Abstract
Two species of giant kelp inhabit the coast of Chile: Macrocystis integrifolia and M. pyrifera, representing important economic resources. As part of our efforts to domesticate these kelps for mariculture, and to obtain superior cultivars, we studied their biological relationship. Hybridization experiments with clonal gametophyte cultures showed reciprocal cross-fertility and produced fertile hybrid sporophytes with intermediate morphological characters. This hybridization potential in the laboratory contrasts with the persistence of two morphologically well-defined sister taxa in natural habitats on the Pacific coast of South America. We conclude that M. integrifolia and M. pyrifera are conspecific and speculate that unknown mechanisms support the co-existence of two morphologically distinct taxa on the subspecific level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent studies using molecular signals suggest taxonomic revisions in the brown algal order Laminariales on the family, genus and species level (Lane et al. 2006). This new approach includes the genus Macrocystis (giant kelp) on the Pacific coasts of North and South America, where M. pyrifera (L.) C. Agardh and M. integrifolia Bory are traditionally recognized as well defined sister species. In their study on evolution in the genus Macrocystis, Coyer et al. (2001) suggested that these two taxa may be unseparable at the molecular level.
Along the coast of Chile, Macrocystis pyrifera and M. integrifolia occur in geographically well separated populations, and are clearly distinguished by their holdfast morphology. Both species represent important economic resources in Chile for alginate production, and as forage for maricultured abalone (Haliotis spp.), high value herbivorous molluscs.
M. pyrifera has a southern distribution, ranging from Tierra del Fuego, Patagonia, Chiloé, Puerto Montt, Osorno, Concepción to Valparaiso. It reappears in the North on the coast of Perú (Neushul 1971). In contrast, M. integrifolia is reported from Concepción northward to Arica, and to co-exist with M. pyrifera further North along the coast of Perú (Neushul 1971; Ramírez and Santelices 1991; Hoffmann and Santelices 1997). Macrocystis pyrifera and M. integrifolia occur jointly in Pacific North America, while M. pyrifera co-exists with M. angustifolia Bory in South Africa, Australia and New Zealand (Neushul 1971; Womersley 1987).
In anticipation of environmental problems caused by over-exploitation of natural Macrocystis beds in Chile, we have developed mariculture techniques based on clonal gametophyte cultures as permanent stock. By manipulation of temperature and light regimes, successive crops of juvenile sporophytes can be produced under laboratory conditions (Westermeier et al. 2006). With our collection of male and female gametophytes of both Macrocystis species from Chile it has now been possible to study sexual compatibility within this pair of allopatric / sympatric sister taxa. A first series of crossing experiments showed that the two taxa interbreed and produce viable hybrids with intermediate characters. Although this result supports their conspecificity, we suspect that Macrocystis pyrifera and M. integrifolia are separated by unknown post-zygotic mechanisms at the sub-specific level. Experiments on long-term survival of hybrids and molecular studies with higher resolution power will be needed to resolve this question.
Materials and methods
Clonal unialgal cultures of male and female gametophytes were established and propagated following the techniques described by Westermeier et al. (2006). Parent specimens were collected in localities well within the territory of each species (Figure 1), and their identity was confirmed by inspection of holdfast morphology. While the holdfast of M. pyrifera forms a pyramid-like complex of haptera (Figures 2 and 10), the base of M. integrifolia is an elaborate, creeping, flat, ribbon, which produces multiple haptera and upright fronds in lateral positions (Figure 3). We used gametophyte clones from the following locations (Figure 1) and collection dates:
-
M. integrifolia: Caldera (int-Cal, November 2002) and Huasco, December 2003
-
M. pyrifera: Chiloé, (pyr-Cho, December 2002), and Puerto Montt, October 2003
Mating experiments were started by mixing vegetative fragments from one male and one female gametophyte culture in a 60 mm polystyrene Petri dish with 10 mL culture medium. Sexual reproduction was initiated by subjecting gametophytes to +10°C and increased white light irradiance as described by Westermeier et al. (2006). First oogonia, eggs, antheridia and spermatozoids appeared after 7–8 days, and zygote formation culminated at about 2 weeks. Three weeks after initiation numerous juvenile sporophytes were present, and the material was introduced into glass bottles with aeration and gentle agitation by magnetic stirring (Figure 4). All subsequent culture steps up to 10 cm seedlings, their fixation on supporting ropes and culture in 800 L tanks followed the descriptions given in Westermeier et al. (2006).
We carried out the following intraspecific matings and interspecific reciprocal crosses:
-
M. pyrifera female x M. pyrifera male (pyr x pyr)
-
M. integrifolia female x M. integrifolia male (int x int)
-
M. pyrifera female x M. integrifolia male (pyr x int)
-
M. integrifolia female x M. pyrifera male (int x pyr)
In addition, as control experiments we sexualized female gametophytes of M. pyrifera in the absence of a male partner in order to examine the potential significance of parthenogenesis.
Results
Intraspecific matings and parthenogenesis
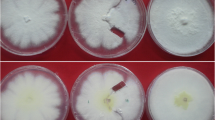
Matings of pyr x pyr have been made on numerous occasions since 2002, because they constituted the basis for the development of our Macrocystis mariculture program. Under suitable light and temperature conditions vegetative gametophyte filaments form oogonia and antheridia in varying densities. In extreme cases, entire female gametophyte thalli transformed into egg biomass. In the presence of a male pyr partner, eggs were fertilized and rapidly developed to juvenile sporophytes (Figure 5). In full-range mariculture experiments, matings pyr-Cho x pyr-Cho developed typical conical holdfasts (Figure 10), reached 14 m in length upon transplantation to the sea, and became fertile (Westermeier et al. 2006).
In parthenogenesis experiments with M. pyrifera, sexualization of potent female gametophyte clones produced large numbers of oogonia and eggs. After 17–20 days, however, when in a bisexual mating eggs had advanced to young sporophytes, the absence of spermatozoids caused massive disturbances. Many eggs expanded, vacuolized, and their cytoplasm contracted. Some eggs died, while others underwent uncoordinated cell divisions resulting in irregular aggregates (Figure 6). A very small proportion of unfertilized eggs developed to more regular multicellular structures, but polarity deficiencies were still present, combined with slow growth. No visible products survived from such parthenogenesis experiments in subsequent mariculture steps.
Intraspecific matings int-Cal x int-Cal were fully fertile, all eggs gave rise to juvenile sporophytes (Figure 7).
Interspecific crosses
In numerous hybridization experiments eggs of Macrocystis pyrifera were clearly fertilized by M. integrifolia spermatozoids. All matings pyr x int-Cal produced rich crops of healthy sporophytes indistinguishable from the results of intraspecific matings in both species (Figure 8). Sporophytes originating from one mating experiment pyr-Cho x int-Cal were subjected to our complete mariculture program up to 800 L tanks in order to study the character of adult hybrid thalli. Reciprocal interspecific crosses int-Cal x pyr-Cho produced similar crops of hybrid sporophytes (Figures 4 and 9).
Morphology and reproductive potential of interspecific hybrids
Hybrids pyr-Cho x int-Cal reached a size of 2 m after 8 months culture in our 800 L tanks. Their holdfast bases were flat and produced series of lateral haptera and new frond initials (Figure 11). From this age onward some individuals became fertile. Sorus areas appeared on somatic phylloids above pneumatocysts, even in young phylloids just below a frond apex (Figure 12). Isolated sorus fragments formed motile spores, which developed normally into gametophytes. Upon proper stimulation they produced rich crops of juvenile sporophytes, which we expect to contain interesting recombinant genotypes for mariculture purposes.
Discussion
Druehl et al. (2005) discussed in great detail the problems connected with the interpretation of inter-generic crossings in Laminariales. Such experiments often produce only low numbers of progeny, and apogamic development of sporophytes cannot be ruled out. Furthermore, in many cases hybridization products were not grown up to full maturity. The authors recommend that in such cases molecular signals be used to confirm the presence of both parental genomes in potential hybrids.
Lewis et al. (1986); Lewis and Neushul (1994) hybridized Macrocystis integrifolia, M. pyrifera and M. angustifolia from the North Pacific and Tasmania. Although various crosses resulted in viable and fertile sporophytes, the authors suggested that their species status by maintained due to clear morphological differences.
Our experiments with Macrocystis pyrifera and M. integrifolia from Chile demonstrate beyond doubt that these two taxa are inter-fertile and form viable hybrids. The total failure of unfertilized eggs in our female pyr-Cho cultures, and the dramatic effect of fertilization by int-spermatozoids in the corresponding sexual crossing (Figures 6 and 8) is self-evident. The introduction of holdfast characters by M. integrifolia spermatozoids into M. pyrifera eggs and the resulting sporophytes further confirms their hybrid character. The formation of sorus tissue in somatic phylloids above pneumatocysts in our hybrids is described for M. integrifolia (Hoffmann and Santelices 1997), but we have also seen it in M. pyrifera (R. Westermeier, unpublished observations).
According to literature reports the two Macrocystis species co-exist on the coast of Peru, and possibly in a transition zone in Central Chile. North- and south-ward drift of floating specimens into their sister species’ territory by coastal currents is very likely to occur naturally. Considering the easy and luxurious way in which the two species produce viable hybrids under laboratory conditions, the conclusion that interspecific cross-fertilization occurs frequently in natural habitats at the Pacific coast of South America seems justified. This notion contrasts strongly with the lack of documented hybrids from field collections. Instead, the two sister taxa seem to conserve their identity. Such taxon separation may be caused by a post-zygotic block that prevents long-term survival of hybrids. Since our interspecific hybrids produce functional sorus tissue, meiospores and fully functional gametophytes meiotic incompatibility must be excluded.
Abiotic factors such as water temperature and daylength show continuous north–south gradients along the coast of Chile, making it unlikely that they are responsible for the separation of the two taxa. The embracement of M. integrifolia territory by M. pyrifera at both its northern and southern boundaries supports this conclusion.
In summary, our experimental finding of luxurious hybridization between Macrocystis pyrifera and M. integrifolia suggests that these two taxa are conspecific. Nevertheless, they are morphologically distinct and maintain their separation in natural habitats in allopatric and sympatric fashion along the coast of Chile. We speculate that hitherto unknown mechanisms exist that support the separation of the two taxa in the natural habitat. Further studies at the biological, taxonomic, biogeographic and molecular levels will be necessary to resolve this question.
Over-exploitation of natural kelp biomass is an urgent problem in Chile. Our results contribute to the clarification of the relationship between the two local Macrocystis taxa, and thus have implications for the translocation of live kelp material by human activities, and in policies for emerging kelp mariculture at various locations along the Chilean coast. Hybrid formation and meiotic recombination of two genetically different parent taxa opens a new approach to generate kelp cultivars with desirable properties for different sites along the 5,000 km length of the Chilean coast. Further work, including full growth of reciprocal crosses int x pyr, and the selection of recombinant genotypes with favorable characteristics, is in progress.
References
Coyer JA, Smith GJ, Andersen RA (2001) Evolution of Macrocystis spp. (Phaeophyceae) as determined by ITS1 and ITS2 sequences. J Phycol 37:574–585
Druehl LD, Collins JD, Lane, CE, Saunders GW (2005) An evaluation of methods used to assess intergeneric hybridization in kelp using Pacific Laminariales (Phaeophyceae). J Phycol 41:250–262
Hoffmann A, Santelices B (1997) Flora marina de Chile Central. Ediciones Universidad Catolica de Chile, Santiago
Lane CE, Mays C, Druehl LD, Saunders GW (2006) A multi-gene molecular investigation of the Kelp (Laminariales, Phaeophyceae) supports substantial taxonomic re-organization. J Phycol 42:493–512
Lewis RJ, Neushul M (1994) Northern and southern hemisphere hybrids of Macrocystis (Phaeophyceae). J Phycol 30:346–353
Lewis RJ, Neushul M, Harger BWW (1986) Interspecific hybridization of the species of Macrocystis in California. Aquaculture 57:203–210
Neushul M (1971) The species of Macrocystis with particular reference to those of North and South America. In: WJ North (ed) Biology of giant kelp beds (Macrocystis) in California. Beih Nova Hedwigia 32. J Cramer Lehre, pp 211–222
Ramírez ME, Santelices B (1991) Catálogo de las algas marinas bentónicas de la costa temperada del Pacífico de Sudamérica. Monografías Biológicas No 5. Publicaciones Periódicas Pontificia Universidad Católica de Chile, Santiago
Westermeier R, Patiño D, Piel MI, Maier I, Müller D (2006) A new approach to kelp mariculture in Chile: Production of free-floating sporophyte seedlings from gametophyte cultures of Lessonia trabeculata and Macrocystis pyrifera. Aquaculture Res 37:164–171
Womersley HBS (1987) The marine benthic flora of southern Australia. Part II. Southern Australian Government Printing Division, Adelaide. pp 484
Acknowledgements
This work has been supported by FONDEF de CONICYT-CHILE, Projects D00I 1144 and D04I 1288. We thank Helga Müller, Constance for digital image processing, and anonymous reviewers for constructive remarks.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Westermeier, R., Patiño, D. & Müller, D. Sexual compatibility and hybrid formation between the giant kelp species Macrocystis pyrifera and M. integrifolia (Laminariales, Phaeophyceae) in Chile. J Appl Phycol 19, 215–221 (2007). https://doi.org/10.1007/s10811-006-9126-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-006-9126-7