Abstract
Growing algae in darkness for biodiesel production eliminates the challenges of evaporation and light penetration reported for open ponds and the costs and fouling that plague photobioreactors. The current study demonstrated that Chlorella kessleri str. UTEX 263 could grow heterotrophically in the dark on pure sugars or lignocellulosic hydrolysates of plant biomass. Hydrolysates of a prairie grass native to Kansas, Big Bluestem (Andropogon gerardii), supported the growth of C. kessleri in the dark. Nitrogen limitation stimulated the accumulation of biodiesel lipids by 10-fold in heterotrophic cultures grown on pure sugars or Big Bluestem hydrolysate. Limiting P in the growth medium also was shown to increase cellular lipid accumulation in C. kessleri. Iron limitation was not sufficient to increase cellular lipid content. Crude biomass extracts may have levels of N that cannot be easily removed, which are high enough to relieve N limitations in growth media. This initial study suggests that P might be more easily removed from biomass extracts than N for increasing cellular lipid production by nutrient limitation and further that native prairie grasses are potentially suitable as sources of lignocellulosic sugars.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biofuels are renewable clean energy alternatives that might reduce CO2 emissions and mitigate global warming (Lincoln 2005; Demirbas 2009; Naik et al. 2010). Liquid fuels for transportation, manufacturing, and domestic heating represent nearly 70% of total global energy usage (Gouveia and Oliveira 2009). Food crops are commonly exploited as feedstocks to support production of biofuels by fungi or bacteria, which has raised sustainability concerns (Milne et al. 1990; Hill et al. 2006; Moore 2008; Brennan and Owende 2010; Havlík et al. 2011). Alternatively, manufacturers can utilize non-food lignocellulose feedstocks such as agricultural biomass, food processing wastes, forest residues, and grassy crops, as sources of sugars that can be liberated through enzymatic or acid hydrolysis. Biofuels produced by algae using the energy of sunlight offer an attractive alternative to biofuels supported by land-based agriculture (Abou-shanab et al. 2006; Dragone et al. 2010; Smith et al. 2010; Verma et al. 2010; Lee and Lavoie 2013). Algae have been used to produce biofuels such as methane from anaerobic digestion of biomass, biodiesel from cellular lipids, and bio-hydrogen in photobioreactors (Belarbi et al. 2000). In general, the physical and fuel properties (density, acidity, and heating value) of biodiesel from algae are comparable to those of conventional diesel (Miao and Wu 2004, 2006).
It is technically feasible, but difficult at large scales, to reach high algal biomass densities autotrophically, due to limitations imposed by poor light penetration (Chen and Johns 1991, 1996; Liang et al. 2009). Shallow production ponds with large surface areas are needed in warm sunny locations, and these rapidly lose water by evaporation, greatly decreasing the economic feasibility of algal biodiesel production. While closed photobioreactors are an alternative to open ponds, these invariably have high start-up and maintenance costs, are easily fouled by algae growing on the clear surfaces, and often become contaminated with fungi or bacteria that are difficult to eliminate (Chisti 2007; Carvalho et al. 2008; Xu et al. 2009; Richardson et al. 2012; Louw et al. 2016; Kern et al. 2017). An appropriate alternative is to grow algae heterotrophically in dark bioreactors supplemented with sugars, preferably from waste biomass hydrolysates (Perez-Garcia et al. 2011; Nagarajan et al. 2018). Heterotrophic algal growth obviates the problems imposed by light penetration and water loss, while producing biodiesel lipids of high quality.
Knowledge of heterotrophic growth of algae dates to the first description of Chlorella by Beijerinck (Beyerinck 1890), with a variety of organic substrates shown to support growth in the dark (Roach 1926, 1927; Skinner and Gardner 1930; Barker 1935). Algal growth has been observed in dark natural habitats such as covered Antarctic lakes and deep soil layers and in dark manmade environments such as water mains and cooling towers (Rodhe 1953; Parker et al. 1961; Seilheimer and Jackson 1963). For many algal species, heterotrophic growth outpaces photoautotrophic growth (Miao and Wu 2004, 2006; Xu et al. 2006; Li et al. 2007). Culture densities of > 100 g dry wt L−1 have been reported for Chlorella, Crypthecodinium, and Galdieria (De Swaaf et al. 2003; Graverholt and Eriksen 2007; Rosenberg et al. 2008; Heredia-Arroyo et al. 2010).
Our initial study with Chlorella kessleri str. UTEX 263 examines its growth and lipid production on a variety of sugar substrates under different lighting regimes. This Chlorella strain is noted for its high lipid content, particularly under conditions of N limitation (Piorreck et al. 1984; Kay and Barton 1991). Chlorella species can grow heterotrophically and mixotrophically with acetate, glucose, glycerol, and other organic compounds (Schneegurt et al. 1997; Heredia-Arroyo et al. 2010; Wan et al. 2011; Pagnanelli et al. 2014). Previous reports demonstrated that Chlorella could produce biodiesel under heterotrophic conditions using a variety of carbon substrates (Miao and Wu 2006; Xu et al. 2006; Li et al. 2007; Liang et al. 2009). In addition, C. kessleri UTEX 263 grows well at elevated salinities and in simplified media with lower costs (Wagley and Schneegurt 2012a, 2012b). We propose a scheme for biodiesel production that uses dark bioreactors to grow algae heterotrophically on the same feedstocks commonly used for bioethanol production. Here, we explore the effects of N or P starvation on growth and lipid production in algae grown on pure sugars or on a lignocellulosic hydrolysate of Big Bluestem (Andropogon gerardii), a prairie grass that is native to Kansas.
Materials and methods
Organism and media
Chlorella kessleri, str. UTEX 263, was grown as shake-flasks (150-rpm) in proteose medium (in g L−1: proteose peptone, 5.0; NaNO3, 0.25; KH2PO4, 0.175; K2HPO4, 0.075; MgSO4·7H2O, 0.075; NaCl, 0.025; CaCl2·2H2O, 0.025) under artificial cool white fluorescent light (50 μmol photons m−2 s−1) or in darkness. Growth media were prepared with different levels of N (0.0, 0.3, 3.0, and 30.0 mM as KNO3). Similarly, media were prepared with different levels of P (0.0, 0.05, 0.5, and 5.0 mM as KH2PO4) and Fe (0.0, 1.0, 2.0, and 20.0 μM as FeCl2). Once autoclaved and cooled, the media were supplemented with 0.01% w/v carbendazim (from a 10% ethanolic stock) and 0.25 mg L−1 ampicillin (from a 0.25 mg mL−1 stock) to inhibit the growth of fungi and bacteria, respectively.
Preparation of sugar substrates from biomass
Big Bluestem was collected from the Wichita State University Biological Field Station (37°32′03.1″N 97°40′23.1″W), air-dried and pulverized for 2 days using a ball mill (3 lb.; United Nuclear) with steel balls. Pretreatment of biomass (4 g) was with 40 mL of NaOH (1%) at 50 °C for 12 h. The slurry was brought to pH 4.8 with 0.1 M Na citrate solution and supplemented with 100 μL of a 20 g L−1 Na azide solution. The mixture was heated to 50 °C after the addition of 5 mL water and then 100 μL of Accelerase 1500 enzyme was added and allowed to react for 48 h on a rotary shaker (150 rpm) at 50 °C. The hydrolysate was clarified by vacuum filtration (Whatman no. 4 filter paper). Corn and sorghum extracts were kindly provided by Donghai Wang (Kansas State University). Glucose contents of hydrolysates were measured using anthrone reagent as previously described (Seifter et al. 1950; Scott and Melvin 1953).
Growth curves
Growth curves were generated from shake-flask cultures (150 rpm) at 25 °C in continuous light (50 μmol photons m−2 s−1) or darkness. Subcultures were made with a 5% inoculum. BSM medium (Dille et al. 2016) was supplemented with 1% sugar as pure compounds or as biomass hydrolysates. A Neubauer hemocytometer was used for direct microscopic cell counts of 10-μL samples with dilution as necessary. Algal cell counts were taken each day until stationary phase was reached. Arnon’s whole-cell protocol and equations (Arnon 1949) were used to determine chlorophyll content with a Genesys 10S UV-VIS spectrophotometer (Thermo Fisher) using fresh media blanks.
Lipid analyses
Nile Red staining can estimate the neutral lipid content of algal cells (Cooksey et al. 1987). Culture samples from 7 days after inoculation were diluted 1:10 in DMSO, heated to near boiling, and then cooled. An equal volume of Nile Red solution (1 μg mL−1 in 50% DMSO) was added and the mixture incubated for 10 min in darkness. Spectrofluorometric measurements were taken with excitation at 490 nm and emission at 580 nm using a SynergyMx instrument (Biotek). Vegetable oil was used to generate a standard curve for quantification. Since dry weight measurements were not made, lipid content is expressed on a per-cell or culture-volume basis. A modified Bligh and Dyer (1959) protocol was used for lipid extraction prior to FAME analysis (Sturm et al. 2011). Cells from 100 mL of algal culture were harvested by centrifugation at 10,000×g for 10 min, and the pellet was resuspended in 10 mL water, before the addition of 20 mL of 2:1 chloroform/methanol. The mixture was vortexed well and centrifuged again at 10,000×g for 10 min, with the lower liquid phase collected in a fresh tube. The solvent was evaporated under a stream of dry nitrogen gas. Non-polar lipids were extracted with 5 mL ice-cold acetone before drying and dissolution in 1 mL chloroform. A transesterification reaction (with 0.2 M KOH in 1:1 methanol/toluene at 37 °C) was used to create fatty acid methyl esters that were extracted thrice with chloroform and evaporated to dryness. Lipid species were identified and quantified by GC/MS (Agilent 6890 GC with 5793 MS) using an Innowax 15-m polyethylene column (HP; 0.25 mm ID; He carrier gas) with a ramped temperature regime from 120 to 240 °C and an MS quadrupole temperature of 150 °C. Methyl ester identities were verified using a mixture of lipid standards (FAMEMix C8-C24, Supelco, USA), and lipid concentrations were determined based on the response factor and the peak area of the internal standards.
Results
Algal growth on various sugar substrates during N starvation
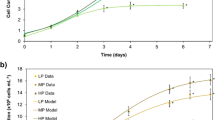
Growth was monitored in heterotrophic cultures of C. kessleri grown in darkness with sucrose as sole carbon and energy source and several levels of fixed N (Fig. 1). Direct microscopic cell counts demonstrated that maximum growth was reduced, when limiting fixed N to 0.3 mM, 1% of the full-strength medium (Fig. 1a). Growth in medium at 10% of the standard N content (3.0 mM) did not show very much inhibition based on cell counts, but when measured by chlorophyll content, 3 days after inoculation accumulation slowed (Fig. 1b). With no added N, cell number decreased (Fig. 1a). Chlorophyll measurements showed clearer trends in part because algal cells tend to bleach with nutrient starvation, so the differences between nutrient levels are amplified (Pal et al. 2011; Gigova and Ivanova 2015). Initial culture densities varied between experiments; however, the inocula were light overall, less than 10% of the final density of mature cultures, and therefore should not considerably affect the response of cultures to starvation.
Growth of C. kessleri cultures supplied with different levels of N in the medium and maintained heterotrophically in the dark on sucrose. Means of triplicates ± SD. diamonds, 0.0 mM N; triangles, 0.3 mM N; squares, 3.0 mM N; circles, 30.0 mM N. a By direct microscopic count; b By chlorophyll content
Similar heterotrophic growth experiments were performed with glucose and fructose. While substantial growth was observed with N limitation, growth was reduced on glucose at 3.0 and 0.3 mM N, relative to the 30.0 mM control (Fig. 2). Control cultures fed fructose and provided 30.0 mM N started at a somewhat lower density but grew rapidly enough to surpass the N-limited cultures (Fig. 3). Little growth was observed on fructose at 0.3 mM N. The glucose-fed cultures had maximum chlorophyll concentrations about twice those of the sucrose or fructose cultures, which also was reflected in direct microscopic counts. Cultures of C. kessleri grown autotrophically in continuous light also were dependent on added fixed N. When exogenously added fixed N was reduced 10- or 100-fold, growth was slowed and maximum culture density was reduced (Fig. 4). For laboratory growth experiments, it was convenient to reduce microbial contamination by adding antibiotics, thereby giving more consistent results. A successful industrial process would likely have to use a cleaner and less expensive alternative to reach an acceptable level of contamination or use multi-species cultures.
Growth of C. kessleri cultures supplied with different levels of N in the medium and maintained heterotrophically in the dark on glucose. Means of triplicates ± SD. diamonds, 0.0 mM N; triangles, 0.3 mM N; squares, 3.0 mM N; circles, 30.0 mM N. a By direct microscopic count; b By chlorophyll content
Growth of C. kessleri cultures supplied with different levels of N in the medium and maintained heterotrophically in the dark on fructose. Means of triplicates ± SD. diamonds, 0.0 mM N; triangles, 0.3 mM N; squares, 3.0 mM N; circles, 30.0 mM N. a By direct microscopic count. b By chlorophyll content
Hydrolysates of lignocellulosic materials from Big Bluestem were used as sole carbon and energy sources for C. kessleri grown heterotrophically in the dark. Growth was reduced, especially when ≤ 0.3 mM N was added (Fig. 5). It is interesting to note that toward the end of the incubation period, the culture with 0.3 mM N began to increase in cell number, although no corresponding increase in chlorophyll was observed. Cultures reached approximately the same density as those fed pure sugars. While the available N content of Big Bluestem hydrolysate was not measured, it must be relatively small since growth was effectively inhibited by N starvation at even 3.0 mM (10% of full-strength medium).
Heterotrophic growth of C. kessleri cultures in the dark on hydrolysate of Big Bluestem supplied with different levels of N in the medium. Means of triplicates ± SD. diamonds, 0.0 mM N; triangles, 0.3 mM N; squares, 3.0 mM N; circles, 30.0 mM N. a By direct microscopic count. b By chlorophyll content
Algal growth on various sugar substrates during P or Fe limitation
Cultures of C. kessleri were grown heterotrophically on sucrose in the dark in media supplemented to various P levels. Very clear growth inhibition was observed as P levels were decreased 10- or 100-fold (Fig. 6). Cultures with no added P grew weakly, indicating residual P was either added with the inoculum or was a contaminant of the media components.
Heterotrophic growth in the dark on Big Bluestem hydrolysates was measured at various P levels (Fig. 7). Growth inhibition with P limitation was not as apparent in these experiments. However, it seems that the Big Bluestem extract may bind free P, since typical growth was not observed at 0.5 mM, the P level of full-strength media. Growth was enhanced at 5 mM P, although growth inhibition by P limitation was still not as clear as when cultures were fed sucrose.
Reducing the iron concentration of media did not result in a substantial inhibition of heterotrophic growth in the dark on sucrose, as measured by chlorophyll content or direct microscopic counts (Fig. 8). Iron levels were reduced from 2 to 0.2 μM with no clear effects on growth. Robust growth in cultures with no added iron suggests that more stringent methods (such as acid-washing glassware) would be needed to reduce iron levels below the trace levels required for growth.
Lipid content under nutrient limitation
Nutrient limitation leads to an increase in the content of lipids in cells of C. kessleri. Reducing the concentration of exogenously added N to 10% (3.0 mM) of the full-strength medium concentration led to a nearly 3-fold increase in the cellular lipid content of cells fed sucrose (Fig. 9a). Cellular lipid content continued to increase with greater N limitation, such that in cultures with no added N, cellular lipid content was nearly 8-fold greater than in cells grown in standard medium. The increase in cellular lipid content was more abrupt for C. kessleri grown on Big Bluestem hydrolysates (Fig. 9b). There was a doubling of cellular lipid content when N was reduced to 3.0 mM. However, at ≤ 1.5 mM N, cellular lipids were ~ 10-fold higher than in cells grown in full-strength medium.
There was not a substantial increase in cellular lipid content until P levels were decreased 10-fold (0.05 mM) from the concentration of full-strength medium (0.5 mM), stimulating a 3-fold increase in lipid content (Fig. 9c). With no exogenously added P, cellular lipid contents were 5-fold greater than for cells in full-strength medium. Cellular lipids increased about 2-fold with P starvation when big Bluestem hydrolysate was used (Fig. 9d). Depleting iron in the medium did not increase cellular lipid content in cells grown on sucrose (Fig. 9e). From a practical perspective, the total lipid concentration of algal cultures reflects both cellular lipid content and culture density. While starving cultures may increase cellular lipid production, the growth inhibition caused by starvation might lower total yields of lipids.
Lipid profiles in C. kessleri grown on biomass hydrolysates
A key task of the current work was to demonstrate that lipids suitable for biodiesel could be produced by C. kessleri grown on hydrolysates of Big Bluestem, a native prairie plant. For comparison, growth experiments were performed with hydrolysates of corn mash, sorghum juice, and sorghum mash (Fig. 10). The initial inoculum was small for this experiment, but it is clear that all of the sugar sources supported the growth of C. kessleri in the dark. Lipid profiles of algal extracts were determined as fatty acid methyl esters. The values are expressed for each lipid as the fraction of total lipids in extracts of cultures grown on hydrolysates of Big Bluestem, corn mash, and sorghum mash (Table 1), which were 20, 20, and 6% sugar, respectively. The most abundant lipids recovered were 18:1 and 18:2 fatty acids, regardless of biomass source. Cultures grown on Big Bluestem hydrolysate were higher in 18:1 and lower in 18:2 fatty acids than cultures grown on corn or sorghum mash. Other lipids were detected at less than 1% of the total lipids extracted, including 14:0, 16:1, 16:2, 16:3, 20:0, 22:0, 22:1, 24:0 fatty acids.
Discussion
It is well known that photosynthetic algae can robustly grow heterotrophically in the dark or mixotrophically in the light, with carbon substrates that include acetate, carboxylic acids, glucose, and glycerol (Schneegurt et al. 1997; Heredia-Arroyo et al. 2010; Wan et al. 2011; Pagnanelli et al. 2014). Chlorella spp. have been shown to produce copious biodiesel lipids in the dark when grown on glycerol or glucose (Endo et al. 1977; Wu et al. 1994; Liang et al. 2009). However, high lipid production is not always observed with Chlorella under these conditions (Neilson and Lewin 1974; O’Grady and Morgan 2011).
Nitrogen limitation is often used to increase lipid yields from algal cultures (Richardson et al. 1969; Converti et al. 2009; Mandal and Mallick 2009; Mutlu et al. 2011; Liang et al. 2013; Ruangsomboon et al. 2013; Fan et al. 2014; Procházková et al. 2014; Singh et al. 2016). A study of 30 species of Chlorophyceae and diatoms found that N limitation led to greater storage of fatty acids (Shifrin and Chisholm 1981). A 75% reduction in the amount of N added to cultures of Nannochloropsis oculata or C. vulgaris led to a doubling of cellular lipid contents (Converti et al. 2009). It has been suggested that under N limitation, cells partition N into functional proteins and use fixed C for making carbohydrates and lipids (Richardson et al. 1969). However, N limitation also can lower maximum culture density and decrease chlorophyll a content, yellowing cultures (Pal et al. 2011; Gigova and Ivanova 2015). The current report demonstrates similar outcomes with N limitation, a decrease in final culture density and an increase in cellular lipid content. Growth inhibition was often modest, with < 2-fold differences between replete and starved cultures, while cellular lipid content was seen to increase 10-fold or more with starvation. Further, we have shown that algal cultures grown on Big Bluestem biomass hydrolysate show similar effects with N limitation as cultures grown on pure sugars.
It might be difficult to control the level of N when feeding cultures biomass hydrolysates. Depleting hydrolysates of N seems challenging given the many forms of fixed N found in cells. Phosphorus however, while also variable in biomass hydrolysates, seems more easily removed from solution. Functionalized beads or precipitation of insoluble P compounds are potential methods for reducing P levels in hydrolysates. We have demonstrated that phosphorus limitation can increase the accumulation of biodiesel lipids by C. kessleri cells grown heterotrophically in the dark, whether pure sugars or biomass hydrolysates were supplied as the carbon source. The hydrolysates used in the current study did not have N or P levels high enough to relieve the nutrient limitations.
Limitation or starvation for P has been shown to increase cellular lipid content in Chlorella and other microalgae, often to the same degree as N starvation (Khozin-Goldberg and Cohen 2006; Mutlu et al. 2011; Přibyl et al. 2012; Liang et al. 2013; Ruangsomboon et al. 2013; Adenan et al. 2016; Su et al. 2016), although responses vary (Reitan et al. 1994; Deng et al. 2011). However, lower maximum culture densities with P limitation may keep total lipids yields near the values obtained with P-replete media. It appears that P limitation leads to the same sequestration of N into proteins and concomitant increase in lipid production as N limitation (Rhee 1978). Furthermore, it has been suggested that algae under P limitation remodel cellular membranes to recycle P from phospholipids while increasing P uptake and mobilization (Mühlroth et al. 2017).
The current study did not achieve sufficient iron limitation to alter cellular lipid content. Microalgae previously have been demonstrated to increase lipid production with release from iron limitation (Liu et al. 2008; Ruangsomboon et al. 2013). Other studies have reported small increases in cellular lipid content with iron limitation (Deng et al. 2011; Fan et al. 2014).
Biodiesel production often competes for biomass from food crops such as corn and sorghum. Lignocellulosic sources of sugars might alleviate this drain on food crops, since hydrolysates of the non-edible portions of plants or non-food plants are commonly used. Beyond its impact on food agriculture, supporting algal cultures on pure sugars and small acids and alcohols represents a principal cost hurdle to biodiesel production (García Sánchez et al. 2003; Louw et al. 2016; Kern et al. 2017). It is common to see suggestions that biodiesel production should rely on plants such as switchgrass, which are non-native, potentially invasive species. Using plants native to a given region seems more sustainable. The current preliminary study demonstrated that hydrolysates of a common native grass in Kansas, Big Bluestem, may potentially be used as a source of sugars for algal biodiesel production in the dark. In a similar fashion, Big Bluestem and other native prairie grasses represent sustainable sources of biomass for lignocellulosic bioethanol production.
Behrens (2005) compared the costs of photoautotrophic and heterotrophic algal production schemes using glucose as the carbon source. While energy costs were higher for dark bioreactors than photobioreactors, the total cost to produce a kg of biomass was 20% lower, since productivity was much higher in the dark bioreactors. In addition, construction costs were lower and scale-up was easier than with photobioreactors. Technologies are available to make either type of system feasible for biodiesel production (Davis et al. 2011; Richardson et al. 2012). Producing algal biodiesel in the dark avoids the problems of light penetration, water loss by evaporation, and fouling common to photobioreactors.
References
Abou-shanab RAI, Jeon BH, Song H, Kim Y, Hwang JH (2006) Algae-biofuel: potential use as sustainable alternative green energy. Online J Power Energ Eng 1:4–6
Adenan NS, Yusoff FM, Medipally SR, Shariff M (2016) Enhancement of lipid production in two marine microalgae under different levels of nitrogen and phosphorus deficiency. J Environ Biol 37:669–676
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Barker HA (1935) The metabolism of the colorless alga, Prototheca zopfii Krüger. J Cell Comp Physiol 7:73–93
Behrens PW (2005) Photobioreactors and fermentors: the light and dark side of growing algae. In: Andersen RA (ed) Algal culturing techniques. Academic Press, Burlington, pp 189–204
Belarbi EH, Molina E, Chisti Y (2000) A process for high yield and scaleable recovery of high purity eicosapentaenoic acid esters from microalgae and fish oil. Enzym Microb Technol 26:516–529
Beyerinck MW (1890) Culterversuche mit Zoochlorellen, Lichenengonidien und anderen niederen Algen. I. Das Isoliren niederer Algen durch die Gelatinemethode. Bot Z 47:725–739
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Brennan L, Owende P (2010) Biofuels from microalgae – a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Carvalho AP, Meireles LA, Malcata FX (2008) Microalgal reactors: a review of enclosed system designs and performances. Biotechnol Prog 22:1490–1506
Chen F, Johns MR (1991) Effect of C/N ratio and aeration on the fatty acid composition of heterotrophic Chlorella sorokiniana. J Appl Phycol 3:203–209
Chen F, Johns MR (1996) Relationship between substrate inhibition and maintenance energy of Chlamydomonas reinhardtii in heterotrophic culture. J Appl Phycol 8:15–19
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process Process Intensif 48:1146–1151
Cooksey KE, Guckert JB, Williams SA, Callis PR (1987) Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J Microbiol Methods 6:333–345
Dandinpet KK, Schneegurt MA (2013a) Dark-grown algae fed corn, sorghum, and lignocellulosic hydrolysates for biodiesel production. Trans KS Acad Sci 116:71–72
Dandinpet KK, Schneegurt MA (2013b) Dark-grown algae fed corn and sorghum hydrolysates for biodiesel production. Abstracts and Program, 113th General Meeting of the American Society for Microbiology, Denver, May 2013
Dandinpet KK, Schneegurt MA (2014) Dark-grown algae fed grain and lignocellulosic hydrolysates for biodiesel production. Abstracts and Program, 114th General Meeting of the American Society for Microbiology, Boston, May 2014
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy 88:3524–3531
De Swaaf ME, Sijtsma L, Pronk JT (2003) High-cell-density fed-batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. Biotechnol Bioeng 81:666–672
Demirbas A (2009) Progress and recent trends in biodiesel fuels. Energ Convers Manage 50:14–34
Deng X, Fei X, Li Y (2011) The effects of nutritional restriction on neutral lipid accumulation in Chlamydomonas and Chlorella. Afr J Microbiol Res 5:260–270
Dille JW, Rogers CM, Schneegurt MA (2016) Isolation and characterization of bacteria isolated from the feathers of wild Dark-eyed Juncos (Junco hyemalis). Auk 133:155–167
Dragone G, Fernandes BD, Vicente A, Teixeira JA (2010) Third generation biofuels from microalgae. In: Méndez-Vilas A (ed) Current research, technology and education topics in applied microbiology and microbial biotechnology. Formatex, Badajoz, pp 1355–1366
Endo H, Hosoya H, Koibuchi T (1977) Growth yields of Chlorella regularis in dark-heterotrophic continuous cultures using acetate. J Ferment Technol 55:369–379
Fan J, Cui Y, Wan M, Wang W, Li Y (2014) Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnol Biofuels 7:17
García Sánchez JL, Berenguel M, Rodríguez F, Fernández Sevilla JM, Brindley Alias C, Acién Fernández FG (2003) Minimization of carbon losses in pilot-scale outdoor photobioreactors by model-based predictive control. Biotechnol Bioeng 84:533–543
Gigova L, Ivanova NJ (2015) Microalgae respond differently to nitrogen availability during culturing. J Biosci 40:365–374
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274
Graverholt OS, Eriksen NT (2007) Heterotrophic high-cell-density fed-batch and continuous-flow cultures of Galdieria sulphuraria and production of phycocyanin. Appl Microbiol Biotechnol 77:69–75
Havlík P, Schneider UA, Schmid E, Böttcher H, Fritz S, Skalský R, Aoki K, De Cara S, Kindermann G, Kraxner F, Leduc S, McCallum I, Mosnier A, Sauer T, Obersteiner M (2011) Global land-use implications of first and second generation biofuel targets. Energ Policy 39:5690–5702
Heredia-Arroyo T, Wei W, Hu B (2010) Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Appl Biochem Biotechnol 162:1978–1995
Hill J, Nelson E, Tilman D, Polasky S, Tiffany D (2006) Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Natl Acad Sci U S A 103:11206–11210
Kay RA, Barton LL (1991) Microalgae as food and supplement. Crit Rev Food Sci Nutr 30:555–573
Kern JD, Hise AM, Characklis GW, Gerlach R, Viamajala S, Gardner RD (2017) Using life cycle assessment and techno-economic analysis in a real options framework to inform the design of algal biofuel production facilities. Bioresour Technol 225:418–428
Khozin-Goldberg I, Cohen Z (2006) The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 67:696–701
Lee RA, Lavoie JM (2013) From first- to third-generation biofuels: challenges of producing a commodity from a biomass of increasing complexity. Anim Front 3:6–11
Li X, Xu H, Wu Q (2007) Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol Bioeng 98:764–771
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic, and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Liang K, Zhang Q, Gu M, Cong W (2013) Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J Appl Phycol 25:311–318
Lincoln SF (2005) Fossil fuels in the 21st century. Ambio 34:621–627
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722
Louw TM, Griffiths MJ, Jones SMJ, Harrison STL (2016) Techno-economics of algal biodiesel. In: Bux F, Chisti Y (eds) Algae biotechnology. Springer, Cham, pp 111–141
Mandal S, Mallick N (2009) Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol 84:281–291
Miao X, Wu Q (2004) High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J Biotechnol 110:85–93
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97:841–846
Milne TA, Evans RJ, Nagle N (1990) Catalytic conversion of microalgae and vegetable oils to premium gasoline, with shape-selective zeolites. Biomass 21:219–232
Moore A (2008) Biofuels are dead: long live biofuels(?) – part one. New Biotechnol 25:6–12
Mühlroth A, Winge P, El Assimi A, Jouhet J, Maréchal E, Hohmann-Marriott MF, Vadstein O, Bones AM (2017) Mechanisms of phosphorus acquisition and lipid class remodeling under P limitation in a marine microalga. Plant Physiol 175:1543–1559
Mutlu YB, Işik O, Uslu L, Koç K, Durmaz Y (2011) The effects of nitrogen and phosphorus deficiencies and nitrite addition on the lipid content of Chlorella vulgaris (Chlorophyceae). Afr J Biotechnol 10:453–456
Nagarajan D, Lee DJ, Chang JS (2018) Heterotrophic microalgal cultivation. In: Liao Q, Chang JS, Herrmann C, Xia A (eds) Bioreactors for microbial biomass and energy conversion. Springer, Singapore, pp 117–162
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev 14:578–597
Neilson AH, Lewin RA (1974) The uptake and utilization of organic carbon by algae: an essay in comparative biochemistry. Phycologia 13:227–264
O’Grady J, Morgan JA (2011) Heterotrophic growth and lipid production of Chlorella protothecoides on glycerol. Bioprocess Biosyst Eng 34:121–125
Pagnanelli F, Altimari P, Trabucco F, Toro L (2014) Mixotrophic growth of Chlorella vulgaris and Nannochloropsis oculata: interaction between glucose and nitrate. J Chem Technol Biotechnol 89:652–661
Pal D, Khozin-Goldberg I, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90:1429–1441
Parker BC, Bold HC, Deason TR (1961) Facultative heterotrophy in some Chlorococcacean algae. Science 133:761–763
Perez-Garcia O, Escalante FME, de-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36
Piorreck M, Baasch KH, Pohl P (1984) Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry 23:207–216
Přibyl P, Cepák V, Zachleder V (2012) Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl Microbiol Biotechnol 94:549–561
Procházková G, Brányiková I, Zachleder V, Brányik T (2014) Effect of nutrient supply status on biomass composition of eukaryotic green microalgae. J Appl Phycol 26:1359–1377
Reitan KI, Rainuzzo JR, Olsen Y (1994) Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J Phycol 30:972–979
Rhee GY (1978) Effects of N:P atomic ratios and nitrate limitation on algal growth, cell composition, and nitrate uptake. Limnol Oceanogr 23:10–25
Richardson B, Orcutt DM, Schwertner HA, Martinez CL, Wickline HE (1969) Effects of nitrogen limitation on the growth and composition of unicellular algae in continuous culture. Appl Microbiol 18:245–250
Richardson JW, Johnson MD, Outlaw JL (2012) Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Res 1:93–100
Roach BMB (1926) On the relation of certain soil algae to some soluble carbon compounds. Ann Bot 40:149–201
Roach BMB (1927) On the carbon nutrition of some algae isolated from soil. Ann Bot 41:509–517
Rodhe W (1953) Can plankton production continue during winter darkness in subarctic lakes? Verh Intern Ver Limnol 12:117–122
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ (2008) A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19:430–436
Ruangsomboon S, Ganmanee M, Choochote S (2013) Effects of different nitrogen, phosphorus, and iron concentrations and salinity on lipid production in newly isolated strain of the tropical green microalga, Scenedesmus dimorphus KMITL. J Appl Phycol 25:867–874
Schneegurt MA, Sherman DM, Sherman LA (1997) Growth, physiology, and ultrastructure of a diazotrophic cyanobacterium, Cyanothece sp. strain ATCC 51142, in mixotrophic and chemoheterotrophic cultures. J Phycol 33:632–642
Scott TA Jr, Melvin EH (1953) Determination of dextran with anthrone. Anal Chem 25:1656–1661
Seifter S, Dayton S, Novic B, Muntwyler E (1950) The estimation of glycogen with the anthrone reagent. Arch Biochem 25:191–200
Seilheimer JA, Jackson DF (1963) A comparison of algal growth in cultures exposed and unexposed to solar radiation. Trans Am Microsc Soc 82:78–83
Shifrin NS, Chisholm SW (1981) Phytoplankton lipids: interspecific differences and effects of nitrate, silicate and light-dark cycles. J Phycol 17:374–384
Shrestha N, Schneegurt MA (2016) Phosphorus limitation enhances biodiesel production in dark-grown algae fed grain or lignocellulosic hydrolysates. Abstracts and Program, 116th Annual Meeting of the American Society for Microbiology, Boston, June 2016
Singh P, Kumari S, Guldhe A, Misra R, Rawat I, Bux F (2016) Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew Sust Energ Rev 55:1–16
Skinner CE, Gardner CG (1930) The utilization of nitrogenous organic compounds and sodium salts of organic acids by certain soil algae in darkness and in the light. J Bacteriol 19:161–179
Smith VH, Sturm BSM, deNoyelles FJ, Billings SA (2010) The ecology of algal biodiesel production. Trends Ecol Evol 25:301–309
Sturm BSM, Peltier E, Smith V, deNoyelles F (2011) Controls of microalgal biomass and lipid production in municipal wastewater-fed bioreactors. Env Progr Sustain Energ 31:10–16
Su G, Jiao K, Li Z, Guo X, Chang J, Ndikubwimana T, Sun Y, Zeng X, Lu Y, Lin L (2016) Phosphate limitation promotes unsaturated fatty acids and arachidonic acid biosynthesis by microalgae Porphyridium purpureum. Bioprocess Biosyst Eng 39:1129–1136
Verma NM, Mehrotra S, Shukla A, Mishra BN (2010) Prospective of biodiesel production utilizing microalgae as the cell factories: a comprehensive discussion. Afr J Biotechnol 9:1402–1411
Wagley PK, Schneegurt MA (2012a) Microbial community analysis of open ponds for algal biodiesel production. In: Proceedings: 8th annual symposium: graduate research and scholarly projects. Wichita, KS: Wichita State University, pp 145–146
Wagley PK, Schneegurt MA (2012b) Molecular analysis of microbial community structure in open ponds for algal biodiesel production. Trans KS Acad Sci 115:78
Wan M, Liu P, Xia J, Rosenberg JN, Oyler GA, Betenbaugh MJ, Nie Z, Qiu G (2011) The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl Microbiol Biotechnol 91:835–844
Wu QY, Yin S, Sheng GY, Fu JM (1994) New discoveries in study on hydrocarbons from thermal degradation of heterotrophically yellowing algae. Sci China Ser B 37:326–335
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Xu L, Weathers PJ, Xiong XR, Liu CZ (2009) Microalgal bioreactors: challenges and opportunities. Eng Life Sci 3:178–189
Acknowledgments
The authors appreciate the technical support of James Crisler, Timothy Eberl, Casper Fredsgaard, Devon Miller, and Robert Tolley. We thank Belinda Sturm (University of Kansas) for performing lipid analyses. Biomass hydrolysates were kindly provided by Donghai Wang and Ke Zhang (Kansas State University) and Accelerase enzyme was a gift from Stephen Crawford, Deborah Dodge, and Chris Nguyen (DuPont). Special thanks to Gregory Houseman and Leland Russell for collecting Big Bluestem biomass. Preliminary accounts of this work have been presented previously and abstracted (Dandinpet and Schneegurt 2013a, b, 2014; Shrestha and Schneegurt 2016). This is publication no. 33 in the series of reports from the Wichita State University Biological Field Station.
Funding
This work was supported by an award from Kansas National Science Foundation (NSF) Established Program to Stimulate Competitive Research (EPSCoR) (EPS-0903806). Additional student support was from Kansas Institutional Development Award (IDeA) Networks of Biomedical Research Excellence (KINBRE) of the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) (P20 GM103418).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shrestha, N., Dandinpet, K.K. & Schneegurt, M.A. Effects of nitrogen and phosphorus limitation on lipid accumulation by Chlorella kessleri str. UTEX 263 grown in darkness. J Appl Phycol 32, 2795–2805 (2020). https://doi.org/10.1007/s10811-020-02144-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02144-x