carbohydrate and starch could be acquired in a short span of time upon nitrogen starvation. Starch is the main stored carbohydrate and accounts for 66.7 % of total carbohydrate, endowing this microalga with excellent superiority in saccharification process. The highest contents of carbohydrate and starch were 66.9 and 43.4 % of DW, which were obtained after only 1 day of nitrogen starvation. Two-stage cultivation strategy was demonstrated to be an effective way to enhance both the carbohydrate content and biomass productivity of C. zofingiensis to serve as a feedstock for bioethanol production. In this strategy, the optimal biomass, carbohydrate, and starch productivities of C. zofingiensis obtained by 5-day cultivation were 699, 407, and 268 mg L−1 day−1, respectively, making it a good potential bioethanol feedstock.
References
Dragone, G., Fernandes, B. D., Abreu, A. P., Vicente, A. A., & Teixeira, J. A. (2011). Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Applied Energy, 88(10), 3331–3335.
Doan, Q. C., Moheimani, N. R., Mastrangelo, A. J., & Lewis, D. M. (2012). Microalgal biomass for bioethanol fermentation: implications for hypersaline systems with an industrial focus. Biomass and Bioenergy, 46, 79–88.
Sun, Y., & Cheng, J. Y. (2002). Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresource Technology, 83, 1–11.
Chen, C. Y., Zhao, X. Q., Yen, H. W., Ho, S. H., Cheng, C. L., Lee, D. J., Bai, F. W., & Chang, J. S. (2013). Microalgae-based carbohydrates for biofuel production. Biochemical Engineering Journal, 78, 1–10.
Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M., Seibert, M., & Darzins, A. (2008). Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant Journal, 54(4), 621–639.
Wijffels, R. H., & Barbosa, M. J. (2010). An outlook on microalgal biofuels. Science, 329(5993), 796–799.
Ho, S. H., Huang, S. W., Chen, C. Y., Hasunuma, T., Kondo, A., & Chang, J. S. (2013). Characterization and optimization of carbohydrate production from an indigenous microalga Chlorella vulgaris FSP-E. Bioresource Technology, 135, 157–165.
Markou, G., Angelidaki, I., & Georgakakis, D. (2012). Microalgal carbohydrates: an overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Applied Microbiology and Biotechnology, 96(3), 631–645.
Choix, F. J., de Bashan, L. E., & Bashan, Y. (2012). Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: I. Autotrophic conditions. Enzyme and Microbial Technology, 51(5), 294–299.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356.
Branyikova, I., Marsalkova, B., Doucha, J., Branyik, T., Bisova, K., Zachleder, V., & Vitova, M. (2011). Microalgae-novel highly efficient starch producers. Biotechnology and Bioengineering, 108(4), 766–776.
Recht, L., Zarka, A., & Boussiba, S. (2012). Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Applied Microbiology and Biotechnology, 94(6), 1495–1503.
Lv, J. M., Cheng, L. H., Xu, X. H., Zhang, L., & Chen, H. L. (2010). Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresource Technology, 101, 6797–6804.
Wang, L., Li, Y. G., Sommerfeld, M., & Hu, Q. (2013). A flexible culture process for production of the green microalga Scenedesmus dimorphus rich in protein, carbohydrate or lipid. Bioresource Technology, 129, 289–295.
González-Fernández, C., & Ballesteros, M. (2012). Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnology Advances, 30(6), 1655–1661.
Roessler, P. G. (1987). Udpglucose pyrophosphorylase activity in the diatom Cyclotella cryptica—pathway of chrysolaminarin biosynthesis. Journal of Phycology, 23(3), 494–498.
Yao, C., Ai, J., Cao, X., Xue, S., & Zhang, W. (2012). Enhancing starch production of a marine green microalga Tetraselmis subcordiformis through nutrient limitation. Bioresource Technology, 118, 438–444.
Fan, J. L., Yan, C. S., Andre, C., Shanklin, J., Schwender, J., & Xu, C. C. (2012). Oil accumulation is controlled by carbon precursor supply for fatty acid synthesis in Chlamydomonas reinhardtii. Plant and Cell Physiology, 53(8), 1380–1390.
Zhu, S., Huang, W., Xu, J., Wang, Z., Xu, J., & Yuan, Z. (2014). Metabolic changes of starch and lipid triggered by nitrogen starvation in the microalga Chlorella zofingiensis. Bioresource Technology, 152, 292–298.
Li, Y. T., Han, D. X., Sommerfeld, M., & Hu, Q. (2011). Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresource Technology, 102, 123–129.
Wan, L. L., Han, J., Sang, M., Li, A. F., Wu, H., Yin, S. J., & Zhang, C. W. (2012). De novo transcriptomic analysis of an oleaginous microalga: pathway description and gene discovery for production of next-generation biofuels. Plos One, 7, e35142.
Foy, R. H., & Smith, R. V. (1980). The role of carbohydrate accumulation in the growth of planktonic Oscillatoria species. British Phycological Journal, 15(2), 139–150.
Ballicora, M., Iglesias, A., & Preiss, J. (2004). ADP-glucose pyrophosphorylase: a regulatory enzyme for plant starch synthesis. Photosynthesis Research, 79(1), 1–24.
Rodolfi, L., Zittelli, G. C., Bassi, N., Padovani, G., Biondi, N., Bonini, G., & Tredici, M. R. (2009). Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnology and Bioengineering, 102(1), 100–112.
Ho, S. H., Chen, W. M., & Chang, J. S. (2010). Scenedesmus obliquus CNW-N as a potential candidate for CO2 mitigation and biodiesel production. Bioresource Technology, 101, 8725–8730.
Han, D. X., Li, Y. T., & Hu, Q. (2013). Astaxanthin in microalgae: pathways, functions and biotechnological implications. Algae, 28(2), 131–147.
Ho, S. H., Li, P. J., Liu, C. C., & Chang, J. S. (2013). Bioprocess development on microalgae-based CO2 fixation and bioethanol production using Scenedesmus obliquus CNW-N. Bioresource Technology, 145, 142–149.
Griffiths, M. J., & Harrison, S. T. L. (2009). Lipid productivity as a key characteristic for choosing algal species for biodiesel production. Journal of Applied Phycology, 21(5), 493–507.
Ho, S. H., Chen, C. Y., & Chang, J. S. (2012). Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresource Technology, 113, 244–252.
Yao, C. H., Ai, J. N., Cao, X. P., & Xue, S. (2013). Characterization of cell growth and starch production in the marine green microalga Tetraselmis subcordiformis under extracellular phosphorus-deprived and sequentially phosphorus-replete conditions. Applied Microbiology and Biotechnology, 97(13), 6099–6110.
Sassano, C. E. N., Gioielli, L. A., Ferreira, L. S., Rodrigues, M. S., Sato, S., Converti, A., & Carvalho, J. C. M. (2010). Evaluation of the composition of continuously-cultivated Arthrospira (Spirulina) platensis using ammonium chloride as nitrogen source. Biomass and Bioenergy, 34(12), 1732–1738.
De Philippis, R., Sili, C., & Vincenzini, M. (1992). Glycogen and poly-β-hydroxybutyrate synthesis in Spirulina maxima. Journal of General Microbiology, 138(8), 1623–1628.
Acknowledgments
This research was financially supported by National Natural Science Foundation of China (No. 31100189), the National Basic Research Program of China (973 Program) (2011CB200905), the 12th Five Year Support Plan of the Ministry of Science and Technology, China (2011BAD14B03), National High-tech R&D Program (2013AA065803), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, S., Wang, Y., Huang, W. et al. Enhanced Accumulation of Carbohydrate and Starch in Chlorella zofingiensis Induced by Nitrogen Starvation. Appl Biochem Biotechnol 174, 2435–2445 (2014). https://doi.org/10.1007/s12010-014-1183-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1183-9