Abstract
Algal biodiesel production presents a possible carbon-neutral source of transportation fuel. Whilst algal biodiesel circumvents some of the issues arising from the use of crop- and waste–biomass-based fuels, the lack of commercial success raises questions regarding the feasibility of the process. Numerous economic and environmental impact assessments have produced highly variable results, predicting costs from as little as 0.42–72 USD L−1. A meta-analysis of these assessments reveals that areal productivity and provision of nutrients, as well as energy and water usage, are the key challenges to algal biodiesel production. A consideration of maximum achievable photosynthetic activity indicates that some scope exists for increasing areal productivity; hence, the factors influencing productivity are discussed in detail. Carbon dioxide supply may represent the single most important challenge to algal biodiesel, while recycling of other nutrients (specifically nitrogen and phosphate) is essential. Finally, a careful balance must be struck between energy and water consumption; this balance is primarily influenced by bioreactor design. It is unlikely that algal biodiesel will supply a substantial portion of the world’s transportation energy demand, but it may fill niche markets such as aviation fuel. Process economics are enhanced by integrating biodiesel production into a biorefinery, producing a suite of products.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The need for renewable energy has prompted the investigation of biologically derived renewable fuels. First-generation biofuels rely on food crops and are in direct competition with food production for arable land. The use of corn ethanol has already impacted food prices (Zilberman et al. 2012) and the first-generation biofuels pose a serious threat to food security (Escobar et al. 2009). Second-generation biofuels avoid these issues by utilizing nonedible (primarily lignocellulosic) biomass as feedstocks. Second-generation biofuels are limited by unreliable biomass supply rates and low conversion efficiency (Sims et al. 2010). Algal-based “third generation” biofuels seem to overcome these challenges and potentially fulfill all sustainability requirements (Brownbridge et al. 2014). Algae are extremely versatile, with the ability to adapt to a range of environments and to produce different forms of biofuels, including bioethanol, biohydrogen, and biodiesel (Jones and Mayfield 2012). Rapid growth rates and high lipid yields make algal biomass ideally suited to biodiesel production (Chisti 2007).
The apparent promise of algal biodiesel is in stark contrast to the lack of commercial success. To date, no facility is producing biodiesel derived from algae on an industrial scale. The scientific community is divided as to whether any further resources should be spent on the concept (see, for instance, the articles “The nonsense…” and “The rationality…” [of biofuels] (Michel 2012; Horta Nogueira et al. 2013). Large-scale algal biofuel production faces a number of technical and economic challenges (Brownbridge et al. 2014), including high energy demands, operating expenses, and capital investment (Richardson et al. 2012; Rawat et al. 2013).
Economic assessments of algal biodiesel vary across a large range of estimates, hindering commercial interest (Liu et al. 2012). Projected costs for algal biodiesel range from 0.42 (Nagarajan et al. 2013) to 72 USD L−1 (Harun et al. 2011). Costs could be decreased by producing biodiesel as one of the commodities in a suite of different bioproducts in a biorefinery approach (Wijffels and Barbosa 2010), including (i) high-value coproducts such as pigments (Ferreira et al. 2013); (ii) bioremediated wastewater (Park et al. 2011; Pittman et al. 2011); (iii) ethanol or methane produced by the fermentation or digestion of spent biomass, respectively (Jones and Mayfield 2012); and (iv) carbon credits. Algal biodiesel has the potential to be a carbon-neutral fuel, but current analyses indicate greenhouse gas (GHG) emissions comparable to crop-based biofuels (Liu et al. 2012). A recent innovative study speculated on the future role of algal biodiesel with respect to carbon policies (Takeshita 2011). Strict regulations would decrease the use of fossil fuels and, subsequently, decrease the availability of anthropogenic carbon dioxide point sources for enhanced algal cultivation, potentially rendering algal biodiesel unfeasible. On the other hand, if carbon restrictions are held at moderate levels, algal biodiesel is predicted to be essential to the future energy mix.
One of the major technical arguments against algal biodiesel is the low efficiency of photosynthesis. The maximum theoretical efficiency for the photosynthetic conversion of water and carbon dioxide to glucose and oxygen, using the sun’s light spectrum, is approximately 11 % (Walker 2009). The Shockley–Queisser limit for single-junction photovoltaic (PV) cells on the other hand is close to 30 % (Shockley and Queisser 1961); this limit can be raised using multi-junction cells. The key difference between PV cells (and other major sources of renewable energy such as wind- and hydropower) and microalgae is energy storage: whereas PV cells generate electricity that must be connected to a grid or secondary storage device, photoautotrophs store energy in chemical bonds. Energy storage is especially important when considering transportation energy, which currently accounts for approximately 20 % of global energy consumption (U.S. Energy Information Administration 2014). The gravimetric energy density of liquid fuels (~46 MJ kg−1 for biodiesel) is an order of magnitude greater than hydrogen (~6 MJ kg−1 at 70 MPa, including the required container) and more than a hundred times greater than lithium ion batteries (~0.6 MJ kg−1) (Zhang 2011). Current electrical vehicles would need a 670 L battery to have a range similar to that of a diesel car with a 46 L tank (Eberle and von Helmolt 2010). Liquid biofuels are essential to applications which require high energy densities; aviation fuels in particular present a potential niche market for algal biofuels (Chisti 2013a).
Hydrogen represents the current energy storage alternative. A recent study compared the annual production of hydrogen by the electrolysis of water, driven by PV cells, to the annual biomass production by photosynthesis. It was found that the PV-electrolysis system had an energy efficiency of 11 %, whereas the annual biomass energy efficiency was 3 % (excluding downstream processing) (Blankenship et al. 2011). Current PV systems exceed the theoretical upper limit of algal biodiesel production efficiency and the PV-photosynthesis efficiency gap is set to grow in the future. While many see this as convincing evidence against the feasibility of algal biofuels, others argue for the use of genetic modifications, synthetic biology, and cell-free technologies (Blankenship et al. 2011; Zhang 2011). These approaches require significant further research.
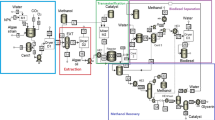
In spite of the apparent inefficiencies, biofuels have an important role in the future energy economy, in part due to their immediate application in internal combustion engines. Unlike hydrogen, the global biodiesel market and infrastructure is well established (Sims et al. 2010). The International Energy Agency Technology Roadmap (International Energy Agency 2011) predicts that biofuels will constitute approximately 27 % of transportation fuels by 2050 and specifically emphasizes the need for the commercial deployment of advanced biofuel production facilities, including algal biodiesel. The success of algal biofuel depends on a holistic methodology, illustrated in Fig. 1. Biodiesel is a single product in an integrated biorefinery, and it will fill a niche, medium-term role in the future energy mix. This chapter investigates the major challenges and opportunities in the techno-economics of algal biodiesel.
Qualitative pie chart illustrating the integrated approach to biodiesel. Algal biodiesel is a single product from an integrated biorefinery. Both biodiesel and spent biomass represent future renewable energy sources, but biodiesel is one of the only future energy sources appropriate for transportation
2 Economic Assessment
Numerous studies have attempted to develop an economic model for algal biofuel production [recent reviews include Brownbridge et al. (2014), Nagarajan et al. (2013) and Williams and Laurens (2010)]. A list of recent economic analyses, with key assumptions, is presented in Table 1. Estimated costs of algal biodiesel production range from 0.42 to 7.50 USD L−1 in open ponds and 1.25 to 72 USD L−1 in closed PBRs. Low-cost estimates follow from optimistic assumptions, e.g., Nagarajan et al. (2013) estimated a final cost of algal biodiesel in the range of 0.42–0.97 USD L−1, assuming growth rates between 30 and 60 g m−2 day−1 and a lipid content of 50 %, neither of which have been achieved independently or in combination in long-term outdoor cultivation. The wide range of cost estimates is due to the limited availability of industrial information regarding this immature technology (Brownbridge et al. 2014), and presents a major challenge to economic analyses.
Brownbridge et al. (2014) conducted an in-depth sensitivity analysis and found that the majority of the process cost is in the production of algal biomass, harvesting, and the price of inputs (e.g., fertilizer), while the total cost was most sensitive to variations in algal lipid content, annual areal productivity, plant production capacity, and the price of carbon. Davis et al. (2012) attempted to combine data from the resource assessment, techno-economic, and life-cycle analysis models of major US national laboratories into a harmonized baseline model representing near-term production of algal biodiesel. They found that models are most sensitive to estimates of algal productivity, lipid content and composition, and downstream processing.
The cost of CO2 supply is a major challenge to economic feasibility (Williams and Laurens 2010). It is often assumed that the algal plant will be situated close to a point source of waste CO2 and therefore carbon supply will be free. In reality, there are significant costs associated with transport and distribution of gas, technical problems related to the effect of flue gas contaminants on algal productivity, and sustainability concerns regarding the availability of CO2 point sources in the future (Chisti 2013a).
Water, energy, and nutrient usage and recycle affect cost and environmental impact. Brownbridge et al. (2014) based their economic analysis on the assumption that the oil-extracted algal biomass would be gasified to produce further crude oil and electricity, while many others have included anaerobic digestion (AD) as a method to recycle nutrients (Harun et al. 2011; Sun et al. 2011; Davis et al. 2012; Nagarajan et al. 2013), and some consider both options (Delrue et al. 2012). Although there is extensive published knowledge and experience on the application of AD to human and animal waste, there is relatively little information on its application to algal biomass (Sialve et al. 2009; Ward et al. 2014).
Until recently, reports consistently pointed to harvesting as a major area of uncertainty that has large economic implications (Williams and Laurens 2010), but recent studies indicate progress in this area. Davis et al. (2012) assumed a 95 % harvesting, 85 % extraction, and 78–85 % conversion efficiencies, resulting in an overall efficiency of ~65 % conversion of algal lipids to fuel. There is potential for substantial improvements in economics if the conversion efficiency can be improved. Harvesting methods such as flocculation followed by centrifugation (Nagarajan et al. 2013), settling followed by dissolved air flotation and centrifugation (Davis et al. 2012), belt filter press (Delrue et al. 2012), and OriginOil’s ‘Electro Water Separation’ technology (Brownbridge et al. 2014) have been proposed as viable options.
New technologies with the potential to revolutionize biomass production have been explored, but are yet to be demonstrated at scale. For example, Norsker et al. (2011) estimate the production cost to be between 5.5 and 7.9 USD kg−1 biomass dry weight, but with improvements in solar radiation through site selection, a reduction in the flow velocity of mixing, free sources of nutrients, and CO2, and an increase in photosynthetic efficiency of 60 %, the price of biomass production could be reduced to around 1.7 USD kg−1 dry weight for open ponds and 0.9 USD kg−1 dry weight in closed reactors—a price at which production of bulk commodity products (e.g., chemicals, fuels, materials, feed) becomes feasible. The likelihood of these improvements all occurring on a sufficiently large scale is uncertain.
Brownbridge et al. (2014) summarized the overall sentiment of the literature with the conclusion that “with current technologies, prices, and forecasts it is unlikely that a plant that has algal biodiesel as its primary product can be commercially feasible.” Accurately assessing the economic feasibility of algal biodiesel in a biorefinery setting requires a fundamental understanding of the limiting factors, which literature indicates to be areal productivity, carbon dioxide and nutrient supplies, and energy requirements in cultivation as well as downstream processing.
3 Environmental Impact Assessment
One of the main driving forces behind research and development of algal biodiesel is its potential as an environmentally sustainable transport fuel (Slade and Bauen 2013). Rigorous analysis of the production process is required to determine the environmental impacts of algal biodiesel before it can be deemed sustainable. A popular method for assessing the environmental impact is life-cycle analysis (LCA). Many LCAs have been performed for algal biodiesel production under a variety of conditions and assumptions. These studies show that global warming potential, carbon footprint, greenhouse gas emissions, nutrient use, water consumption, and fossil energy requirements are the primary environmental concerns. Table 2 summarizes LCA results from a selection of reports on algal biodiesel.
Algae capture CO2 during photosynthesis, which is released back into the atmosphere on combustion of algal biodiesel. Thus, the production of algal biodiesel can result in a carbon-neutral CO2 cycling system at best, having no net effect on atmospheric CO2. Biomass cultivation (mixing, pumping, and aeration), harvesting, and drying constitute the major fossil energy requirements for algal biodiesel production (Slade and Bauen 2013) and contribute to GHG emissions. Energy requirements may be offset by anaerobic digestion and combustion of spent algal biomass after lipid extraction. If fossil fuels are utilized in the production of algal biodiesel, more CO2 will be emitted than consumed during algal cultivation. Similarly, if CO2 from industrial flue gas is fed to algal cultures, this CO2 will be released on combustion of the biodiesel and so will not result in a net reduction of fossil carbon and GHG emissions (Stephenson et al. 2010; Chisti 2013a; Azadi et al. 2014), although the associated carbon cycling may facilitate enhanced energy provision per unit CO2 emissions formed. Understanding the relationship between CO2 generation, supply, and consumption in the algal biofuel process is critical.
In addition to methane from anaerobic digestion, other coproducts include syngas, ethanol, glycerol, and nutrient-rich feed. The reduction in GHG emissions and other environmental burdens associated with producing these coproducts from algae as opposed to fossil sources has a significantly positive outcome on the overall LCA (Batan and Quinn 2010; Sander and Murthy 2010; Stephenson et al. 2010). AD, with associated recycle of nitrogen and phosphorus in the digestate, could aid in the reduction of eutrophication which occurs when nitrogen or phosphate is released into the environment, changing the natural concentration of nutrients, and particularly dissolved oxygen, in surrounding rivers and lakes and threatening ecosystems. Fresh water is a very scarce commodity, and overuse has serious environmental and social sustainability issues.
4 Key Challenges to Feasibility of Algal Biodiesel
The economic and environmental impact of algal biodiesel is influenced by the use of technology, biology, and process design to address a set of key technical challenges. These challenges include (i) areal productivity, limited by light utilization; (ii) nutrient supply, including carbon, nitrogen, and phosphate; and (iii) water and energy consumption.
4.1 Areal Productivity
Algal productivity is influenced by the characteristics of the strain as well as environmental parameters such as nutrient availability, temperature, pH, and salinity, but is fundamentally limited by light availability. Available sunlight provides the ultimate constraint to biofuel production and, as such, arguments against the feasibility of biofuels usually refer to “maximum theoretical productivity” based on light utilization. This maximum productivity is dependent on the insolation, photosynthetic efficiency, and bioreactor design; an understanding of these factors will illuminate the discussion.
4.1.1 Insolation
The amount of solar energy (SSE) reaching the earth’s surface is the total insolation (measured in W m−2). SSE is abundant (120 × 103 TW) but diffuse, with annual average intensities of 200–280 W m−2 in regions of interest. Insolation is significantly less than the extraterrestrial irradiance due to atmospheric light scattering and absorption. Insolation data are used to evaluate the potential productivity of a site. Satellite-derived data for insolation calculations are freely available from the NASA Langley Research Center Atmospheric Science Data Center Surface Meteorological and SSE web portal supported by the NASA LaRC POWER Project (https://eosweb.larc.nasa.gov/sse/) (NASA 2013). Once a site has been selected, the only method of enhancing solar insolation is by adjusting the tilt of the insolated plane. This is impossible for open raceway ponds, but is an important consideration for closed (specifically flat panel) PBRs. See RETScreen International (2005) and Duffie and Beckman (2013) for an introduction to interpreting and applying insolation data in the assessment of SSE sources.
4.1.2 Photosynthesis
Only a portion of the solar irradiance spectrum is utilized during photosynthesis. A large amount of energy is absorbed by atmospheric gases, most importantly, oxygen and ozone, resulting in distinct absorption bands in the terrestrial insolation spectrum. Insolation spectrum data are freely available from ASTM (2003).
Green algae host two separate photoreaction centers activated by 680 and 700 nm wavelength photons, respectively (Zhu et al. 2008). Light-harvesting complexes allow photosystems to utilize energy from photons with shorter wavelengths by resonance energy transfer (RET), but photons with longer wavelengths have insufficient energy to cause excitation in the photoreaction center. Due to this limitation, photosynthetically active radiation (PAR) is most often defined as the insolation in the 400–700 nm bandwidth, with some sources adjusting the limits to 380–740 nm. The energy contained in the 400–700 nm range is approximately 42 % of the total insolation energy (using data provided by ASTM (2003)). Bacteria often contain photosystems with the ability to absorb near-infrared light with wavelengths up to 1000 nm, thereby increasing the useable light spectrum (Blankenship et al. 2011).
Light-harvesting complexes can absorb photons with shorter wavelengths and higher energy content than the 680/700 nm photons required by the reaction centers, but the excess energy is dissipated as heat during RET. This process represents a significant energy loss: given the terrestrial insolation spectrum (ASTM 2003), RET amounts to a decrease in energy of approximately 20 %.
The photosynthetic machinery of green algae and higher plants requires at least eight photons to fix a single carbon dioxide molecule (Zhu et al. 2008), or 48 photons to form glucose (that is, 24 at 680 nm and 24 at 700 nm), in a process commonly known as the Z-scheme. The 48 mole of photons contain 8323.40 kJ of energy, while the heat of combustion of glucose is 2805 kJ mole−1, resulting in a 66 % energy reduction when operating in optimal light utilization conditions. This energy reduction is intrinsic to the photosynthesis Z-scheme.
4.1.3 Absorption and Photoinhibition
Not all of the incident lights over a given area of algal culture reach the photosynthetic machinery of an algal cell. A proportion is lost due to light scattering and reflection on the reactor surface, as well as absorption by non-photosynthetic material. The absorption coefficient is dependent on the wavelength (Hoepffner and Sathyendranath 1993). The total PAR is often weighted by \( a\left( \lambda \right) \) to yield the photosynthetically useable radiation (PUR) (Morel 1978) or the yield photon flux (YPF) (Barnes et al. 1993), which provides a more realistic approximation to the photosynthetic action spectrum. The relationship between PUR and PAR is dependent on many factors including the water column depth, biomass concentration, and the state of photoacclimation. Spectrally resolved models have been developed to account for these effects (Behrenfeld and Falkowski 1997; Kyewalyanga et al. 1997). All these aspects can (and should) be controlled by PBR design, but it is impossible to assign a fixed value to the ratio PUR:PAR. Zhu et al. (2008) used a value (without explanation) of 10 % for “inefficient absorption,” the same value is adopted here, but significant variation is likely.
It is well known that the rate of photosynthesis is linearly proportional to the absorbed photon flux in the light-limited regime, but a maximum fixation rate is achieved once the irradiation crosses a certain threshold (light saturated regime). A further increase in light intensity can cause photoinhibition. The relationship between photon flux density and photosynthetic rate is commonly referred to as the photosynthesis-irradiance (PE) response curve. The PE curves have been studied intensively by MacIntyre and Kana (2002).
Algae can modify their PE characteristics to avoid photoinhibition in a process known as photoacclimation, primarily by modifying pigment content. Photosynthesis utilizes light most efficiently in the light-limited regime, and thus it is this region that is of interest to PBR design. In the light-limited region, the photosynthetic rate is approximately linearly proportional to the photon flux, with proportionality constant \( \alpha = a\phi_{m} \), where \( a \) is the specific absorption coefficient and \( \phi_{m} \) is the quantum yield (MacIntyre and Kana 2002). Both the quantum yield and the absorption coefficient are nearly constant in the light-limited region, although the quantum yield can decrease at higher levels of photon flux due to non-photosynthetic absorption. The quantum yield is limited to a maximum of 0.125 mol of oxygen produced per mole of photons absorbed due to the photosynthesis Z-scheme, as discussed above.
4.1.4 Photorespiration and Respiration
Algae consume energy and produce carbon dioxide by photorespiration as well as mitochondrial respiration. Photorespiration occurs when oxygenation instead of carboxylation is catalyzed by the Rubisco enzyme. At atmospheric conditions (380 ppm CO2 and 21 % O2), photorespiration can result in an approximately 49 % decrease in energy efficiency (Zhu et al. 2008). However, Rubisco is highly selective toward carbon dioxide and photorespiration can effectively be eliminated by maintaining a high CO2 concentration around the enzyme (Sousa 2013).
Respiration is necessary to perform a host of biological activities. Some 10 % of the oxygen generated by photosynthesis is used in respiration; higher reported values can often be attributed to the presence of respiring bacteria (Talling 1957; Li et al. 2003). As with photorespiration, respiration can be minimized by decreasing oxygen concentration.
4.1.5 Theoretical Areal Productivity
The primary factors influencing the efficiency of light utilization are illustrated in Fig. 2. Approximately, 89 % of the energy lost can be attributed to the inherent photosynthetic machinery. Genetic modification is required to address these issues, and a number of suggestions are made in the literature. Notably, Chen and Blankenship (2011) suggest expanding the PAR range through the incorporation of red-shifted chlorophylls, which will result in the capture of a major portion of the solar irradiance spectrum. However, absorbed photons with wavelengths longer than 700 nm cannot be utilized by algal reaction centers. To overcome this problem, some authors suggest coculture with bacterial species able to utilize photons with wavelengths up to 900 nm (Pilon et al. 2011). Blankenship et al. (2011) suggested a complete re-engineering of photosystem I to absorb photons close to 1100 nm.
Factors influencing energy loss in photosynthesis. Left The total percentage of energy lost due to individual processes. Energy losses due to the photosynthetic machinery are fixed and can only be addressed through substantial genetic modification (GM). The energy losses due to photorespiration, respiration, and inefficient/non-photosynthetic absorption are dependent on environmental factors; estimates of minimum and maximum losses are shown. These effects can be addressed through PBR design. Right Cumulative effect of energy losses on total energy efficiency, showing an estimated final efficiency between 3 and 11 %
Other factors can be minimized through PBR design. Eliminating photorespiration could double photosynthetic efficiency: PBR oxygen concentrations typically exceed saturation levels (Molina et al. 2001), and strategies for degassing have been explored (Sousa 2013). Respiration is an essential cellular process and cannot be removed completely, but ratios of respiration to photosynthesis of as low as 5 % have been observed in nature (Talling 1957). Similarly, inefficient- and non-photosynthetic absorption could potentially be minimized if PBRs can be designed to operate purely in the light-limited regime. Proper PBR design can increase the efficiency of light utilization from 3 to 11 %, but this is still significantly lower than current PV cell efficiencies.
4.1.6 Biomass and Lipid Productivity
The photosynthetic efficiencies discussed above pertain to the conversion of SSE into chemical energy contained in glucose, which does not translate directly into areal biomass productivity. The biomass productivity (g m−2 day−1) can be calculated through an elemental carbon balance: Carbonaceous Biomass Accumulation = Carbon Fixation − Respiration = 0.4 × Net Glucose Production (the mass fraction of carbon in glucose is 0.4). The net glucose production is determined using the photosynthetic efficiencies above, and the areal biomass productivity is calculated using the following equation:
where \( {\text{d}}X^{\prime}/{\text{d}}t \) is the rate of biomass accumulation per unit area (g m−2 s−1), \( f_{C} \approx 0.5 \) is the mass fraction of carbon in the biomass (Mirón et al. 2003), \( \Delta H_{c} \) is the heat of combustion of glucose (15.57 kJ g−1), \( \eta_{P} \) is the photosynthetic efficiency, and I is the insolation (W m−2). The \( {{0.4} \mathord{\left/ {\vphantom {{0.4} {f_{C} }}} \right. \kern-0pt} {f_{C} }} \) term varies between 65 and 90 %, depending on the biomass composition. An annual average insolation of 250 W m−2 and photosynthetic efficiency of 3 or 11 % will result in a biomass productivity of 16 or 61 g m−2 day−1, respectively.
Comparison in Table 1 shows that estimates of actual productivities are in general much lower than the theoretical limit. This indicates that there is substantial room for improvement in PBR design to minimize the productivity gap. The decreased productivity can be attributed partly to seasonal variation. Algal productivities were estimated to range from 6.2 (winter) to 16.5 g m−2 day−1 (spring) in the region of the US most suitable for algal production (Davis et al. 2012). Seasonal variations are often neglected in economic models, but affect process cost greatly. Facilities large enough to accommodate peak season productivities are oversized for the rest of the year. It is essential that data on algal productivities in large-scale outdoor facilities over seasonal variations be generated, both from experimental observations and using the techniques described above, and made available to allow R&D to consider seasonal costs, energy consumption, and productivities rather than annual averaged values (Davis et al. 2012). Site and strain selection is critical in minimizing seasonal fluctuations.
The feasibility of algal biodiesel is heavily dependent on biomass productivity as well as lipid content and composition. In the Davis et al. (2012) economic model, an estimated algal productivity of 25 g m−2 day−1 and a lipid content of 25 % was used. However, these assumptions resulted in higher estimates of lipid productivity than many developers felt justified at the time. Reducing productivity to a more realistically achievable season-dependent annual average of 13.2 g m−2 day−1 resulted in a selling price of 19.6 USD gal−1 (5.2 USD L−1). Williams and Laurens (2010) suggest that 35 % lipid appears to be a maximum realistic target. They also point out that an increase in lipid content results in a decreased fraction of other cell components (e.g., protein, nucleic acid, and carbohydrate), which in turn leads to reduced growth rates. Correlating growth and lipid content data from a variety of studies (e.g., see the analysis of Griffiths and Harrison 2009), it was estimated that an increase in lipid content from 15 to 30 % would lead to a reduction in growth rate of 50 % or more. In addition, non-triacylglycerides unsuited to biodiesel production can account for a sizeable portion of the lipid fraction (Davis et al. 2012). These factors are often unaccounted for in models that predict a concurrently high lipid content and growth rate. Genetic modification of strains to improve productivity, particularly with concomitant high triacylglycerol content, and aid recovery of lipids, could have major impacts on algal biodiesel feasibility (Chisti 2013a). Robustness of such modifications, particularly for systems not operated as monoseptic, also needs consideration.
Seasonal variations and lipid conversion rates notwithstanding the theoretical limit to productivity are nearly 200 % greater than that which is currently achieved. Insolation and the fundamental limits to photosynthetic efficiency do not render algal biodiesel infeasible, as is commonly believed, and there is great scope for improvement in areal productivity.
4.2 Nutrient Supply: Carbon, Nitrogen, and Phosphate
Generating algal biomass requires, at minimum, the stoichiometric equivalent of its average elemental composition: CH1.7O0.4N0.15P0.0094 (Oswald 1988). CO2 is often the major or only source of carbon in algal cultivation (Chisti 2007). Algae utilize CO2 from the air, but supplementary CO2 in cultivation systems is essential for commercially viable growth and lipid productivities (Brennan and Owende 2010; Pate et al. 2011). Purchasing supplementary CO2 can increase operating costs by 3–10 % (Takeshita 2011; Chisti 2013a; Nagarajan et al. 2013; Richardson et al. 2014). The cost and availability of CO2 is affected by policies aiming to stabilize atmospheric CO2 and the environmental impacts of CO2 release. Sequestration of captured CO2 will be an essential means of meeting climate stabilization constraints in the next 50–100 years. Production of biodiesel, which is eventually be combusted, at best results in carbon-neutral cycling.
Generating 1 t of algal biomass requires approximately 1830 kg of CO2, 50–80 kg of nitrogen, and 5 kg of phosphate (Chisti 2007; Pate et al. 2011; Borowitzka and Moheimani 2013). Therefore, nitrogen and phosphate are two additional nutrients of particular concern due to the volumes required, cost, and long-term sustainability (Chisti 2013a). Species selection can affect nutrient requirements (Harrison et al. 2013). Selection of algal species with a low nitrogen content, or species that can maintain biomass productivity with an enhanced lipid content under nitrogen limiting conditions, could assist in minimizing the nitrogen input required. The application of nitrogen limiting conditions, known to enhance lipid content of many algal species (Griffiths and Harrison 2009; Griffiths et al. 2012), can reduce the nitrogen use of the overall process. Lardon et al. (2009) estimated that the energy required for provision of fertilizer can be reduced, under nitrogen-limited conditions, to 6–9 % of total process energy (compared to 15–25 % under nitrogen sufficient conditions). Despite potential reductions in nutrient requirements, feed and fertilizer requirements are central to assessing algal biodiesel feasibility.
4.2.1 Availability
Atmospheric CO2 exists in abundance. Algae are more efficient at fixing CO2 than terrestrial plants, which results in higher lipid yields (Chisti2013a; Borowitzka and Moheimani 2013). However, atmospheric CO2 levels remain insufficient for growing large concentrations of algae. Genetic engineering could be used to improve algal carbon concentrating mechanisms (Savile and Lalonde 2011), but gas–liquid CO2 mass transfer remains limiting. Reports indicate that heterotrophic cultivation can result in very high biomass and lipid production (Rosenberg et al. 2008; Chisti 2013a); however, the organic carbon is ultimately derived from plants and so reintroduces many of the limitations to terrestrial plant-derived biodiesel (including land use, water, and productivity), unless it can be sourced from waste resources.
Industrial waste, such as flue gas, represents an inexpensive source of concentrated CO2. Flue gas contains up to 20 % CO2 from conventional sources, far exceeding atmospheric concentrations of 0.036 % (Brennan and Owende 2010). This decreases cost but restricts the location of the algae facility to the vicinity of CO2 point sources. Reports indicate that the amount of CO2 required to produce meaningful amounts of algal biodiesel far outweighs the CO2 availability at point sources (Pate et al. 2011; Chisti 2013a). According to an analysis by Pate et al. (2011), 140 million tons of CO2 are required to produce 40 billion L year−1 of algal biodiesel (only 2–3 % of liquid fuel consumption in the USA), but only 193 million tons of CO2 emissions are available at stationary sources in the region best suited to algal cultivation in the USA. As production of energy from renewable sources is set to increase, CO2 point sources such as coal burning power stations will decrease, further limiting CO2 availability (Chisti 2013a). On the other hand, CO2 will become available from new sources such as anaerobic digesters and bioethanol production plants (Takeshita 2011). These may also represent cleaner and more concentrated CO2 sources.
Atmospheric nitrogen is almost unlimited, but current production methods of fixed nitrogen are energy intensive (Chisti 2013a). The provision of nutrients, particularly nitrogen, to bioprocesses not only represents a major cost of the process, but also greatly increases the carbon footprint and fossil fuel requirement due to the manufacture of ammonia, urea, or nitrate fertilizers (Harding 2009). The provision of fertilizer has been estimated to account for 15–25 % of the total energy requirement per unit algal biodiesel (Lardon et al. 2009). Clarens and Resurreccion (2010) attribute up to 50 % of the energy requirement for biomass production in open ponds to nutrient provision. In addition, at current production levels, any significant use of fertilizer for production of fuel would reduce the availability of fertilizer for food agriculture.
In contrast to CO2 and nitrogen, the global supply of phosphate is scarce and finite. Due to its nongaseous environmental cycle, there is no means of production other than mining of phosphate rock, a nonrenewable resource. It is estimated that terrestrial global reserves may be depleted in 50–100 years (Cordell et al. 2009). Due to its finite supply, there is an absolute necessity to reclaim phosphate from waste sources and recycle phosphate within all agricultural processes.
4.2.2 Carbon Dioxide Mass Transfer
In addition to the limitations of CO2 supply to the cultivation plant, another key consideration is the rate of CO2 transfer from the gas phase into the liquid culture. In raceway ponds, a paddlewheel creates turbulence that enhances CO2 absorption from the surrounding air. However, the mass transfer in raceways is low in comparison to other PBRs, and is one of the limiting factors of these systems (Mata et al. 2010; Brennan and Owende 2010; Christenson and Sims 2011). Bubbling CO2 into the ponds increases mass transfer and improves productivity (Brennan and Owende 2010), but much of the CO2 is lost to the atmosphere due to short bubble residence times (Mata et al. 2010; Christenson and Sims 2011). Christenson and Sims (2011) suggest using rotating gas–liquid contactors to increase mass transfer.
Closed PBRs commonly use gas sparging to provide CO2. CO2 losses from aeration are lower in closed PBRs compared to open systems, and mixing and mass transfer tend to be superior (Mata et al. 2010; Singh et al. 2011). In this analysis, the combined consideration of gas–liquid mass transfer and CO2 uptake rates by the algae is essential to maximize the efficiency of CO2 utilization (Langley et al. 2012). The compression of gas for sparging is energy intensive and thus limits the feasibility for large-scale algal biodiesel production. Carvalho et al. (2006) report the use of an open gas exchange unit at the bottom of a flat-panel reactor for gas–liquid mass transfer. However, this system is open to contamination, which is usually one of the advantages of closed PBRs. Carvalho et al. (2006) describe membrane aeration in which CO2 diffuses through a silicone or hollow-fiber membrane tubing. This prevents CO2 losses that occur with bubbling, and also allows for accurate control of transfer rates and the use of pure CO2. However, large membrane surface areas are required which contributes to the cost of cultivation. High salt media, as used with marine algae, limits membrane diffusion, and bacterial cells can cause fouling (Carvalho et al. 2006). Further development of low energy PBRs with high mass transfer will greatly enhance the feasibility of algal biodiesel.
4.2.3 Alternative Fertilizer Sources
There are three approaches to reducing the need for fertilizers in algal biodiesel production: (1) using a source of waste nutrients, e.g., domestic wastewater, (2) using nitrogen-fixing algae or bacteria, and (3) recycling the nutrients in the non-lipid portion of the biomass. Wastewater sources are commonly suggested as a means of supplying cheap nutrients to algal culture, but wastewater streams vary in composition over time, making reliable production challenging, and the nutrient concentrations are often far below that required for optimal algal growth (Peccia et al. 2013). Wastewaters frequently contain toxic levels of ammonia (Borowitzka and Moheimani 2013) and pose a high risk of contamination with unwanted algae and other eukaryotes, bacteria, and viruses that may compete with or reduce the productivity of oleaginous algae (Peccia et al. 2013). Most problematically, the volumes of waste nutrient sources available globally will constrain the amount of biofuel produced (Chisti 2013a; Borowitzka and Moheimani 2013). It is likely that waste sources of nutrients (including sewage, urine, manure, waste biomass, ash, and bone meal) will be in high demand for other bioprocesses, as well as conventional agriculture, in the future.
Eukaryotic microalgae cannot utilize N2 directly (Peccia et al. 2013). The ability to fix nitrogen could potentially be engineered into a lipid producing algae, or a naturally nitrogen-fixing strain of cyanobacteria could be used as the fuel producer. Alternatively, a coculture or series of cultures could be developed utilizing cyanobacteria (e.g., Anabaena or Synechococcus) or other nitrogen-fixing bacteria (e.g., Rhizobium) (Dawson and Hilton 2011; Borowitzka and Moheimani 2013). This kind of artificial symbiosis between oleaginous strains of microalgae and ammonium-producing bacteria has been demonstrated (Lipman and Peakle 1925; Ortiz-Marquez et al. 2012), and genetic modification to enhance nitrogen fixation has been successful in Anabaena (Chaurasia and Apte 2011). Nitrogen fixation as well as O2 removal is energetically costly operation for the organism, indicating that an engineered strain would require an increased photosynthetic capacity in order to maintain both nitrogen fixation and lipid production (Peccia et al. 2013; Chisti 2013a).
4.3 Nutrient Recycling
Efficient recycling of CO2, nitrogen, and phosphates within the process is likely to be an absolute requirement for sustainable production of algal biofuels (Chisti 2013a). This precludes the production of coproducts such as food, feed, or fertilizer that would result in net export of large amounts of nutrients from the process, unless the income or reduction in alternative production of those products offsets the energy and fertilizer use in the algal process.
After extraction of lipids (largely carbon and hydrogen), the residual nutrient-rich biomass can be fed to an AD, which produces methane for the generation of heat and power, as well as CO2 and a liquid effluent rich in ammonia and phosphates (Sialve et al. 2009; Cai et al. 2013; Sheets et al. 2014; Ward et al. 2014). The liquid AD effluent currently appears to be the most feasible option for nutrient recycling (Chisti 2013a). The CO2 produced by the AD and methane combustion could be fed to the algal culture, effectively recycling the majority of carbon not exported in the lipid fraction (Fig. 3).
Thermo-catalytic conversions (e.g., hydrothermal liquefaction or gasification) are alternatives to AD in recycling nutrients, but these thermal technologies are more often performed on whole biomass, rather than biomass residue from lipid extraction, because the benefits lie in reducing the capital and life-cycle costs associated with lipid extraction and conversion (Peccia et al. 2013). Hydrothermal liquefaction has been shown to convert 80 % of the energy in algal biomass to biocrude and generate a waste stream containing the majority of nitrogen and phosphate that has potential to be recycled (Valdez et al. 2012).
Nutrient recycling is critical to successful algal biodiesel production. However, product export and system inefficiencies will require constant input of carbon, nitrogen, and phosphate. CO2 supplies are the limiting factor, particularly at the scales required to ensure algal biodiesel contributes to the future energy mix.
4.4 Water and Energy Consumption
The production of algal biofuels, whether in closed reactors or open ponds, requires large amounts of water and energy. Water is used in algal cultivation for replacing evaporative and process losses, with smaller amounts used in biodiesel production (Harto et al. 2010). At scales large enough to make a significant contribution to the future world energy mix, the supply of freshwater quickly becomes limiting, particularly in warm areas with high insolation (Borowitzka and Moheimani 2013). It is very unlikely that freshwater species will be used to produce algal biofuels in significant quantities, unless grown in wastewater, where production will be limited by wastewater availability. Even if marine algal species are used, the freshwater required to replace that lost to evaporation and therefore to prevent an increase in the salinity of the medium will become a limiting factor (Chisti 2013a).
The energy used to cultivate algae and produce biodiesel must be significantly lower than the energy that can be harnessed from the resulting biodiesel, i.e., a favorable net energy recovery. If fossil fuel energy is used in the process, the environmental impacts of this must be weighed against the benefits of the resulting algal biodiesel. According to a review of studies on algal biodiesel sustainability, major contributing factors to energy intensity include PBR design, nutrient source, dewatering and biomass drying, and lipid extraction (Lam and Lee 2012; Harrison et al. 2013).
4.4.1 Algal Cultivation
In regions of high insolation, evaporation rates are generally greater than 1.5 m year−1 or approximately 4 mm day−1 (Borowitzka and Moheimani 2013). It will be advantageous to minimize evaporative water loss by careful selection of location. The major factors determining evaporation rate are solar irradiance, temperature, wind speed, and humidity (Chisti 2013b). Temperature and irradiance are likely to be chosen for optimal algal productivity rather than minimizing water loss (higher algal productivity per unit volume also assists in decreasing the water requirement per unit product); the key criteria for site selection in terms of evaporation are therefore low wind speeds and high humidity. Evaporation could potentially be controlled or contained to an extent using closed reactors or covered ponds, with a concomitant increase in capital costs. There is also a difficult balance between minimizing evaporation and maximizing mass transfer of CO2 into and O2 out of the culture medium.
It is likely that replacement of evaporative water will need to be with saline water, leading to a gradual increase in the salinity of the culture medium. Algal cells will incur a metabolic cost at high salt concentrations due to energy required to export excess salt or produce compounds that maintain the osmotic balance (Chisti 2013a). If the increase in salinity affects microalgal performance, a portion of the pond water must either be purged regularly, or the entire pond contents replaced, leading to loss of nutrients and increased costs. Some success has been achieved in finding algal species that are tolerant of a wide range of salinities so that productivity can remain high while the pond salinity increases (Sing et al. 2014).
Currently, algal cultivation requires the majority of share of the energy input in biodiesel production (Stephenson et al. 2010; Harun et al. 2010; Slade and Bauen 2013). Several studies indicate that raceway ponds have substantially lower energy requirements than tubular PBRs, the latter frequently demonstrating unfavorable net energy ratios (NER) (more energy being used to produce the algae than can be harvested from the biodiesel) (Stephenson et al. 2010; Lam and Lee 2012; Rawat et al. 2013; Slade and Bauen 2013). Jones and Harrison (2014) discuss key factors affecting NER in vertical tubular reactors which may allow improved NER with improved reactor design and operation. Specifically, the NER is sensitive to the CO2 partial pressure and can be lowered substantially by sparging with enriched CO2. Power requirements for circulating the algal culture (paddle wheels, pumps, aeration) usually account for the majority of the energy demand. Stephenson et al. (2010) suggest alternative PBR designs, such as flat panels or oscillating reactors to improve the NER of algal biodiesel production from closed systems. However, as long as extensive sparging is required for mixing and/or gas transfer, energy requirements will remain a critical consideration. Jorquera et al. (2010) demonstrated a much-improved NER for a flat-panel reactor in comparison to a tubular PBR, due to the substantially lower power requirements for pumping air (2500 W m−3 for tubular PBR; and 53 W m−3 for flat-panel PBR).
4.4.2 Downstream Processing
Downstream processing usually makes a smaller contribution to the NER of algal biodiesel production in comparison to cultivation, with the exception of drying (Harun et al. 2010; Slade and Bauen 2013). Centrifugation and filtration are energy-intensive harvesting methods (Lam and Lee 2012), but gravity sedimentation, flocculation, and flotation have minor energy requirements, usually attributed to pumping (Sander and Murthy 2010; Stephenson et al. 2010; Brennan and Owende 2010; Campbell et al. 2011; Rawat et al. 2013). Lam and Lee (2012) report energy consumption for bioflocculation to be 9.8 kJ kg−1 biodiesel.
A major cause of water loss is associated with harvesting. Minimizing the loss of water in the harvested algal biomass is limited by the economic and energetic feasibility of achieving efficient solid–liquid separation of small algal cells from the medium. Based on an algae plant producing 100,000 bbl of lipids year−1, Borowitzka and Moheimani (2013) calculated evaporative water loss to be between 490 and 1307 ML and harvest loss between 13,415 and 35,772 ML. In contrast, Harto et al. (2010) calculate the evaporative water loss (average 165 L L−1 fuel) to be much greater than the process water consumption (average 50 L L−1 fuel) in open ponds.
Water inhibits lipid extraction and transesterification (Griffiths et al. 2010), thus necessitating drying. Many energy analyses assume the use of solar drying, and so do not discuss the large energy demand associated with other drying methods. Solar drying is only a realistic option under ideal conditions in sunny climates (Brennan and Owende 2010; Lam and Lee 2012), and prohibits water recycling. Sander and Murthy (2010) report in their case study that drying in a natural gas-fired dryer accounted for 69 % of the total energy consumption in the biodiesel production process. Using biodiesel conversion methods that are not inhibited by wet biomass is important for improving sustainability in terms of both water and energy usage (Azadi et al. 2014). Alternative methods of lipid extraction and biodiesel production that require only partial or no drying, such as supercritical fluid extraction, in situ transesterification, and hydrothermal liquefaction (Lam and Lee 2012), are especially attractive.
Lipid extraction accounts for 5–10 % of the energy requirement for biodiesel production (Sander and Murthy 2010; Stephenson et al. 2010). Solvent extraction is the most commonly used method, but requires dry biomass (Lam and Lee 2012). Supercritical fluid extraction can be achieved using wet biomass, and thus eliminates the large energy burden associated with drying. This method requires heat and compression energy (Rawat et al. 2013). A commonly used fluid is supercritical CO2 due to its low critical temperature. However, the energy requirement for capturing CO2 from the atmosphere and recompressing it after each extraction needs to be assessed (Lam and Lee 2012).
Transesterification of the algal oil into biodiesel accounts for a small portion of energy requirements (0.036 J J−1 fuel) (Lardon et al. 2009; Sander and Murthy 2010). Acid–base and heterogeneously catalyzed transesterification are the conventional methods, but in situ transesterification is an emerging technology that has several advantages. In situ transesterification allows extraction and conversion to occur in one step in which biomass is used directly for conversion to diesel, removing the energy demands associated with lipid extraction as well as biomass drying (Griffiths et al. 2010; Lam and Lee 2012; Rawat et al. 2013). This may impact the application of the residual biomass.
Water may be recycled after the biomass has been harvested and processed, but recycling introduces additional challenges. Any soluble organic waste products, pond debris, or additives (e.g., chemical flocculants used in harvesting) will accumulate, potentially leading to reduced productivity. Contaminants such as competitors, predators, and pathogens (e.g., viruses) will be recycled if not removed. This may call for additional treatment or sterilization of the recycled medium, which inevitably increases energy requirements and cost (Williams and Laurens 2010).
The total water footprint of algal biodiesel has been estimated by Harto et al. (2010) to be between 216 and 656 L L−1 fuel in open ponds, based on conditions in the southwest US, with reduction to between 30 and 63 L L−1 fuel in closed systems. Yang et al. (2011) calculated a water requirement of 591 L L−1 fuel, assuming that all the water from harvesting was recycled. This is lower than estimates for ethanol from sugar beet or sugar cane (1388 and 2516 L L−1, respectively, Gerbens-Leenes et al. 2009) but higher than that for cellulosic ethanol (between 356 and 423 L L−1, Harto et al. 2010) and significantly higher than that for fossil fuels (2–6 L L−1, Harto et al. 2010). Improvements in algal productivity per unit volume or an increase in cell density would reduce the water footprint per liter of fuel (Yang et al. 2011); however, algal cell density and culture surface area to volume ratio are constrained by the necessity to provide adequate sunlight to all cells.
Many studies have assessed the NER of algal production processes. The results are best illustrated in Fig. 4, which shows a NER ranging between 0.12 and 5.92. As discussed above, the choice of bioreactor (open ponds, flat or tubular PBRs) seems to be the most important factor influencing the NER. Water and energy requirements clearly illustrate the difficulty in choosing between open ponds and closed PBRs. Energy gains from the use of open ponds must be carefully balanced against water and CO2 losses, as well as decreased productivity.
Comparison of net energy ratios (NER; energy input into process: energy recovered from biodiesel produced) from a selection of life-cycle analyses for algal biodiesel production. RP raceway pond; PBR Photobioreactor; Flat flat panel. The graph shows NER below 1 (positive energy balance) for most biodiesel processes that use raceway ponds; the harvesting method (FP filter press; Cn centrifugation) plays a substantial role in final energy balance; and flat-panel reactors have potential for low NER
5 Conclusions
The composition of 1 L of algal biodiesel in terms of nutrients, solar and fossil energy, and water, as described in the previous sections, is illustrated in Fig. 5. A clear trade-off exists between open and closed systems in terms of NER and water usage, although flat panel closed PBRs show great potential. The required SSE input is extremely large, with nearly 140 m2 required to produce 1 L biodiesel day−1, given an average daily insolation of 250 W m−2. Approximately, 8.45 kg of CO2 is required to produce 1 L of biodiesel; considering that the US requires approximately 2 trillion L of transportation fuel per year, this number quickly becomes staggering.
If algal biodiesel were to fill a niche in the liquid fuels market, such as aviation fuel, these limitations do not eliminate its potential as a feasible renewable liquid fuel source (Lundquist et al. 2010; Norsker et al. 2011; Chisti 2013a). Algal biodiesel is superior to first-generation biofuels for aviation fuel due to the high energy density and low freezing point (Brennan and Owende 2010). The 2008 demand for aviation fuel was 89 billion L year−1. Using conservative estimates of CO2 availability at point sources from Pate et al. (2011), 40 billion L of biodiesel can be produced from algae, supplying 45 % of aviation fuel demand. The production would require 1.5 Mha of land and, if open raceway ponds are used, close to 80,000 ML of water day−1.
Aside from limitations of scale, the immediate determinant of the economic feasibility is a direct comparison between the cost of algal biodiesel and the price of competing fuels. The price of crude oil has varied between 70 USD bbl−1 (0.44 USD L−1) and 114 USD bbl−1 (0.71 USD L−1) over 2014, while the retail price of fossil-derived diesel in the US has averaged 1.02 USD L−1 over 2014 (Ameritrade et al. 2014; InfoMine 2014). Comparing predicted costs (Table 1) in light of the limitations discussed (max 30 % lipid content, <60 g m−2 day−1 biomass), and removing outliers, it seems feasible that biodiesel can be produced at between 2 and 3 USD L−1.
Although the cost of producing biofuels may currently be higher than the cost of petroleum-based fuel, the cost of fossil fuels is artificially low as the environmental and social costs of burning fossil fuels are unaccounted for, particularly the impact of rising CO2 levels in the atmosphere. The price of fossil fuels must include the cost of carbon mitigation; this will contribute substantially toward the competitiveness of sustainable liquid fuels. As liquid biofuels seem to be an essential part of the future energy mix, at least in the short term, the key question becomes twofold: how does the cost of producing algal biodiesel compared to the cost of producing liquid fuels from other sources? which feedstock(s) are most sustainable and cost effective for the production of biofuels? Although the cost of production of algal biodiesel may be higher, and the production process less well developed than that of terrestrial crop plants, crop plants suffer from issues regarding food security and sustainability. Major advantages of algal biodiesel include potentially higher productivity per unit area, production of only oil-containing biomass (i.e., no roots, leaves, and stems when only seeds are required), minimal loss of fertilizer due to runoff, potential to enhance carbon mass transfer through dissolution in liquid medium, and reduced competition with food crops for arable land, although competition at scale for fresh water and nutrients remains.
The economic and energetic feasibility of algal biodiesel will be greatly improved by applying the biorefinery concept in terms of utilizing all component parts of the biomass generated (Wijffels et al. 2010; Williams and Laurens 2010; Harrison et al. 2013). Potential coproducts along with algal oil for biodiesel production include proteins for animal feed, carbohydrates for bioethanol, and high-value pigments. Coproducts resulting in a net export of nutrients, e.g., food, feed, or fertilizer, will only be feasible if it offsets the use of alternative sources, e.g., traditional feed or fertilizer, sufficiently to account for the inputs required to the algal process.
A great deal of uncertainty remains regarding the techno-economic feasibility of algal biodiesel. There are several key factors, highlighted by a review of the literature, to bear in mind when developing processes and models for algal biodiesel production. Progress in downstream processing has the potential to reduce energy consumption, but the algal cultivation NER remains a critical factor, especially in closed systems, and particularly in terms of the energy required to mix and sparge reactors. Complete drying of algal biomass is unlikely to be financially or energetically viable, and therefore conversion to biodiesel and extraction or production of any coproducts must be done using wet biomass. It is unlikely that it will be feasible to use freshwater for algal cultivation or replacing evaporative and process losses, and therefore there should be a focus on halotolerant production strains and minimizing water loss. Recycling of nitrogen and phosphate within the process will be critical and requires development and demonstration of cost- and energy-efficient mechanisms to achieve this. Additionally, coproducts leading to net export of nutrients from the process need to be carefully evaluated in terms of feasibility. Finally, major challenges appear to be in securing sufficient, inexpensive CO2 supply at geographically suitable locations, balancing energy and water usage, and decreasing the gap between current and theoretical photosynthetic efficiencies.
References
Acién, F. G., Fernández, J. M., Magán, J. J., & Molina, E. (2012). Production cost of a real microalgae production plant and strategies to reduce it. Biotechnology Advances, 30, 1344–1353. doi:10.1016/j.biotechadv.2012.02.005.
Amer, L., Adhikari, B., & Pellegrino, J. (2011). Technoeconomic analysis of five microalgae-to-biofuels processes of varying complexity. Bioresource Technology, 102, 9350–9359. doi:10.1016/j.biortech.2011.08.010.
Ameritrade, L. M, Stanley M, et al. (2014). YCharts.
ASTM. (2003). Reference solar spectral irradiance: Air mass 1.5. In: American society for testing and materials (ASTM) terrestrial reference spectra for photovoltaic performance evaluation http://rredc.nrel.gov/solar/spectra/am1.5/. Accessed December 2, 2014.
Azadi, P., Brownbridge, G., Mosbach, S., et al. (2014). The carbon footprint and non-renewable energy demand of algae-derived biodiesel. Applied Energy, 113, 1632–1644. doi:10.1016/j.apenergy.2013.09.027.
Barnes, C., Tibbitts, T., Sager, J., et al. (1993). Accuracy of quantum sensors measuring yield photon flux and photosynthetic photon flux. HortScience, 28, 1197–1200.
Batan, L., & Quinn, J. (2010). Net energy and greenhouse gas emission evaluation of biodiesel derived from microalgae. Environmental Science and Technology, 44, 7975–7980.
Behrenfeld, M., & Falkowski, P. G. (1997). A consumer’s guide to phytoplankton primary productivity models. Limnology and Oceanography, 42, 1479–1491. doi:10.4319/lo.1997.42.7.1479.
Blankenship, R. E., Tiede, D. M., Barber, J., et al. (2011). Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science, 332, 805–880. doi:10.1126/science.1200165.
Borowitzka, M. A., & Moheimani, N. R. (2013). Sustainable biofuels from algae. Mitig Adapt Strateg Glob Chang, 18, 13–25. doi:10.1007/s11027-010-9271-9.
Brennan, L., & Owende, P. (2010). Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renewable and Sustainable Energy Reviews, 14, 557–577. doi:10.1016/j.rser.2009.10.009.
Brownbridge, G., Azadi, P., Smallbone, A., et al. (2014). The future viability of algae-derived biodiesel under economic and technical uncertainties. Bioresource Technology, 151, 166–173. doi:10.1016/j.biortech.2013.10.062.
Cai, T., Park, S. Y., Racharaks, R., & Li, Y. (2013). Cultivation of Nannochloropsis salina using anaerobic digestion effluent as a nutrient source for biofuel production. Applied Energy, 108, 486–492. doi:10.1016/j.apenergy.2013.03.056.
Campbell, P. K., Beer, T., & Batten, D. (2011). Life cycle assessment of biodiesel production from microalgae in ponds. Bioresource Technology, 102, 50–56. doi:10.1016/j.biortech.2010.06.048.
Carvalho, A., Meireles, L. A., & Malcata, F. X. (2006). Microalgal reactors: A review of enclosed system designs and performances. Biotechnology Progress, 22, 1490.
Chen, M., & Blankenship, R. E. (2011). Expanding the solar spectrum used by photosynthesis. Trends in Plant Science, 16, 427–431. doi:10.1016/j.tplants.2011.03.011.
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25, 294–306. doi:10.1016/j.biotechadv.2007.02.001.
Chisti, Y. (2013a). Constraints to commercialization of algal fuels. Journal of Biotechnology, 167, 201–214. doi:10.1016/j.jbiotec.2013.07.020.
Chisti, Y. (2013b). Raceways-based production of algal crude oil. Green. doi:10.1515/green-2013-0018.
Christenson, L., & Sims, R. (2011). Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnology Advances, 29, 686–702. doi:10.1016/j.biotechadv.2011.05.015.
Clarens, A., & Resurreccion, E. (2010). Environmental life cycle comparison of algae to other bioenergy feedstocks. Environmental Science and Technology, 44, 1813–1819.
Cordell, D., Drangert, J.-O., & White, S. (2009). The story of phosphorus: Global food security and food for thought. Glob Environ Chang, 19, 292–305. doi:10.1016/j.gloenvcha.2008.10.009.
Davis, R., Aden, A., & Pienkos, P. T. (2011). Techno-economic analysis of autotrophic microalgae for fuel production. Applied Energy, 88, 3524–3531. doi:10.1016/j.apenergy.2011.04.018.
Davis, R., Fishman, D., Frank, E., & Wigmosta, M. (2012). Renewable diesel from algal lipids: An integrated baseline for cost, emissions, and resource potential from a harmonized model.
Dawson, C. J., & Hilton, J. (2011). Fertiliser availability in a resource-limited world: Production and recycling of nitrogen and phosphorus. Food Policy, 36, S14–S22. doi:10.1016/j.foodpol.2010.11.012.
Delrue, F., Setier, P., Sahut, C., et al. (2012). An economic, sustainability, and energetic model of biodiesel production from microalgae. Bioresource Technology, 111, 191–200. doi:10.1016/j.biortech.2012.02.020.
Duffie, J. A., Beckman, W. A. (2013). Solar engineering of thermal processes (Vol. 936). New York: Wiley.
Eberle, D. U., & von Helmolt, D. R. (2010). Sustainable transportation based on electric vehicle concepts: A brief overview. Energy & Environmental Science, 3, 689. doi:10.1039/c001674h.
Escobar, J. C., Lora, E. S., Venturini, O. J., et al. (2009). Biofuels: Environment, technology and food security. Renewable and Sustainable Energy Reviews, 13, 1275–1287. doi:10.1016/j.rser.2008.08.014.
Ferreira, A. F., Ribeiro, L. A., Batista, A. P., et al. (2013). A biorefinery from Nannochloropsis sp. microalga—energy and CO2 emission and economic analyses. Bioresour Technol 138, 235–244. doi:10.1016/j.biortech.2013.03.168.
Gerbens-Leenes, W., Hoekstra, A. Y., & van der Meer, T. H. (2009). The water footprint of bioenergy. Proceedings of the National Academy of Sciences USA, 106, 10219–10223. doi:10.1073/pnas.0812619106.
Griffiths, M., van Hille, R., & Harrison, S. (2012). Lipid productivity, settling potential and fatty acid profile of eleven microalgal species grown under nitrogen replete and limited conditions. Journal of Applied Phycology, 24, 989–1001.
Griffiths, M. J., van Hille, R. P., & Harrison, S. T. L. (2010). Selection of direct transesterification as the preferred method for assay of fatty acid content of microalgae. Lipids, 45, 1053–1060.
Griffiths, M. J., & Harrison, S. T. L. (2009). Lipid productivity as a key characteristic for choosing algal species for biodiesel production. Journal of Applied Phycology, 21, 493–507. doi:10.1007/s10811-008-9392-7.
Harding, K. (2009). A generic approach to environmental assessment of microbial bioprocesses through life cycle assessment. South Africa: University of Cape Town.
Harrison, S., Richardson, C., & Griffiths, M. (2013). Analysis of microalgal biorefineries for bioenergy from an environmental and economic perspective focus on algal biodiesel. In F. Bux (Ed.), Biotechnol. Applied Microalgae Biodiesel Value-Added Prod (pp. 113–136). USA: CRC Press.
Harto, C., Meyers, R., & Williams, E. (2010). Life cycle water use of low-carbon transport fuels. Energy Policy, 38, 4933–4944. doi:10.1016/j.enpol.2010.03.074.
Harun, R., Davidson, M., Doyle, M., et al. (2011). Technoeconomic analysis of an integrated microalgae photobioreactor, biodiesel and biogas production facility. Biomass and Bioenergy, 35, 741–747. doi:10.1016/j.biombioe.2010.10.007.
Harun, R., Singh, M., Forde, G. M., & Danquah, M. K. (2010). Bioprocess engineering of microalgae to produce a variety of consumer products. Renewable and Sustainable Energy Reviews, 14, 1037–1047. doi:10.1016/j.rser.2009.11.004.
Hoepffner, N., & Sathyendranath, S. (1993). Determination of the major groups of phytoplankton pigments from the absorption spectra of total particulate matter. Journal Geophysical Research, 98, 22789. doi:10.1029/93JC01273.
Horta Nogueira, L. A., Moreira, J. R., Schuchardt, U., & Goldemberg, J. (2013). The rationality of biofuels. Energy Policy, 61, 595–598. doi:10.1016/j.enpol.2013.05.112.
InfoMine. (2014) Infomine.
International Energy Agency. (2011). Technology roadmap: Biofuels for transport. doi:10.1787/9789264118461-en.
Jones, C. S., & Mayfield, S. P. (2012). Algae biofuels: Versatility for the future of bioenergy. Current Opinion in Biotechnology, 23, 346–351. doi:10.1016/j.copbio.2011.10.013.
Jones, S. M., & Harrison, S. T. L. (2014). Aeration energy requirements for lipid production by Scenedesmus in airlift bioreactors. Algal Research 5, 249–257. doi:10.1016/j.algal2014-.03.003.
Jorquera, O., Kiperstok, A., Sales, E. A., et al. (2010). Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresource Technology, 101, 1406–1413. doi:10.1016/j.biortech.2009.09.038.
Kyewalyanga, M., Platt, T., & Sathyendranath, S. (1997). Estimation of the photosynthetic action spectrum: implication for primary production models. Oceanogr Lit Rev, 44, 207–223.
Lam, M. K., & Lee, K. T. (2012). Microalgae biofuels: A critical review of issues, problems and the way forward. Biotechnology Advances, 30, 673–690. doi:10.1016/j.biotechadv.2011.11.008.
Langley, N., Harrison, S. T. L., & van Hille, R. P. (2012). The effect of CO2 availability on the growth of Chlorella vulgaris. Biochemical Engineering Journal, 68, 70–75. doi:10.1016/j.bej.2012.-07.013.
Lardon, L., Hélias, A., Sialve, B., et al. (2009). Life-cycle assessment of biodiesel production from microalgae. Environmental Science and Technology, 43, 6475–6481.
Li, J., Xu, N. S., & Su, W. W. (2003). Online estimation of stirred-tank microalgal photobioreactor cultures based on dissolved oxygen measurement. Biochemical Engineering Journal, 14, 51–65. doi:10.1016/S1369-703X(02)00135-3.
Liu, X., Clarens, A. F., & Colosi, L. M. (2012). Algae biodiesel has potential despite inconclusive results to date. Bioresource Technology, 104, 803–806. doi:10.1016/j.biortech.2011.10.077.
Lundquist, T., Woertz, I., Quinn, N., & Benemann, J. (2010). A realistic technology and engineering assessment of algae biofuel production. Energy Biosci Inst, 1, 1–178.
MacIntyre, H., & Kana, T. (2002). Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. Journal of Phycology, 38, 17–38.
Mata, T. M., Martins, A. A., Caetano, N. S. (2010). Microalgae for biodiesel production and other applications: A review. Renew Sustain Energy Rev 14, 217–232. doi:10.1016/j.rser.2009.07.020.
Michel, H. (2012). Editorial: The nonsense of biofuels. Angewandte Chemie (International ed. in English), 51, 2516–2518. doi:10.1002/anie.201200218.
Mirón, A. S., Garcı́a, M. C. C, Gómez, A. C., et al. (2003). Shear stress tolerance and biochemical characterization of Phaeodactylum tricornutum in quasi steady-state continuous culture in outdoor photobioreactors. Biochem Eng J 16:287–297. doi:10.1016/S1369-703X(03)00072-X.
Molina, E., Fernández, J., Acién, F. G., & Chisti, Y. (2001). Tubular photobioreactor design for algal cultures. Journal of Biotechnology, 92, 113–131.
Morel, A. (1978). Available, usable, and stored radiant energy in relation to marine photosynthesis. Deep Sea Research, 25, 673–688.
Nagarajan, S., Chou, S. K., Cao, S., et al. (2013). An updated comprehensive techno-economic analysis of algae biodiesel. Bioresource Technology, 145, 150–156. doi:10.1016/j.biortech.2012.11.108.
NASA. (2013). Surface meteorology and Solar Energy (SSE) Release 6.0 Methodology.
Norsker, N.-H., Barbosa, M. J., Vermuë, M. H., & Wijffels, R. H. (2011). Microalgal production–a close look at the economics. Biotechnology Advances, 29, 24–27. doi:10.1016/j.biotechadv.2010.08.005.
Oswald, W. J. (1988). Micro-algae and waste-water treatment. In M. Borowitzka & L. Borowitzka (Eds.), Microalgal Biotechnol (pp. 305–328). Cambridge: Cambridge University Press.
Park, J. B. K., Craggs, R. J., Shilton, A. N. (2011). Wastewater treatment high rate algal ponds for biofuel production. Bioresource Technology, 102, 35–42. doi:10.1016/j.biortech.2010.06.158.
Pate, R., Klise, G., & Wu, B. (2011). Resource demand implications for US algae biofuels production scale-up. Applied Energy, 88, 3377–3388. doi:10.1016/j.apenergy.2011.04.023.
Peccia, J., Haznedaroglu, B., Gutierrez, J., & Zimmerman, J. B. (2013). Nitrogen supply is an important driver of sustainable microalgae biofuel production. Trends in Biotechnology, 31, 134–138. doi:10.1016/j.tibtech.2013.01.010.
Pilon, L., Berberoğlu, H., & Kandilian, R. (2011). Radiation transfer in photobiological carbon dioxide fixation and fuel production by microalgae. J Quant Spectrosc Radiat Transf, 112, 2639–2660. doi:10.1016/j.jqsrt.2011.07.004.
Pittman, J. K., Dean, A. P., & Osundeko, O. (2011). The potential of sustainable algal biofuel production using wastewater resources. Bioresource Technology, 102, 17–25. doi:10.1016/j.biortech.2010.06.035.
Rawat, I., Ranjith Kumar, R., Mutanda, T., & Bux, F. (2013). Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Applied Energy, 103, 444–467. doi:10.1016/j.apenergy.2012.10.004.
RETScreen International. (2005). Clean energy project analysis.
Richardson, J. W., Johnson, M. D., & Outlaw, J. L. (2012). Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Res, 1, 93–100. doi:10.1016/j.algal.2012.04.001.
Richardson, J. W., Johnson, M. D., Zhang, X., et al. (2014). A financial assessment of two alternative cultivation systems and their contributions to algae biofuel economic viability. Algal Res, 4, 96–104. doi:10.1016/j.algal.2013.12.003.
Rincón, L. E., Jaramillo, J. J., Cardona, C. A. (2014). Comparison of feedstocks and technologies for biodiesel production: An environmental and techno-economic evaluation. Renewable Energy 69,479–487. doi:10.1016/j.renene.2014.03.058.
Rosenberg, J. N., Oyler, G. A., Wilkinson, L., Betenbaugh, M. J. (2008). A green light for engineered algae: Redirecting metabolism to fuel a biotechnology revolution. Current Opinion in Biotechnology 19, 430–436. doi:10.1016/j.copbio.2008.07.008.
Sander, K., & Murthy, G. (2010). Life cycle analysis of algae biodiesel. International Journal of Life Cycle Assessment, 2008, 704–714. doi:10.1007/s11367-010-0194-1.
Savile, C. K., & Lalonde, J. J. (2011). Biotechnology for the acceleration of carbon dioxide capture and sequestration. Current Opinion in Biotechnology, 22, 818–823. doi:10.1016/j.copbio.2011.06.006.
Sheets, J. P., Ge, X., Park, S. Y., & Li, Y. (2014). Effect of outdoor conditions on Nannochloropsis salina cultivation in artificial seawater using nutrients from anaerobic digestion effluent. Bioresource Technology, 152, 154–161. doi:10.1016/j.biortech.2013.10.115.
Shockley, W., & Queisser, H. J. (1961). Detailed balance limit of efficiency of p-n junction solar cells. Journal of Applied Physics, 32, 510. doi:10.1063/1.1736034.
Sialve, B., Bernet, N., & Bernard, O. (2009). Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnology Advances, 27, 409–416. doi:10.1016/j.biotechadv.2009.03.001.
Sims, R. E. H., Mabee, W., Saddler, J. N., Taylor, M. (2010). An overview of second generation biofuel technologies. Bioresour Technol 101:1570–1580. doi:10.1016/j.biortech.2009.11.046.
Sing, S. F., Isdepsky, A., Borowitzka, M. A., & Lewis, D. M. (2014). Pilot-scale continuous recycling of growth medium for the mass culture of a halotolerant Tetraselmis sp. in raceway ponds under increasing salinity: A novel protocol for commercial microalgal biomass production. Bioresour Technol 161, 47–54. doi:10.1016/j.biortech.2014.03.010.
Singh, A., Nigam, P. S., & Murphy, J. D. (2011). Mechanism and challenges in commercialisation of algal biofuels. Bioresource Technology, 102, 26–34. doi:10.1016/j.biortech.2010.06.057.
Slade, R., & Bauen, A. (2013). Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass and Bioenergy, 53, 29–38. doi:10.1016/j.biombioe.2012.12.019.
Sousa, C. E. (2013). Oxygen accumulation in photobioreactors. The Netherland: Wageningen University.
Stephenson, A., Kazamia, E., Dennis, J., et al. (2010). Life-cycle assessment of potential algal biodiesel production in the United Kingdom: a comparison of raceways and air-lift tubular bioreactors. Energy & Fuels, 24, 4062–4077. doi:10.1021/ef1003123.
Sun, A., Davis, R., Starbuck, M., et al. (2011). Comparative cost analysis of algal oil production for biofuels. Energy, 36, 5169–5179. doi:10.1016/j.energy.2011.06.020.
Takeshita, T. (2011). Competitiveness, role, and impact of microalgal biodiesel in the global energy future. Applied Energy, 88, 3481–3491. doi:10.1016/j.apenergy.2011.02.009.
Talling, J. (1957) Photosynthetic characteristics of some freshwater plankton diatoms in relation to underwater radiation. New Phytologist 29–50.
U.S. Energy Information Administration. (2014). How much energy is consumed in the world by each sector? http://www.eia.gov/tools/faqs/faq.cfm?id=447&t=1. Accessed November 26, 2014.
Valdez, P. J., Nelson, M. C., Wang, H. Y., et al. (2012). Hydrothermal liquefaction of Nannochloropsis sp.: Systematic study of process variables and analysis of the product fractions. Biomass and Bioenergy, 46, 317–331. doi:10.1016/j.biombioe.2012.08.009.
Walker, D. A. (2009). Biofuels, facts, fantasy, and feasibility. Journal of Applied Phycology, 21, 509–517. doi:10.1007/s10811-009-9446-5.
Ward, A. J., Lewis, D. M., & Green, F. B. (2014). Anaerobic digestion of algae biomass: A review. Algal Res, 5, 204–214. doi:10.1016/j.algal.2014.02.001.
Wijffels, R. H., Barbosa, M. J. (2010) An outlook on microalgal biofuels. Science (80-) 329,796–769. doi:10.1126/science.1189003.
Wijffels, R. R. H., Barbosa, M. J., & Eppink, M. H. (2010). Microalgae for the production of bulk chemicals and biofuels. Biofuels, Bioprod Biorefining, 4, 287–295. doi:10.1002/bbb.
Williams, P. J. L. B., & Laurens, L. M. L. (2010). Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy & Environmental Science, 3, 554. doi:10.1039/b924978h.
Yang, J., Xu, M., Zhang, X., et al. (2011). Life-cycle analysis on biodiesel production from microalgae: water footprint and nutrients balance. Bioresource Technology, 102, 159–165. doi:10.1016/j.biortech.2010.07.017.
Zhang, Y.-H. P. (2011). What is vital (and not vital) to advance economically-competitive biofuels production. Process Biochemistry, 46, 2091–2110. doi:10.1016/j.procbio.2011.08.005.
Zhu, X.-G., Long, S. P., & Ort, D. R. (2008). What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Current Opinion in Biotechnology, 19, 153–159. doi:10.1016/j.copbio.2008.02.004.
Zilberman, D., Hochman, G., Rajagopal, D., et al. (2012). The Impact of Biofuels on Commodity Food Prices: Assessment of Findings. American Journal of Agricultural Economics, 95, 275–281. doi:10.1093/ajae/aas037.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Louw, T.M., Griffiths, M.J., Jones, S.M., Harrison, S.T. (2016). Techno-economics of Algal Biodiesel. In: Bux, F., Chisti, Y. (eds) Algae Biotechnology. Green Energy and Technology. Springer, Cham. https://doi.org/10.1007/978-3-319-12334-9_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-12334-9_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12333-2
Online ISBN: 978-3-319-12334-9
eBook Packages: EnergyEnergy (R0)