Abstract
Lessonia berteroana is one of the most exploited seaweeds in the Southeastern Pacific and its populations are recurrently facing overexploitation in northern Chile. Since germplasms are not available, we decided to start gametophyte biobanking to support conservation measures for this important resource in the future. Spores of L. berteroana from nine localities at the Atacama coast were used to establish clonal male and female gametophyte cultures. Unexpectedly, after isolation and under low light conditions, juvenile sporophytes originated from somatic cells via apogamy in most female gametophyte strains. In addition, eggs from solitary female gametophytes from Caleta Cisnes and Torres del Inca had a strong tendency to generate sporophytes by parthenogenesis, some of them even under low light regimes. Contrarily, female gametophytes of the sister species Lessonia spicata from southern Chile showed no evidence for apomixis. When one of these L. berteroana strains is cross-fertilized with L. spicata, true hybrids emerged based on the presence of eggs and subsequent sperm attraction. These observations contrast with (i) kelp recruitment assumed to be majorly by sexual reproduction and (ii) the strict reproductive separation of the two taxa reported for natural populations within their contact zone at 30°S and highlight their consideration for future repopulation and breeding programs of Lessonia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The brown algal genus Lessonia grows in large kelp beds along the coast of Chile. Some of them represent natural resources of great economic and social importance. The subtidal Lessonia trabeculata is predominantly used as forage for the mollusk abalone (blades), which is a high-value export product, but its hard stipes are rich in low-M/G-ratio alginates (Peteiro 2018). Lessonia berteroana and L. spicata occupy wave-exposed intertidal stretches and offer a high-quality alginate, rich in high M:G ratio (Percival et al. 1983). These two kelps are highly exploited seaweeds in Chile and currently subjected to severe over-exploitation. This has inspired numerous studies on natural kelp beds, motivated by efforts to understand population dynamics as a base for the development of management plans and harvesting strategies (summarized in Westermeier et al. 2018). However, a serious deficit of fundamental data remains for Chilean kelps: there is an almost complete lack of knowledge on their microscopic stages, which are obligatory parts of their life cycles. The majority of published studies deals with kelp beds on a macro-scale, taxonomy, and molecular population genetics. Consequently, life histories and sexual reproduction, including Mendelian genetics, are un-explored, although knowledge in these fields is an indispensable basis for crop improvement and future breeding programs.
Since no details beyond these general features were known for Chilean kelps, we have started years ago to initiate pilot-scale clonal gametophyte cultures of Lessonia trabeculata and Macrocystis pyrifera (Westermeier et al. 2006). We found that such cultures can be maintained for many years and used as stock material for mariculture projects. Subsequently, we showed that heterosis breeding in M. pyrifera is possible by mating parents of complementary genotypes (Westermeier et al. 2011), and we also detected diploid male gametophytes in natural habitats (Müller et al. 2016). Breeding programs for Lessonia species are lacking in Chile, although culture techniques have been described to obtain small sporophytes in the laboratory (Hoffmann and Santelices 1982).
Brown algal kelps are members of the order Laminariales and exhibit haplo-diploid life histories: a haploid microscopic dioecious gametophyte generation produces eggs and spermatozoids. Zygotes develop to the macroscopic diploid kelp sporophyte, which re-establishes the haplophase via meiosis. Haploid meio-spores are formed in sori located on reproductive parts of the thallus. Sometimes, however, gametophytes can undergo deviations towards asexual reproduction (Oppliger et al. 2007). The term apomixis stands for non-sexual origin of a sporophyte in haplo-diploid life histories. This deviation from the standard life history pattern has been described for a plethora of terrestrial seed plants (Bicknell and Koltunow 2004) and is also not uncommon in kelps (Nakahara and Nakamura 1973; Le Gall et al. 1996; Oppliger et al. 2007). Two different mechanisms may be distinguished: parthenogenesis, where an unfertilized egg produces a haploid, genetically female sporophyte, and apogamy, where a sporophyte emerges directly from a somatic cell of a gametophyte. Since apomixis products are often developed abnormally and die in the early stages of life, their actual contribution to ecological aspects is under-documented. However, sometimes parthenogenetic kelps may indeed perform better than sporophytes derived from sexual reproduction, which suggested an unexplored role in the field and a potential opportunity for mariculture.
Lessonia berteroana and L. spicata are endemic to the southeastern Pacific. Due to their habitats in the rocky exposed high-energy intertidal zone, mariculture attempts are difficult. Up to the present, harvest depends exclusively on natural stocks, endangering the availability of the resource. In analogy to our previous strategies with Macrocystis, we decided to evaluate the basis for a germplasm collection, in order to conserve seedstock of both species, and to study their life history in detail. In these screenings, we recurrently found deviations of the life cycle towards apomixis, which we aim to describe in this study.
Materials and methods
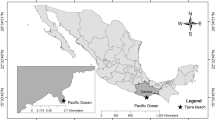
Fertile sporophytes of Lessonia spicata were collected at Mar Brava, Chiloé, in January 1997 and September 1999 (Fig. 1, Table S1). Gametophyte isolation and maintenance proceeded as stated by Westermeier et al. (2006) for Macrocystis and Lessonia trabeculata. One male and one female gametophyte was axenized in July 2002 for long-term maintenance on agar (Müller et al. 2008).
Coast of Chile showing collection sites of Lessonia berteroana in northern Chile, ranging from North of the townships Caldera to Huasco in the South, and of L. spicata in Chiloé (for strain details, see Table S1). The distribution of both species (after González et al. 2012) is indicated in colored rectangles in the left side
A sporophyte of Lessonia berteroana was collected at Caleta Cisne, Atacama Región, in May 2015. From this specimen, two pairs of clonal male and female gametophytes were selected for experiments (Table S1). Additional clonal gametophyte cultures were established from specimens collected in Jan 2017 at the following locations along the Atacama coast ranging from North of the township Caldera to Huasco: Los Médanos, Flamenco, Torres del Inca, Chorillos, Bahia Salado, Caleta Pajonales, Carrizal Bajo, and Totoral (Fig. 1; Table S1). These strains are maintained in our culture collection, since they represent a first attempt to preserve different genotypes of L. berteroana from localities that are presently under severe exploitation. In total, our study included 17 male and 21 female gametophyte clones of L. berteroana from 9 localities. Cultures were maintained in 60-mm polystyrene Petri dishes at 10 °C, under illumination with 0.5–3 μmol photons m−2 s−1 (hereafter low light condition) from daylight type fluorescent lamps (Osram DuluxStar Lumilux 11 W cool White) for 14 h day−1. Agar cultures were transferred in 6-month intervals. Prior to experiments, material from agar cultures was transferred to and maintained in liquid culture medium for at least 1 month. Cultures in liquid medium were maintained by bi-weekly transfer intervals. Artificial sea water was prepared from a commercial salt mixture (Tropic Marin Pro-Reef; www.tropic-marin.com) in deionized water, and adjusted to 30 PSU salinity with a sodium chloride refractometer (ATAGO, Sodium chloride 0–10%). After autoclaving, 20 mL L−1 Provasoli enrichment (Starr and Zeikus 1993) was added.
In order to induce gametogenesis, suspensions of few-celled gametophyte fragments were prepared by cutting with a scalpel or mild homogenization in a 1.5-mL Eppendorf tube. Exposure in fresh culture medium at 10 °C under white light at 40 μmol photons m−2 s−1 (hereafter high light condition) resulted in formation of eggs and spermatozoids, followed by fertilization and appearance of juvenile sporophytes. Using this approach, we made conspecific and interspecific crosses using one locality representative from each species (Table 1). All unialgal and cross-fertilization experiments were repeated two to three times (otherwise is stated in Table 1). Additionally, we worked with strains from localities distant enough that were Lessonia species in the intertidal that can be recognized as L. berteroana and L. spicata, respectively, based on González et al. (2012) observations (see Fig. 1).
Results
Lessonia spicata gametophytes
Spore samples from field specimens of Lessonia spicata produced equal numbers of male and female gametophytes with clear dimorphism in cell dimensions and branching pattern (Fig. 2). Under low light conditions, they grow in unlimited manner, and reproduction was not triggered whatsoever. In absence of a partner from the opposite sex, gametophytes may remain growing somatically (Fig. 3). In case that eggs/sperm are formed (as in high light treatment), they eventually die. Therefore, sporophytes are not formed from our L. spicata unialgal cultures.
Diversity of reproductive outcomes in Lessonia gametophytes. 2: Mixed sexually mature female and male gametophytes of L. spicata (L nig MB 97-23-4-f x L nig MB 99–61 m) after 6 days. Open arrowheads: antheridia; solid arrowheads: zygotes developing on oogonium apertures. 3: Solitary female gametophyte of L. spicata (L nig MB 97-23-4-f) derived from axenic agar culture after 37 days under gametogenesis-inducing conditions at high light. No apomictic sporophytes present. 4: L. berteroana conspecific cross from Caleta Cisne (L bert CC 11 f x L bert CC 7 m) after 13 days of culture. 5: Early apogamic sporophyte (arrow) developing from a somatic female gametophyte cell of L. berteroana from Chorrillos (L bert Cho 05 f) at low light intensity (image from stock culture). 6: Multiple apogamic sporophytes developing on a female gametophyte of L. berteroana from Caleta Totoral (L bert CT 01 f) at low light intensity (image from stock culture). 7: Solitary clonal female gametophyte of L. berteroana from Torres del Inca (L bert TI 1 f), forming oogonia, eggs (double arrowheads) and apomictic sporophytes at reduced light intensity (image from stock culture). 8: Parthenogenetic sporophyte originating from an un-fertilized egg of a clonal female gametophyte of L. berteroana from Caleta Cisne (L bert CC 11 f), 38 days after the parent was transferred from low to high light. 9 and 10: Parallel batches of female L. spicata at 37 days producing zygotic sporophytes in presence of fertile male gametophytes. 9: Conspecific cross L. spicata (L nig MB 97-23-4-f x L nig MB 99–61 m). 10: Hybrid cross L. spicata × L. berteroana (L nig MB 97-23-4-f x L bert CC 7 m). Scale bars: 100 μm for Figs. 2, 4, 5, 6, 7, and 8 and 0.5 mm for Figs. 3, 9, and 10
Lessonia berteroana gametophytes
In principle, gametogenesis and zygote development in Lessonia berteroana followed the same development pattern (Fig. 4). However, in contrast to L. spicata, female gametophytes of L. berteroana from almost all localities showed a tendency to apogamy (Table 1). Apogamic sporophytes emerged from apical as well as intercalary cells of somatic gametophyte filaments and started to form multicellular foliose sporophyte thalli (Fig. 5). They were variable in cell shapes and growth patterns, but appeared to be fully viable and grew up to several mm in our laboratory conditions. The potential to apogamy seems to vary between localities. We found it in 2 out of 5 female gametophytes from Los Médanos, while in all female gametophyte clones from Flamenco, Chorrillos (Fig. 5), Caleta Cisne, Bahia Salado, C. Pajonales, Carrizal Bajo, and Totoral (Fig. 6). These strains normally showed massive production of apogamic sporophytes in low light conditions and account to the 67% of all L. berteroana female strains used in this study.
In addition, some clonal female gametophyte cultures of L. berteroana produced eggs, which in the absence of spermatozoids developed to juvenile sporophytes via parthenogenesis (Table 1; Figs. 7 and 8). Female gametophytes from Torres del Inca were especially light-sensitive and produced eggs and partheno-sporophytes even under low light (Fig. 7). Only two strains from Los Medanos (LM 02 f and LM 05 f) remained growing and lacked any apomixis outcome. On the contrary, all male gametophytes of L. berteroana generally showed very slow growth and appeared as compact packages (see Fig. 4). They responded well to gametogenesis conditions, forming dense packets of antheridia-releasing spermatozoids. No hints of apomixis were observed for any of them (17 strains; Table S1) in our experiments.
Conspecific crosses
From L. spicata crosses, eggs produced zygotic sporophytes in presence of conspecific spermatozoids (Fig. 2). Release of eggs and spermatozoids in L. spicata occurred from the sixth day onward. Zygotes immediately started to develop, forming uniseriate, and later multicellular foliose juvenile sporophytes, which grew up to 8 mm under our culture conditions (Fig. 9). Lessonia spicata cultures did not show deviations from normal sexual life history. Unfertilized eggs were generally moribund. Occasional cell divisions of unfertilized eggs resulted in irregular cell complexes, but we did not see parthenogenetic nor apogamic development of viable sporophytes (see Fig. 3 for comparison). Consequently, we consider sporophytes resulting from matings of L. spicata eggs with L. spicata spermatozoids (Fig. 9) as heterozygous (Table 1).
After one attempt to cross L. berteroana female and male gametophytes (Fig. 4), we observed predominant apomixis going on even before inducing gametogenesis. We did not continue using female gametophytes of L. berteroana conspecific crosses onwards, since an unseparable mixture of zygotic and apomictic sporophytes was expected (Table 1).
Interspecific crosses
Gametophytes of female L. spicata from Mar Brava and male L. berteroana from Caleta Cisne and the reciprocal combination were mixed in two independent experiments and subjected to gametogenesis treatment (Table 1). In both crosses, we observed egg formation and sperm attraction, which normally are used as proxies for fertilization in brown algae. Oogonia and eggs, antheridia and spermatozoids appeared and at 37 days numerous juvenile sporophytes were recorded, mostly in dense groups emerging from remnants of a female gametophyte in the center (Fig. 10). These observations were less recurrent in the reciprocal cross L bert CC 11 f x L nig MB 99–61 m, where both parthenogenesis and apogamy are constitutively happening under low and high light regimes. Based on the controlled parameters (e.g., light conditions, temperature, and culture medium) of this experiment, we concluded that the resulting sporophytes were true sexual hybrids.
Discussion
Apomixis in L. berteroana female gametophytes and its putative contribution to L. berteroana ecology
Normally, kelp gametophytes can be maintained for decades as stock cultures in liquid culture medium or in axenic condition on agar, with low temperature and irradiance (Lüning and Neushul 1978). While cultures on agar are routinely transferred twice per year, gametophytes may survive for several years untouched in liquid culture medium (Müller et al. 2008). Typically, these gametophytes may be sensitive at high irradiance levels, and therefore, isolation and maintenance at low light/red light are suggested to avoid undesirable fertility (Lüning and Dring 1975). Our L. berteroana strains from northern Chile were even more sensitive and became fertile under extremely low light levels, where members of any other Laminarialean species, including L. spicata and Macrocystis pyrifera from several latitudes and heterogeneous climates, remained sterile (Hoffmann and Santelices 1982; Westermeier et al. 2006).
In land plants, it is quite well documented that apomixis is spatially structured and linked with heterogeneous habitats (Cosendai and Hörandl 2010). The drivers for apomixis are not yet widely explored in seaweeds, but some findings shed light into this question. Marginal populations of Laminaria digitata exhibit a higher prevalence of geographical parthenogenesis based on population genetic structures (Oppliger et al. 2014). In our study, a strong tendency for apomixis is particularly characteristic for a significant proportion of L. berteroana strains (26–28 °S), but non-existent in L. spicata (42 °S). This is inconsistent with the observations made by Oppliger et al. (2007). They reported that Lessonia nigrescens from Central Chile (Las Cruces, 33°S, L. spicata based on current Lessonia systematics) is able to produce sporophytes by parthenogenesis, which during early development tended to diploidize. Temporal-fluctuating factors may also affect gametophyte performance. Murúa et al. (2013) determined that fertility in female L. trabeculata changes dramatically between annual seasons, and even inter-annually after comparing with analogous non-contemporaneous studies. Tatarenkov et al. (2005) found a parthenogenetic dwarf morph of Fucus vesiculosus, as the likely result of prolonged exposure to low salinity levels. Genetic/environmental mediation of apomixis has been better studied in plant models. Eragrostis curvula (weeping lovegrass) distributes in biotypes that may be obligate or facultatively apomictic (Voigt and Bashaw 1976). Sexual diploids are rare (Voigt 1971); however, towards later generations, some purely sexual individuals reformed into apomictic, which involved genetic and epigenetic changes (Zappacosta et al. 2015). We therefore cannot rule out that apomixis in L. berteroana may be linked with our focalized sampling (majorly in 2017) and that both gametophyte viability and apomictic deviations are genetic and/or environmentally driven.
Kelp asexuality in natural populations is a topic poorly integrated in seaweed ecology, but has demonstrated to be more common and relevant than previously thought. In Ecklonia radiata, two morphs have been identified with contrasting different reproductive strategies, one of them vegetative (Coleman and Wernberg 2018). As in plants, in E. radiata, asexuality contributes to fix phenotypes and genotypes (especially heterozygous). Asexual reproduction has often been correlated with range expansions (Krueger-Hadfield et al. 2016), substrate colonization (Westermeier et al. 2016), senescence recovery (Murúa et al. 2017), and better acclimation under unfavorable conditions (Demes and Graham 2011). For plants, apomictic reproduction leading to healthy progeny indeed offers ecological advantages that can be extrapolated to Lessonia, such as reduction of costs for male sexual selection and outcrossing sex (reviewed in Richards 2003). In our culture experiments, female gametophytes of L. berteroana were able to perform both apogamy and parthenogenesis deviations. In consequence, the sporophytes produced by a bisexual mixture of gametophytes is expected to contain regular diploid heterozygous as well as genetically female apomictic sporophytes. A high apomictic tendency in gametophytes may reflect the results observed by Tellier et al. (2011), who pointed out that genetic diversity (e.g., allele richness, gene diversity) is significantly more reduced in L. berteroana than L. spicata, suggestive of different histories between populations. Vegetative propagation from big sporophytes has not been reported in Lessonia like in other kelps; therefore, a higher apomixis extent provides a probable hypothesis to explain such low levels of genetic diversity. The consequences for such particular development need to be elucidated.
L. berteroana cross-fertilization with L. spicata
Based on molecular and morphological evidence, González et al. (2012) sub-divided the former taxon Lessonia nigrescens into two separate species: L. spicata and L. berteroana. In parallel, Tellier et al. (2011) studied 12 field populations along 50 km of the contact zone between the two taxa using molecular markers. They reported complete reproductive isolation with strict geographic segregation and total lack of spatial co-existence. The factors responsible for this rigid separation in the natural habitat are unknown. On laboratory scale, however, our study showed ambiguous results: the two species L. spicata and L. berteroana are sexually compatible, and we are sure that at least in one direction (with L. spicata as female partner), 100% of the obtained progeny was viable hybrids. Gamete interaction and recognition mechanisms worked perfectly, up to the production of almost cm-size sporophytes. Hybridization in kelps with contrasting phenotypes/genotypes and well-delimited separation in natural habitats has also been studied for the two ecomorphs of Macrocystis pyrifera (Westermeier et al. 2007). Since later studies demonstrated that they belong to the same species (Macaya and Zuccarello 2010), the factors regulating such spatial separation are unknown. Our study may indicate that two Lessonia taxa are phylogenetically close and/or their cell architecture/molecular clues are similar enough to make them sexually compatible, allowing cross-fertilization and production of viable hybrid sporophytes at least under laboratory conditions.
Implications of apomixis and hybrid viability for Lessonia fishery and breeding programs
As asexual reproduction strategies in natural kelp populations are starting to be documented, its erroneous prevalence estimation in the field may conduct to design inaccurate policies for kelp management. This ultimately may lead to negative consequences for the fishery/ecology of highly exploited resources, such as L. berteroana and L. spicata. As pointed out before, populations dominated by asexual propagation may perform better. However, this is not always the case and sometimes asexuality led to maladaptation (Oppliger et al. 2014). How these processes are now varying in the current climate change scenario is virtually unexplored, even for higher plants (Walther et al. 2009).
In sharp contrast, apomixis is a potent tool in modern agronomy of terrestrial seed plants, stabilizing desirable phenotypes (Barcaccia and Albertini 2013). Particularly, seaweeds have also been suggested to facilitate mariculture in the kelp Undaria pinnatifida (Shan et al. 2013), whereby parthenosporophytes showed high attachment to culture ropes and more attractive yields. Our study opens the chance to generate apomictic kelp seedlings of selected stable genotypes. It also provides materials and knowledge for pilot scale mariculture experiments making use of Mendelian genetics including heterosis breeding. The limitation of our observations to laboratory scale does not allow to judge the survival potential of apomictic sporophytes in the natural habitat. However, our study presents a well-defined basis for a search for factors supporting species segregation in the natural habitat. We plan to grow up interspecific hybrid sporophytes L. spicata female × L. berteroana male to maturity in our pilot scale mariculture facilities. Jointly with a study using molecular sex markers, this strategy will offer a chance to verify their heterozygous character, determine if our Lessonia sporophytes may be product of pseudogamous and/or autonomous apomixis, and to identify factors such as meiotic failure responsible for the observed species separation in the natural habitat.
References
Barcaccia G, Albertini E (2013) Apomixis in plant reproduction: a novel perspective on an old dilemma. Plant Reprod 26:159–179
Bicknell RA, Koltunow AM (2004) Understanding apomixis: recent advances and remaining conundrums. Plant Cell 16:S228–S245
Coleman M, Wernberg T (2018) Genetic and morphological diversity in sympatric kelps with contrasting reproductive strategies. Aquat Biol 27:65–73
Cosendai A-C, Hörandl E (2010) Cytotype stability, facultative apomixis and geographical parthenogenesis in Ranunculus kuepferi (Ranunculaceae). Ann Bot 105:457–470
Demes KW, Graham MH (2011) Abiotic regulation of investment in sexual versus vegetative reproduction in the clonal kelp Laminaria sinclairii (Laminariales, Phaeophyceae). J Phycol 47:463–470
González A, Beltrán J, Hiriart-Bertrand L, Flores V, de Reviers B, Correa JA, Santelices B (2012) Identification of cryptic species in the Lessonia nigrescens complex (Phaeophyceae, Laminariales). J Phycol 48:1153–1165
Hoffmann AJ, Santelices B (1982) Effects of light intensity and nutrients on gametophytes and gametogenesis of Lessonia nigrescens Bory (Phaeophyta). J Exp Mar Biol Ecol 60:77–89
Krueger-Hadfield SA, Kollars NM, Byers JE, Greig TW, Hammann M, Murray DC, Murren CJ, Strand AE, Terada R, Weinberg F, Sotka EE (2016) Invasion of novel habitats uncouples haplo-diplontic life cycles. Mol Ecol 25:3801–3816
Le Gall Y, Asensi A, Marie D, Kloareg B (1996) Parthenogenesis and apospory in the Laminariales: a flow cytometry analysis. Eur J Phycol 31:369–380
Lüning K, Dring MJ (1975) Reproduction, growth and photosynthesis of gametophytes of Laminaria saccharina grown in blue and red light. Mar Biol 29:195–200
Lüning K, Neushul M (1978) Light and temperature demands for growth and reproduction of laminarian gametophytes in southern and Central California. Mar Biol 45:297–309
Macaya EC, Zuccarello GC (2010) DNA barcoding and genetic divergence in the giant kelp Macrocystis (laminariales). J Phycol 46:736–742
Müller DG, Gachon CMM, Küpper FC (2008) Axenic clonal cultures of filamentous brown algae: initiation and maintenance. Cah Biol Mar 49:59–65
Müller DG, Maier I, Marie D, Westermeier R (2016) Nuclear DNA level and life cycle of kelps: evidence for sex-specific polyteny in Macrocystis (Laminariales, Phaeophyceae). J Phycol 52:157–160
Murúa P, Westermeier R, Patiño DJ, Müller DG (2013) Culture studies on early development of Lessonia trabeculata (Phaeophyceae, Laminariales): seasonality and acclimation to light and temperature. Phycol Res 61:145–153
Murúa P, Müller DG, Patiño DJ, Westermeier R (2017) Giant kelp vegetative propagation: adventitious holdfast elements rejuvenate senescent individuals of the Macrocystis pyrifera “integrifolia” ecomorph. J Phycol 53:230–234
Nakahara H, Nakamura Y (1973) Parthenogenesis, apogamy and apospory in Alaria crassifolia (Laminariales). Mar Biol 18:327–332
Oppliger LV, Correa JA, Peters AF (2007) Parthenogenesis in the brown alga Lessonia nigrescens (Laminariales, Phaeophyceae) from Central Chile. J Phycol 43:1295–1301
Oppliger LV, von Dassow P, Bouchemousse S, Robuchon M, Valero M, Correa JA, Mauger S, Destombre C (2014) Alteration of sexual reproduction and genetic diversity in the kelp species Laminaria digitata at the southern limit of its range. PLoS One 9:e102518
Percival EE, Venegas Jara MF, Weigel H (1983) Carbohydrates of the brown seaweed Lessonia nigrescens. Phytochemistry 22:1429–1432
Peteiro C (2018) Alginate production from marine macroalgae, with emphasis on kelp farming. In: Rehm B, Moradali M (eds) Alginates and their biomedical applications. Springer, Singapore, pp 27–66
Richards AJ (2003) Apomixis in flowering plants: an overview. Philos Trans R Soc B 358:1085–1093
Shan TF, Pang SJ, Gao SQ (2013) Novel means for variety breeding and sporeling production in the brown seaweed Undaria pinnatifida (Phaeophyceae): crossing female gametophytes from parthenosporophytes with male gametophyte clones. Phycol Res 61:154–161
Starr RC, Zeikus JA (1993) UTEX-the culture collection of algae at the University of Texas at Austin 1993. List of cultures. J Phycol 29:1–106
Tatarenkov A, Bergström L, Jönsson RB, Serrao EA, Kautsky L, Johannesson K (2005) Intriguing asexual life in marginal populations of the brown seaweed Fucus vesiculosus. Mol Ecol 14:647–651
Tellier F, Tapia J, Faugeron S, Destombe C, Valero M (2011) The Lessonia nigrescens species complex (Laminariales, Phaeophyceae) shows strict parapatry and complete reproductive isolation in a secondary contact zone. J Phycol 47:894–903
Voigt PW (1971) Discovery of sexuality in Eragrostis curvula (Schrad.) Nees. Crop Sci 11:424
Voigt PW, Bashaw EC (1976) Facultative apomixis in Eragrostis curvula. Crop Sci 16:803
Walther G-R, Roques A, Hulme PE, Sykes MT, Pysek P, Kühn I, Zobel M, Bacher S, Botta-Dukát Z, Bugmann H, Czúcz B, Dauber J, Hickler T, Jarosík V, Kenis M, Klotz S, Minchin D, Moora M, Nentwig W, Ott J, Panov VE, Reineking B, Robinet C, Semenchenko V, Solarz W, Thuiller W, Vilà M, Vohland K, Settele J (2009) Alien species in a warmer world: risks and opportunities. Trends Ecol Evol 24:686–693
Westermeier R, Patino D, Piel MI, Maier I, Müller DG (2006) A new approach to kelp mariculture in Chile: production of free-floating sporophyte seedlings from gametophyte cultures of Lessonia trabeculata and Macrocystis pyrifera. Aquac Res 37:164–171
Westermeier R, Patiño D, Müller DG (2007) Sexual compatibility and hybrid formation between the giant kelp species Macrocystis pyrifera and M. integrifolia (Laminariales, Phaeophyceae) in Chile. J Appl Phycol 19:215–221
Westermeier R, Patiño DJ, Murúa P, Müller DG (2011) Macrocystis mariculture in Chile: growth performance of heterosis genotype constructs under field conditions. J Appl Phycol 23:819–825
Westermeier R, Murúa P, Patiño DJ, Muñoz L, Müller DG (2016) Holdfast fragmentation of Macrocystis pyrifera (integrifolia morph) and Lessonia berteroana in Atacama (Chile): a novel approach for kelp bed restoration. J Appl Phycol 28:2969–2977
Westermeier R, Murúa P, Patiño DJ, Manoli G, Müller DG (2018) Evaluation of kelp harvest strategies: recovery of Lessonia berteroana (Phaeophyceae, Laminariales) in Pan de Azucar, Atacama, Chile. J Appl Phycol. https://doi.org/10.1007/s10811-018-1500-8
Zappacosta DC, Ochogavía AC, Rodrigo JM, Romero JR, Meier MS, Garbus I, Pessino SC, Echenique VC (2015) Increased apomixis expression concurrent with genetic and epigenetic variation in a newly synthesized Eragrostis curvula polyploid. Sci Rep 4:4423
Acknowledgements
Sincere thanks are due to C. Atero for collection of field samples, U. Hueppeler and I. Maier for support with art-work and to the three anonymous reviewers for their contributions that helped to improve the earlier version of this MS. This work was done in the framework of the projects FIC 2013 33-91-243, FIC 2015 33-01-244 and FIC 2016 40486116 (GORE Atacama), FONDEF D04I1288 and VW Foundation, granted to the Universidad Austral de Chile (RW).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Table S1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Müller, D.G., Murúa, P. & Westermeier, R. Reproductive strategies of Lessonia berteroana (Laminariales, Phaeophyceae) gametophytes from Chile: Apogamy, parthenogenesis and cross-fertility with L. spicata. J Appl Phycol 31, 1475–1481 (2019). https://doi.org/10.1007/s10811-018-1625-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1625-9