Abstract
Alginates are produced industrially from marine macroalgae (also called seaweeds) belonging to the taxonomic group of brown algae (phylum Ochrophyta, class Phaeophyceae). In particular, the seaweeds commonly known as kelps (order Laminariales) are the most widely exploited worldwide as raw materials for alginate production. Alginophytes (i.e. alginate-yielding seaweeds) are mainly harvested from wild populations, although some of the raw material that is used in the alginate industry comes from the cultivation of the kelp Saccharina japonica. The demand for alginate production has increased over time, and it is likely to increase significantly in the future, particularly for the use of alginates in current and future biomedical and bioengineering applications. However, alginophyte resources are limited, and the natural kelp resources have declined worldwide in recent years. One way to meet the current and future demands of alginate-using industries is to encourage alginate production via kelp farming. The mariculture of the kelp S. japonica has already been well developed in Asia, and the cultivation of other kelp species is currently also being attempted in Europe and the Americas. This chapter provides an overview of seaweeds as a feedstock for alginate production, with emphasis on kelp farming to ensure a sustainable supply of alginates required for many applications. It describes the major stages for the cultivation of Saccharina and any other kelp, as well as the economic and environmental benefits of integrated kelp aquaculture to produce alginates, in addition to other value-added products.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Alginate extraction
- Alginate production
- Alginophytes
- Aquaculture
- Brown seaweeds
- Kelp farming
- Marine macroalgae
- Seaweed alginate
- Seaweed resources
2.1 Introduction
Alginate, also called algin, is the generic name for the salts of alginic acid or any of the derivatives of this compound. It belongs to the family of linear unbranched polysaccharides, which consists of binary copolymers of β-D-mannuronic acid (M) and α-L-guluronic acid (G) units linked together by 1 → 4 glycosidic bonds (see representation of the two monomeric units of alginic acid in Fig. 2.1). The monomers are mainly arranged in sequences of homopolymeric blocks (MM and GG blocks) and heteropolymeric blocks (MG or GM blocks) [1,2,3]. The block types and their respective chair conformations are shown in Fig. 2.2. The monomer sequence distribution in the copolymer gives rise to a flat ribbonlike structure for the MM blocks, a buckled ribbonlike structure for the GG blocks and a helix-like structure for the MG or GM blocks. The differences in conformation are due to the existence of a linkage in diequatorial position for the MM blocks, a linkage in diaxial position for the GG blocks and an equatorial/axial or axial/equatorial linkage for the MG or GM blocks, respectively. The linkage in the block structure results in varying degrees of stiffness or flexibility in alginates due to a greater or lesser hindrance of rotation around the glycosidic bonds. The polymer chains of alginates containing predominantly GG blocks are stiffer and possess a more extended chain conformation than those containing MM blocks, which in turn are stiffer than MG or GM blocks (i.e. the relative flexibility increasing in the order G block < M block < MG or GM block) [4,5,6].
Alginate structure depends fundamentally on the monomer composition, sequential structure and molecular weight of the polymeric chain. These structural parameters affect the chemical and physical properties of alginate, and these properties in turn have both biological and industrial significance [7,8,9]. Generally, chemical structure of alginate is typically described by the frequencies of monads (one monomer unit: M or G), dyads (blocks containing two monomer units: MM, GG, or MG = GM) and sometimes triads (blocks containing three monomer units: GGG, MGM, or GGM = MGG) [10,11,12,13,14]. The monad frequencies (F M and F G), the dyad frequencies (F MM, F GG and F MG = F GM) and the triad frequencies (F GGG, F MGG and F GGM, = F MGG) are preferably expressed as a mole fraction [15,16,17], although it has previously been reported as a percentage [8, 15, 18]. In addition, commercial alginate is traditionally characterized by the ratio of mannuronic to guluronic acid (M/G), which is also currently estimated from monad frequencies [3, 17, 19]. These key structural elements of alginate are obtained by applying various methods (for more details, see review in ref. [15]). Among all techniques used for the description of alginates, proton nuclear magnetic resonance (1H–NMR) spectroscopy is the most accurate method currently employed to determine both the composition and sequential structure of alginates.
One of the most important and useful properties of alginates is their ability to form gels and stabilize emulsions in the presence of certain metal cations, particularly divalent cations such as calcium (Ca2+), through a cross-linking reaction [20,21,22]. This ability is conventionally described in terms of the so-called “egg-box” model proposed by Grant and co-workers [20]. According to this model, the divalent cations are embedded into cavities formed naturally by two adjacent polymer chains containing GG blocks in a helical conformation. The alginate chains thereby adopt a structure that resembles an “egg-box”, hence the name given to this model. The mechanism for the alginate gelation may involve the ionic-bonding interaction of cations with carboxyl groups and the hydrogen-bonding interaction of these cross-linking agents with oxygen atoms, in both cases between the guluronic acid blocks of two adjacent polymer chains [5, 21, 22]. The alginate gelation process with divalent calcium cations is depicted in Fig. 2.3.
Alginate gel formation is mainly dependent on the type and concentration of cross-linking agents, as well as the composition, sequence and polymer chain length of the alginate; these features determine the physical properties of the gels formed [23,24,25]. For example, the binding affinity of alginates for different divalent cations has been shown to increase in the following order: barium (Ba2+) > strontium (Sr2+) > calcium (Ca2+) > magnesium (Mg2+), as well as increasing with the density of the crosslinkers. In addition, alginates with a high guluronic acid (G) content display a higher affinity towards these crosslinkers than do alginates with high mannuronic acid (M) content [20, 25,26,27]. Essentially, gel strength and viscosity are the two most important physical properties used to assess the gelling capability of alginates [23, 28, 29]. While the gel strength is mainly dependent on the content and length of the guluronic acid (G) in the alginate [26, 28, 30], the viscosity of an alginate solution is directly determined by the alginate concentration and the chain length of the alginate polymer, which is proportional to its molecular weight [17, 31, 32]. Generally, alginates rich in guluronic acid are known to form strong but brittle gels, whereas those rich in mannuronic acid or mixed sequences form weaker but more flexible gels [27, 33, 34]. Thus, gel strength has also been shown to increase in the order of GG block > MG block > MM block [26, 27].
The physical and chemical properties vary considerably among different commercial alginates. This natural variability in alginates provides a wide range of functional properties that determine their use in specific applications and thus also their commercial value [8, 9, 34]. Furthermore, enzymatic and chemical modifications have been used to manipulate the composition, sequential structure and molecular weights of alginates, and their derivatives exhibit novel or improved functional properties for specific high-value applications [35,36,37].
Alginate was discovered in 1881 by the British pharmacist Stanford [38, 39], and it has since become one of the most useful and versatile polymers, used in a wide range of industries. Because of their gelling, thickening, emulsifying and stabilizing properties, alginates have been commonly employed in the food, textile printing, papermaking and pharmaceutical industries, as well as for many other purposes. Alginates are especially important in the food and beverage industry, in which they are used as food additives or functional food ingredients in a vast array of different dairy products [9, 33, 40]. Alginates are internationally accepted food additives and are therefore explicitly listed as human food ingredients by the European Union (EU) and as “generally recognized as safe” (GRAS) by the US Food and Drug Administration (FDA), as well as being recognized as such in the United Nations Codex Alimentarius (Latin for “Food Code”) established by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO). In particular, the reference codes of the European Union for the different alginates used in the food industries are E400 (alginic acid), E401 (sodium alginate), E402 (potassium alginate), E403 (ammonium alginate), E404 (calcium alginate) and E405 (propylene glycol alginate, usually abbreviated as PGA) [15, 40].
More recently, the alginates have found a wide variety of applications in biomedical and bioengineering fields. The interest in and use of alginates for biomedical applications has expanded considerably in recent years because of alginates’ unique and favourable properties such as gelling capacity, biocompatibility, biodegradability and lack of toxicity as well as their biological and pharmacological activities [7, 8, 41]. The current biomedical applications of alginates are the focus of this book, and an updated and detailed review of the subject may be found in the different chapters. Although the food and textile uses are still the most important markets worldwide for alginates, there are growing markets in the bioscience, bioengineering and medical fields. The demand for alginate production has increased during recent years, and it is likely to increase significantly in the future, particularly for their use in current and future biomedical and bioengineering applications worldwide [42,43,44].
Alginates occur naturally as a major structural component in marine macroalgae (also called seaweeds) belonging to the taxonomic group of brown algae (phylum Ochrophyta, class Phaeophyceae) [42, 44, 45] and are also produced as extracellular polysaccharides (exopolysaccharides) by some bacteria belonging to the genera Pseudomonas and Azotobacter [46,47,48]. Currently, all commercial alginates are produced solely from brown seaweeds [43, 44] because most species contain large amounts of alginate [44, 49, 50] and because of the availability of seaweed resources, as they can be harvested from natural populations and farmed in the sea [42, 51, 52]. The industrially most important seaweeds used worldwide for alginate production are the species commonly known as kelp (order Laminariales) [42,43,44]. This review focuses on the source of seaweed alginate, the process for alginate extraction and the availability of seaweed resources and their exploitation. It also summarizes the techniques that have been developed for the commercial-scale farming of kelps as well as describes the important environmental benefits associated with their cultivation.
2.2 Alginate Production from Marine Macroalgae
2.2.1 Seaweeds Used as Alginate Sources
The term algae (singular, alga) is commonly used to refer to a large and diverse group of aquatic photosynthetic organisms that can grow in marine, brackish and freshwater environments. Based on morphology and size, algae are generally grouped into two categories: macroalgae and microalgae. Macroalgae are multicellular forms, often with plant-like structures, ranging in length from a few millimetres up to 50 m, which typically live on hard-bottom substrates (i.e. benthic) of coastal marine habitats. In contrast, microalgae are unicellular or simple forms with a size range of a few micrometres up to hundreds of millimetres, which typically grow suspended in water [53, 54]. Marine macroalgae, so-called seaweeds, are classified primarily on the basis of their photosynthetic pigment composition into three different phyla (taxonomic groups): Ochrophyta (brown algae), Rhodophyta (red seaweed) and Chlorophyta (green algae) [55]. For example, the presence of the pigment fucoxanthin is responsible for the characteristic yellow-brown colour of brown algae. In addition, these taxonomic groups also differ in many ways, particularly in their morphology, life history, storage compounds and cell wall polysaccharides [53, 54].
Alginate is characteristically present in most or all species of brown algae, which belong to the class Phaeophyceae (phylum Ochrophyta, formerly named Phaeophyta), as a structural component of the matrix of the cell wall and intercellular regions. In these seaweeds, alginate is found in the form of insoluble mixed salts of alginic acid, mainly with calcium and to a lesser extent with sodium, potassium, and magnesium, strontium and barium, among other ions naturally found in seawater [1, 56, 57]. Its biological function is primarily skeletal, giving the algae both the mechanical strength and the flexibility necessary to withstand the force of the sea. Indeed, functional differences in the alginate content and structure of seaweeds have been reported. For example, the seaweeds growing in more wave-exposed habitats have alginates with higher mannuronic acid content than those in wave-sheltered habitats, providing greater flexibility to withstand the wave action [58,59,60]. It has also been observed that the part of the thallus (plural, thalli) that attaches the algae to a hard substrate (the so-called holdfast) contained more guluronic acid than in the rest of the thallus, giving it more rigidity and thereby affixing it more firmly to the rock [59,60,61]. In addition, alginate plays important roles in high ion-exchange equilibrium with seawater as well as functioning in retarding desiccation when the seaweeds are exposed to the air during low tide [56, 57, 62].
Brown seaweeds of the class Phaeophyceae (Ochrophyta) and in particular some species of the orders Laminariales and Fucales (commonly known as kelps and fucoids, respectively) have large amounts of alginate, comprising up to 55% of their dry weight (Table 2.1). Both kelps and fucoids are the largest and most structurally complex brown seaweeds. Generally, and in particular in kelps, the body or thallus of the macroalgae consists of a holdfast (root-like), stipe (stem-like) and blade (leaf-like) (see Fig. 2.4, in which some kelp species are illustrated). At present, commercial alginates are produced mainly from brown seaweeds of the genera Laminaria, Saccharina, Lessonia, Macrocystis, Durvillaea, Ecklonia and Ascophyllum [42, 43] (Fig. 2.4). Specifically, the industrially most important alginate-yielding species (alginophytes) are currently the kelps (Laminariales) Macrocystis pyrifera, Laminaria hyperborea, Laminaria digitata, Saccharina japonica, Lessonia nigrescens species complex, Lessonia trabeculata, Ecklonia arborea and Ecklonia radiata as well as the fucoids (Fucales) Durvillaea potatorum and Ascophyllum nodosum [42, 43] (more information on these alginophyte resources will be described in Sect. 2.2.3).
The industrially most important alginate-yielding seaweeds worldwide (Data from Refs. [42, 43]. Taxonomic classification based on Algaebase [55]. The drawings are not completely to scale between the different seaweeds in order to improve the view. Note that, when referring collectively to some or all of the species in a genus, the generic name is followed by spp)
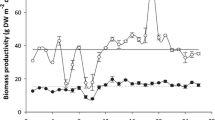
Tables 2.1 and 2.2 present the alginate yield and chemical composition of the main kelp and fucoid species used worldwide for alginate production. These parameters vary considerably between and within brown seaweed species. Based on these data, the highest levels of alginate are found in Durvillaea potatorum and Macrocystis pyrifera, in which alginate represents up to 55% and 45% of the dry seaweed weight, respectively, while the lowest levels are in Ascophyllum nodosum and Laminaria hyperborea, in which alginate represents 12% and 13% of the dry weight. Regarding structural parameters, Lessonia trabeculata and Laminaria hyperborea have the highest fraction of guluronic acid (M/G ratio of <1), while the lowest proportions of guluronic acid are observed in Durvillaea potatorum and D. antarctica and to a lesser extent in Saccharina japonica, Macrocystis pyrifera and Ascophyllum nodosum (all with M/G ratios of >1.5). Brown seaweeds are well known to exhibit some seasonal variation both in alginate chemical structure and alginate content, which can be higher or lower depending on the species [49, 50, 75]. For example, the content of alginate in Macrocystis pyrifera is highly variable throughout the year, in contrast to that in Laminaria digitata, which is much more stable (Fig. 2.5). Similarly, there may also be seasonal differences in the structural characteristics of alginate, as in the case of Saccharina latissima, whose M/G ratio varies seasonally. However, there is hardly any variation in the M/G ratio over the course of a year in species such as Laminaria digitata (Fig. 2.6). The seasonal variability of alginate is related mainly to seasonal changes in temperature as well as to nutrient and light availability, most often influencing seaweed growth [76,77,78]. It has been reported that the highest values of alginate in some kelps and fucoids occur in the summer months [49, 50, 70]. However, in general, it appears that there is no overall pattern of seasonal variation, and the same applies to the composition of alginates. Thus, it is essential to know the seasonal composition of alginates in brown seaweeds in order to determine the optimal harvesting time by which to obtain not only higher quantities but, above all, alginates of better quality, i.e. those with high G content.
Seasonal variation in mannuronic to guluronic acid ratios (M/G) of alginate from the brown seaweeds Saccharina latissima (SL) and Laminaria digitata (LD) (Data from Ref. [64])
Nevertheless, alginate content and structure also depend on the age and part of the seaweed used [50, 59, 61] as well as on the environmental conditions of the habitat in which the seaweed grew [58, 59, 64]. In general terms, thalli from older seaweeds are richer in mannuronic acid than those from younger specimens [17, 33]. In addition, compared with the blades, the stipes of kelp species generally contain a higher amount of alginate rich in guluronic acid [62, 75, 79]. Indeed, the highest content of guluronic acid in alginate is obtained from stipes of the kelps Lessonia trabeculata (F G of 0.78) and Laminaria hyperborea (F G of 0.68) (Table. 2.2). Thus, alginate companies produce guluronic acid-rich alginates (designated high G) prepared from stipes of these species [43]. As mentioned above, differences in alginate composition in relation to the hydrodynamic environment of the seaweeds have also been reported [58,59,60].
2.2.2 Alginate Extraction from Seaweeds
Various commercial types or forms of alginate (sodium alginate, potassium alginate, ammonium alginate, magnesium alginate, calcium alginate and propylene glycol alginate, commonly abbreviated as PGA) are prepared from brown seaweeds [80]. All these derivatives of alginic acid are industrially produced following the same manufacturing process that will be described here based on sodium alginate extraction. The process of alginate extraction from seaweeds is based on the conversion in an alkaline medium of the water-insoluble mixed salts of alginic acid from algal cell wall matrix to water-soluble salts, normally sodium alginate, followed by precipitation and purification [42, 81, 82]. This conventional procedure for alginate extraction has been well studied during the last decade to optimize the yield and quality of alginate for various applications [81, 83, 84]. Today, it is commonly used in the industry to produce alginates from brown seaweeds [42, 80].
The following will present in detail each of the steps constituting the process of producing seaweed alginate. The alginate extraction procedure is schematically illustrated in Fig. 2.7. Generally, it consists of three major steps: (1) pre-extraction, (2) neutralization and (3) precipitation/purification [42, 81, 82].
In the first step, the seaweeds (usually dried) are washed with distilled water and then ground to speed up the chemical reactions for the extraction of alginic acid. Further, 0.1% formaldehyde solution may be added in order to avoid pigments in alginate; this has been seen to increase the alginate yield. The milled algal biomass is then dissolved and stirred with a dilute mineral acid up to pH 4 (usually hydrochloric acid (HCl) or calcium chloride (CaCl2) at 0.1–0.2 M) to remove counter ions (Ca2+, Na+, Mg2+, Sr2+, etc.) of algal alginate by ion exchange with protons from the acid [81, 85]. The acid treatment is also effective in removing potential contaminants or impurities (fucoidans, laminarins, proteins and polyphenols), leading to a higher final yield and purity of alginate. In addition, 85% ethanol can be used to extract pigments and proteins in this process [16]. This pretreatment is often repeated several times to ensure full extraction of alginic acid. At the end of this process, the supernatant (residual algal particles) is eliminated [81, 85].
In the second step, the insoluble alginic acid in the seaweed-water mixture is brought to pH 9–10 with an alkaline solution (usually sodium carbonate (Na2CO3) or sodium hydroxide (NaOH)) to form water-soluble sodium alginate. In this process, the mixture is mechanically stirred, and the temperature is maintained at 60–80 °C. The insoluble algal residues are removed by extensive centrifugation and subsequent filtration (up to 0.2 μm pore size), thereby obtaining the sodium alginate in aqueous solution [16, 81].
In the third step, the sodium alginate solution can be precipitated into sodium alginate, calcium alginate or alginic acid by the addition, respectively, of alcohol (usually ethanol (C2H6O)), calcium chloride (CaCl2) and hydrochloric acid (HCl). These three methods are therefore known as the sodium alginate process or ethanol route, the calcium alginate process or CaCl2 route and the alginic acid process or HCl route [42, 81, 85]. The sodium alginate precipitated via the ethanol route is obtained directly by the addition of ethanol, and it is separated by solvent extraction/evaporation. The CaCl2 route first produces a precipitated calcium alginate that is isolated by sieving and rinsed with distilled water to remove the excess calcium. It is then converted to alginic acid by acid treatment, generally using hydrohydrochloric acid (HCl) as described above in the first step. The HCl route directly yields alginic acid, which is separated from the solution by simple flotation and centrifugation. The resulting alginic acid can also be reconverted by alkaline neutralization to any of the commercial forms of alginate in the same manner as described in the second step. Specifically, sodium carbonate (Na2CO3), potassium carbonate (K2CO3), ammonium carbonate ((NH4)2CO3), magnesium carbonate (MgCO3), calcium carbonate (CaCO3) or propylene oxide (C3H6O) is added in order to obtain the following alginates, respectively: sodium alginate (Na-alginate), potassium alginate (K-alginate), ammonium alginate (NH4-alginate), magnesium alginate (Mg-alginate), calcium alginate (Ca-alginate) and propylene glycol alginate (PGA). Finally, all alginates form a paste that is separated, dried and milled. Commercial alginates produced specifically for biomedical purposes (e.g. ultrapure and amitogenic alginates) are prepared using more rigorous extraction processes to remove any biological and inorganic impurities, and companies consider these processes confidential. However, the alginates obtained from the described methods usually contain some impurities, making them unsuitable for some biomedical applications. In this case, an alternative extraction method using barium ions (Ba2+) is used in the precipitation process due to their high binding affinity and selectivity towards alginates. Subsequently, the Ba2+ from the alginate is exchanged for sodium ions to form sodium alginate, which can be precipitated using ethanol [36].
It is known that the alginate extraction process can influence the yield and chemical compositions as well as rheological properties of the isolated alginates [42, 81, 85]. To illustrate these effects, Table 2.3 summarizes the comparative results of three precipitation methods in the process of alginate extraction from the brown seaweed Macrocystis pyrifera. According to these data, the sodium alginate process or ethanol route gives the highest yield and rheological properties of alginates, although very similar results can be obtained from the alginic acid process or HCl route. Clearly, the calcium alginate process or CaCl2 route results in an alginate with poor viscoelastic properties and low toughness [85].
Overall, it is also important to control both pH and temperature during all extraction processes to improve the yield and quality of the alginate obtained. For example, it is well known that acid treatment at a pH lower than 4 may break bonds of the polymer chain, decreasing the alginate viscosity [81, 84, 85]. In addition, it has been shown that high temperatures (higher than 80 °C) and longer extraction processes increase yield but decrease viscosity [81, 84].
2.2.3 Alginophyte Resources Worldwide
Brown seaweeds have been worldwide resources for alginate extraction since industrial alginate production began in 1929 in California, USA, shortly thereafter, beginning in 1939 in several European countries and Japan and, more recently in the 1980s, in China [42, 43, 81]. Currently, the alginate industry is concentrated into 15 factories in 6 different countries (China, the USA, the United Kingdom, Japan, Chile and Germany), and most of these factories are now in China (see compilation in ref. [86] for more details on commercial companies selling alginates). According to the latest available data for 2009, the world market for alginates is approximately 26,500 tons (dry weight), with an estimated sale value of US$ 318 million annually [43]. Alginate production worldwide is derived from seaweed resources, most of which are harvested from the wild, except in China, where seaweed is sourced mostly from aquaculture [42, 43, 81].
Although there are over 2000 species of brown seaweeds (phylum Ochrophyta, class Phaeophyceae) [55], only some species of the orders Laminariales and Fucales (kelp and fucoid) are exploited worldwide as raw material for alginate production. It is estimated that there currently are at least 38 species of kelps and fucoids grown in 24 countries that are used worldwide to produce alginates [51, 52, 87]. A list of alginate-yielding seaweeds (or alginophytes) and their countries of origin is provided in Table 2.4, which includes the full names of the species and their taxonomic classifications (updated). However, many of these seaweeds are harvested from small local stocks and are therefore not available on the international market. In addition, some seaweed species (e.g. Sargassum species) are only used occasionally for alginate production, when the main commercial sources are unavailable, because their alginate is usually judged to be of “borderline” quality [42, 43]. Thus, the global alginate production worldwide comes from a small number of seaweed species, specifically the kelps Macrocystis pyrifera, Laminaria hyperborea, L. digitata, Saccharina japonica, Lessonia nigrescens species complex, L. trabeculata, Ecklonia arborea, and Ecklonia radiata and the fucoids Durvillaea potatorum and Ascophyllum nodosum [42, 43, 80]. These alginophytes, in addition to containing large amounts of alginate [43, 44, 51], form dense stands on shallow rocky shores commonly referred to as forests or beds. These seaweed species are distributed in cold-temperate waters around the world, and as photosynthetic organisms, they are restricted to habitats with appropriate light levels, primarily from the intertidal zone to a depth of 50 m in the sublittoral zone.
The quantities of alginophytes harvested worldwide in 2009 are summarized in Table 2.5, including the main producer countries and the quality or type of alginate obtained from seaweed species. These statistics are based on the most up-to-date and reliable estimates available [43], but some species names have been updated to the current taxonomy [55]. Indeed, recent revision of kelps based on the application of molecular techniques has greatly improved the taxonomic understanding of this group, resulting changes in the taxonomic identity and nomenclature of some commercialized species. For example, some species of the former Laminaria sensu lato have been transferred to the new genus Saccharina, including the cultivated Laminaria japonica (now Saccharina japonica) [89]. Lessonia species have also undergone taxonomic changes, and the former Lessonia nigrescens is now a species complex, i.e. encompassing multiple species that were hidden under a single name. Currently, there is genetic evidence of the presence of at least two cryptic species: Lessonia berteroana and Lessonia spicata [90, 91]. Moreover, Lessonia flavicans, which has been reported traditionally in the alginate marketplace [43], actually corresponds to either Lessonia nigrescens or L. trabeculata [88, 92]. Finally, all species of the genus Macrocystis are now considered taxonomic synonyms of Macrocystis pyrifera [93, 94]. Independent of the current taxonomic status of seaweeds, the commercial companies selling seaweed or algal products generally continue to maintain the commercial names of their raw materials or products [92]. However, an adequate specific identification of seaweed species used as alginate sources is particularly important because of effects on chemical compositions and properties of the isolated alginate (see Sect. 2.2.1).
Based on data for 2009 from Table 2.5, the world harvest of alginophytes is estimated to be approximately 95,000 tonnes (dry seaweed weight) annually. The main alginophytes harvested worldwide are Lessonia and Laminaria, accounting for 65% of the total production, followed by Saccharina with 21% of the total. It is worth highlighting the drastic reduction in the harvest of Macrocystis and Ascophyllum, which were previously important raw materials for alginate production.
In the year 2009, these seaweeds supplied only 2% of the worldwide production, down from 58% in 1999 [43]. The reason for this change is the marked decrease in the use of these species because their alginate has low guluronic acid (G) content, while the current market is demanding alginates with intermediate or high G content [43, 81]. These commercial-grade alginates are now mainly used in biomedical applications and novel therapies [7, 36, 37], which have increased considerably in the last decade and are currently the most profitable, selling at the high end of the alginate market [43]. Regarding the kelp Macrocystis, harvesting has also been curtailed for ecological reasons due to concern about potential environmental effects of exploitation of kelp forests that provide habitat for many species [43].
The world alginate production is mainly produced from seaweed resources in 13 countries (Table 2.5, Fig. 2.8). Global distribution of alginophytes harvested industrially harvested from wild populations to meet the global demand for the alginate industry. Traditionally, harvesting of natural resources has been performed by hand, but the demand for larger quantities of seaweeds for alginate production at lower costs has led to the development of mechanized harvesters in some areas of California, Norway and France. However, alginophyte exploitation is still performed manually in most countries, such as Chile, Peru, Ireland, the United Kingdom, South Africa, New Zealand and Australia [42, 88, 95,96,97,98]. Macroalgae can be harvested manually on the shore by cutting attached seaweeds as well as by collecting drift or beach-cast seaweeds. For example, in some parts of Europe, the fucoid Ascophyllum nodosum and the kelp Laminaria digitata are cut by hand using a small knife at low tide while the seaweed is uncovered or while it remains submerged in less than a metre of water [42, 97]. Durvillaea and Ecklonia in Australia, and New Zealand and Lessonia in Chile are collected as drift or beach-cast, where they are washed ashore in large quantities by tides and waves [42, 88, 95, 98]. Mechanical harvesting using specially designed boats is done in Southern California (USA) and Baja California (Mexico) with Macrocystis pyrifera, in Norway with Ascophyllum nodosum and in France with Laminaria digitata [42, 99]. The Macrocystis forests are harvested using mowers from special ships that are fitted with underwater cutter bars at the front or rear that cut the seaweeds at 1 metre below the surface and a conveyor belt that moves the biomass into the hold of the vessel (Fig. 2.9) [42]. Small, flat-bottomed boats equipped with suction cutters and driven by paddlewheels or water jets are used for harvesting Ascophyllum nodosum in Norway [42, 99]. Exploitation of Laminaria hyperborea on the coast of Norway is performed using trawler boats with a cutting dredge that is towed through the kelp beds to cut the seaweeds. In France, Laminaria digitata is harvested by boats with a hydraulic arm fitted with an iron hook on the end (called a “scoubidou”) that rotates to wrap the kelp around itself [42, 99].
It has been demonstrated that kelp harvesting in various parts of the world may cause deterioration of natural resources or habitats or disturbance of species [100,101,102]. Kelps act as ecosystem engineers or foundation species, providing habitat, protection and food for numerous organisms in coastal ecosystems, in the same way as terrestrial forests [103, 104]. Over the last several years, there has been an increase in governmental control over the exploitation of natural seaweeds populations to limit their misuse. Some countries, depending on the state of the specific resource, allocate harvest quotas and/or establish different management and control measures to ensure the conservation of kelp forests and to lower the impact of their exploitation on marine ecosystems. Such measures may include establishing fallow periods of several years for areas subject to harvest, allowing only the collection of the upper part of the thallus from perennial seaweeds, limiting harvesting during non-reproductive periods, or even prohibiting the exploitation of endangered species and/or those with high ecological value [102, 105, 106]. In addition, kelp forests are also exposed to a range of disturbances of natural and/or anthropogenic origins. Particularly in recent years, kelp populations have declined in many areas of the world due to environmental stress caused by climate change, among other factors, especially by the increase in sea temperature and disruption in the natural nutrient availability patterns [107, 108].
Seaweed exploitation in Asia intended for alginate production mainly comes from commercial cultivation of the kelp Saccharina japonica (kombu) in China [42,43,44]. Kelp cultivation techniques have been well developed in Japan and China, where several Asian species have been cultivated on a large scale since the 1960s [109, 110]. Today, aquaculture in Asia provides almost all of the global production of S. japonica, reaching over 900 thousand tonnes dry weight of seaweeds (estimated from fresh weight data) in 2014 [111], of which until now only a small part has been used for alginate extraction, but, as we have seen (Table 2.5), accounting for more than 20% of world alginate production [43]. Current practices of kelp farming in Asia have contributed not only to significantly increasing production to meet commercial demands for various uses, including alginate production but also to conserving natural populations and the ecosystems that they produce. Recently, mariculture of kelp species has also generated great interest in Europe and the Americas, as it may lead to increased production for commercial uses and potential applications; in addition, it may help protect the kelp forests from overharvesting [112,113,114]. The current limitations on the availability and use of natural kelp resources are expected to become the major driving force for the growth and development of farming of kelp species for alginate production, as has already been the case in Asia. In fact, it is now widely recognized that a transition from seaweed extraction to aquaculture is needed to meet the growing demand and to avoid the decline or loss of natural populations [115, 116]. At present, cultivation of kelp species is currently also being attempted in several countries of Europe and the Americas [112,113,114]. The techniques and biological basis required for full-cycle cultivation of Saccharina, as well as for other kelp species, will be described in the next section.
2.3 Cultivation of Saccharina and Other Kelp Species
2.3.1 Key Biological Aspects
Kelps are characterized by a heteromorphic life cycle that alternates between a haploid generation formed by microscopic filaments (known as the gametophyte because it produces gametes) and a diploid generation formed by a macroscopic thallus (called the sporophyte because it produces spores) [117]; the different life-history stages are shown in Fig. 2.10. Most kelp species have a perennial (i.e. lasting several years) sporophytic phase, during which the sporophyte may reach a length of several metres depending on the specific seaweed taxon (e.g. up to 50 m in Macrocystis, up to 15 m in Ecklonia, up to 10 m in Durvillaea, up to 4 m in Lessonia, up to 5 m in Saccharina japonica, up to 2 m in Laminaria hyperborea and up to 1 m in Laminaria digitata [55]).
Large sporophytes are commercially exploited for different uses, such as the extraction of alginates [42, 43, 99]. Sporophyte morphology of kelps varies depending on the species and the environmental conditions in which they grow. However, three parts are typically recognized: the holdfast, stipe and blade (described in Sect. 2.2.1; see Figs. 2.4 and 2.10) [55, 118]. The gametophytic phase, in contrast, consists of slightly branched male and female filaments composed of round-shaped cells smaller than 50 μm in diameter. Microscopic gametophytes are a survival strategy for the sporophyte, enabling long-term resistance to adverse environmental conditions while it waits to reproduce and form new sporophytes. The filaments of gametophytes may remain dormant or grow vegetatively, although their growth is generally much reduced [119,120,121].
Most kelps are distributed along the rocky shores of the Arctic and the cold-temperate regions of the Northern and Southern Hemispheres, where temperatures are generally below 20 °C. Temperature is therefore a key environmental factor that affects not only the distribution of kelp species but also their growth [122,123,124]. The perennial sporophytes of kelps generally exhibit strong seasonality in their development with a period of rapid growth during winter and spring and a period of minimal growth during the summer and fall, which coincides with the seasonal temperature and nutrient cycles in cold-temperate waters. In winter, temperatures are lower, and nitrogen levels are higher. In contrast, temperatures are higher and nitrogen levels are often negligible during summer [118, 125]. Another important environmental factor influencing the development of kelp sporophytes is water movement, which affects nutrient assimilation and gas exchange by determining passive transport across the diffusion boundary layer of the algal surface [126].
2.3.2 Background of Kelp Farming
Cultivation practices are rooted in Asia in the eighteenth century, during which different methods were used to expand the populations of edible kelps as a natural resource [127, 128]. However, these practices depended entirely on the natural environment since there was no control over the biological cycle of these seaweeds. The scientific basis for the development of the full-cycle cultivation of Saccharina and other kelp species was first established in the middle of the twentieth century, and since then, techniques have been established in Asia to obtain seedlings from spores under more-or-less controlled laboratory conditions. This Asian technique of seedling production enabled the subsequent development of different types of floating rafts for cultivating kelp in the sea, and beginning in the 1960s, commercial kelp mariculture extended to different regions of Japan, China and Korea, where it was promoted by different governments to meet the demand for human consumption in a context of insufficient natural resources [110, 127,128,129]. Currently, Saccharina japonica is the most extensively cultivated kelp species in these countries.
In Europe and the Americas, different cultivation practices were initiated in the 1980s and 1990s to study the viability of native kelps [120, 130,131,132,133]. As an alternative to the Asian method of seedling production, a European technique was developed to produce kelp seedlings from gametophyte cultures [120, 134]. Research showed that kelp cultivation using simple, relatively low-cost techniques was biologically and technically feasible, but these early attempts at cultivation did not continue due to a lack of interest since the available wild kelp stocks were sufficient to meet commercial demand and their exploitation was considered more profitable than cultivation. However, there is currently a growing interest in the development and optimization of kelp species cultivation in several European and American countries; in fact, early cultivation practices have already begun on a commercial scale. This change is due to the growing demand for these species for different high-value commercial uses as well as the important environmental benefits that their cultivation would provide [112, 113]. Marine macroalgae use carbon dioxide and nutrients to grow and may thus contribute to the reduction of atmospheric CO2, which is a contributing factor to climate change [135,136,137,138], and of the amount of inorganic waste that is discharged into marine coastal areas [139,140,141,142]. In particular, seaweed farming is considered to be the basis for the development of sustainable aquaculture because they can absorb some of the inorganic nutrients that are produced, for example, in the aquaculture of mussels and fish [114, 143,144,145,146,147,148] (Fig. 2.11). To date, sea farming has been tested with success for the following kelp species: Macrocystis pyrifera in Chile [149]; Laminaria digitata in Ireland [150]; Saccharina latissima in several countries in Europe [113], Canada [142] and the USA [139]; Saccharina longicruris in Canada [151]; Lessonia trabeculata in Chile [152]; Alaria esculenta in Canada [142] and in Ireland [153]; and finally Undaria pinnatifida in Japan, China and Korea [110] and in France and Spain [154]. In general, the techniques developed for the commercial-scale farming of the kelp Saccharina in Asia [109] have been adapted to the cultivation of other kelp species in Europe and the Americas [113, 149].
2.3.3 Cultivation Steps and Methods
As with other kelps, full-cycle cultivation of Saccharina consists of two very different phases associated with their characteristic life cycle (Fig. 2.10). In the first step (laboratory-culture stage), kelp seedlings are produced on strings (commonly known as seed-strings) under controlled environmental conditions in the laboratory, and in the second step (sea-culture stage), the seed-strings are attached to ropes on floating rafts for cultivation at sea until the sporophytes reach a certain size and are harvested. The main steps in kelp farming are summarized schematically in Fig. 2.12 and described in more detail.
Diagram summarizing the steps in kelp farming, which include the production of seedlings on strings (laboratory-culture stage) and their subsequent attachment to culture ropes for growth in a floating raft culture (sea-culture stage) (Adapted and reprinted from Ref. [113], Copyright 2016, with permission from Elsevier)
2.3.3.1 Laboratory-Culture Stage
The traditional Asian seedling production method is performed by sowing strings of spores extracted from mature kelp sporophytes from natural populations [110, 129]. However, an alternative method was developed to culture kelp seedlings from “free-living gametophytes” [120, 134], which has important advantages over the traditional approach, such as the possibility of genetic selection, the creation of clones, the cryopreservation of strains and the generation of large quantities of gametophytes through vegetative growth that can produce seedlings at any time of the year [119]. This method is currently being successfully used to cultivate different kelp species in Europe [119, 150, 153], the Americas [151, 155] and in Asia, albeit in a more limited way [156, 157]. Given its extensive role in cultivation, the production of kelp seedlings from gametophyte cultures is specifically described here.
The production of seedling strings under laboratory conditions is divided into two phases: a first phase to create a culture of free-living gametophytes maintained with aeration under controlled environmental conditions (Fig. 2.13) and a second phase where gametophytes are sown on strings and grown in tanks, in which gametogenesis is induced so that, after sexual reproduction, seedlings develop (Fig. 2.14).
Gametophyte cultures are obtained from the germination of spores extracted from the fertile parts (i.e. reproductive structures) of mature sporophytes that are generally obtained from natural populations or cultures. The formation of reproductive structures (called sori or sporophylls) in kelp sporophytes can also be induced in some species under short-day or long-day photoperiods [158, 159]. Spore germination and the subsequent development of gametophyte cultures are performed in culture flasks containing sterile, nutrient-enriched seawater under environmental conditions specific to each kelp species [119, 149, 150, 156]. The entire process is carried out in environmental culture chambers or incubators that are designed to rigorously control temperature and light (considering irradiance, the light spectrum and photoperiod) and to aerate the cultures (Fig. 2.13).
Adequate aeration of free-living gametophytes is maintained by bubbling within the culture flasks (Fig. 2.13), which homogenizes light and promotes nutrient availability in the culture medium. Gametophytes are usually conserved in their dormant or slow-growth states, although vegetative growth can be promoted by filament fragmentation under specific environmental conditions to increase their biomass. However, the growth rate of kelp gametophytes is generally very low, so large quantities of spores are usually obtained and germinated to have sufficient reserves of gametophytes [119]. It is important to note that the gametophyte culture also acts as a germplasm bank for ex situ kelp conservation, as it can be indefinitely maintained in vivo under suitable environmental conditions [119, 121, 160]. Seedlings or early sporophytes can be obtained from the gametophyte collections of a germplasm kelp bank for sea culture and for the repopulation of coastal areas that have been degraded by human activities and/or natural processes [161,162,163,164].
For indoor production of seedlings, a selection of cultures with a suitable proportion of male and female gametophytes (generally a 1:1 sex ratio) are sprayed onto strings that are wound around a rigid coil or frame called a collector. To promote the attachment of gametophytes, the strings are pretreated by boiling followed by successive washes with distilled water, bidirectional sanding and, finally, a surface burn using hot air guns to remove any filaments produced by sanding [119, 165]. The collectors with the strings seeded with gametophytes are immersed in embryogenesis tanks, in which temperature, light (irradiance, the light spectrum and photoperiod) and water movement (by aeration) are under absolute control (Fig. 2.14). In these tanks, which contain sterile seawater enriched with nitrates and phosphates, sexual reproduction is induced under environmental conditions specific to each kelp species [119, 149, 150, 156], which allows zygotes to be fertilized, first giving rise to embryos and later to seedlings (i.e. early sporophytes) (Figs. 2.10 and 2.14). Generally, seedlings are embryos with polystromatic thalli (i.e. composed of many layers of cells) that are normally more than 2 mm long, in which the stipe-blade area begins to differentiate.
2.3.3.2 Sea-Culture Stage
To grow young sporophytes in the sea, the seedling strings are primarily attached to the culture ropes by two methods (Fig. 2.15). In the first, a continuous string is helically wound around the culture rope, while in the second, pieces of cut string are woven into the structure of the culture rope at regular intervals.
The culture ropes are deployed in the sea in floating culture rafts, the main elements of which include an anchoring system, a floating structure and culture lines. Figure 2.16 shows the different culture rope arrangements that are usually used in kelp mariculture [110, 113, 127,128,129, 149]. The hanging rope culture is used in protected areas, while the horizontal rope culture is used in the most exposed areas, as it better resists strong waves and currents. In horizontal culture, the light is homogeneous along the rope; thus, production is greater, and it may be used in shallow areas and highly turbid conditions. In the hanging culture, light decreases with depth, so growth is irregular. To minimize this effect, this approach is usually used in clear water and within the optimal depth range of the species. Culture rafts may be configured to regulate the depth of the culture ropes and thus control light conditions, so rafts can be adapted to the needs of the kelp species or the individual culture.
Floating raft culture with different rope arrangements: hanging rope method (vertical type or garland type) and horizontal rope method (long-line type) (Adapted and reprinted from Ref. [113], Copyright 2016, with permission from Elsevier)
In Saccharina mariculture in Asia, three different methods have been used: two-year cultivation, forced cultivation and cultivation by transplanting [128, 129, 166]. Due to the natural biannual growth cycle of these kelps, two-year cultivation requires approximately 20 months of cultivation in the sea to obtain sporophytes of commercial size. An alternative method has been developed in Asia that involves the production of seedlings during the summer, allowing earlier cultivation and thus reducing the growth period in the sea to 10 months to obtain adult sporophytes. This method of sea cultivation, termed forced cultivation, expanded rapidly and became the main method for the commercial cultivation of Saccharina in Asia. In cultivation by transplanting, young sporophytes obtained by thinning cultures are normally used in combination with the above cultivation methods to increase Saccharina production [128, 129, 166].
The outplanting and harvesting times of kelp cultures vary by species but are mainly related to the temperature and nitrogen concentration in the sea (Fig. 2.17). In addition, there are important differences in these environmental factors between regions (and even localities), which cause variability in outplanting and harvesting periods between different areas for the same species. Generally, the cultivation period in cold waters starts earlier and has a longer duration with several possible harvests; the cultivation period in temperate waters starts later and has a shorter duration with a single final harvest [113, 127, 128]. Moreover, in some regions of China where seawater nitrogen concentrations are very low, sea fertilization is performed as part of the commercial cultivation of Saccharina [167]. Furthermore, the hydrodynamic conditions of the growing site are an important factor to consider when determining the most suitable locations for kelp cultivation, as they affect culture growth and production. Moderately exposed conditions tend to favour increased kelp production [113, 154, 168, 169].
Cultured sporophytes are grown in the sea until they reach commercial size, which varies by kelp species (e.g. up to 5 m in length for Saccharina japonica), at which time the crop is usually harvested from boats. Harvesting can be performed by means of several collections of the larger sporophytes (thinning) or by partially cutting the apical part of the blade, leaving the basal part, which may regrow apically from the basal blade meristem. However, the most common harvesting method is the collection of all sporophytes when most have reached commercial size [113, 127, 128].
The productivity of kelp cultures varies with the species, cultivation method, growing season, environmental conditions at the cultivation site and many other factors. However, the approximate average wet weight biomass yield per hectare of cultivation on a commercial farm has been, for example, 70 tons fresh weight for Macrocystis pyrifera [149], 26 tons for Saccharina japonica [129] and 25 tons for Saccharina latissima [169].
2.4 Conclusions and Perspectives
The commercial alginates that have numerous applications are exclusively extracted from brown seaweeds, and it is estimated that the global production of alginates primarily involves the exploitation of only 9 seaweed species, of which kelps Lessonia, Laminaria and Saccharina are the most commercially important, accounting for 86% of the worldwide production. Most of these marine macroalgae are currently harvested from native populations (over 80%), while Saccharina farming in Asia provides the rest of the resources (approximately 20%) for alginate production.
The demand for alginates is expected to increase in the future; however, natural resources are limited, and kelp forests are decreasing worldwide. Nevertheless, it is expected that the contribution of kelp cultures to global alginate production in the coming years will increase in volume and in the number of species used for this purpose, which would also provide greater security and stability to the market supply of alginates, as it would no longer depend on natural populations. Additionally, this cultivation would enable alginates of commercial interest to be obtained from species whose extraction has not been previously possible due to a lack of available natural resources.
Finally, kelp farming provides significant environmental benefits by capturing atmospheric carbon and recycling inorganic nutrients from the marine environment. Additionally, since kelps have many other applications, the uses of the biomass harvested in cultures could be integrated in biofactories so that other products of commercial value, besides alginates, could be obtained (Fig. 2.18).
Kelp sea farming scheme to produce alginates as well as other value-added bioproducts from the integrated use of the kelp biomass harvested in biofactories (Adapted and reprinted from Ref. [113], Copyright 2016, with permission from Elsevier)
References
Haug A (1964) Composition and properties of alginates. Rep Norw Inst Seaweed Res No. 30. Norwegian Institute of Seaweed Research, Trondheim, pp 1–123
Percival E (1979) The polysaccharides of green, red and brown seaweeds: their basic structure, biosynthesis and function. Br Phycol J 14(2):103–117
Grasdalen H (1983) High-field, 1H-n.m.r. spectroscopy of alginate: sequential structure and linkage conformations. Carbohydr Res 118:255–260
Smidsrød O, Glover RM, Whittington SG (1973) The relative extension of alginates having different chemical composition. Carbohydr Res 27(1):107–118
Braccini I, Grasso RP, Pérez S (1999) Conformational and configurational features of acidic polysaccharides and their interactions with calcium ions: a molecular modeling investigation. Carbohydr Res 317(1/4):119–130
Draget KI, Gåserød O, Aune I, Andersen PO, Storbakken B, Stokke BT, Smidsrød O (2001) Effects of molecular weight and elastic segment flexibility on syneresis in Ca-alginate gels. Food Hydrocoll 15(4/6):485–490
Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37(1):106–126
Andersen T, Strand BL, Formo K, Alsberg E, Christensen BE (2012) Alginates as biomaterials in tissue engineering. Carbohydr Chem 37:227–258
Helgerud T, Gåserød O, Fjæreide T, Andersen PO, Larsen CK (2009) Alginates. In: Imeson A (ed) Food stabilisers, thickeners and gelling agents. Wiley-Blackwell, Oxford, pp 50–72
Murillo-Álvarez JI, Hernández-Carmona G (2007) Monomer composition and sequence of sodium alginate extracted at pilot plant scale from three commercially important seaweeds from Mexico. J Appl Phycol 19(5):545–548
Indergaard M, Skjåk-Bræk G (1987) Characteristics of alginate from Laminaria digitata cultivated in a high-phosphate environment. Hydrobiologia 151(152):541–549
Reyes Tisnado R, Hernández Carmona G, Rodríguez Montesinos E, Arvizu Higuera DL, López Gutiérrez F (2005) Food grade alginates extracted from the giant kelp Macrocystis pyrifera at pilot-plant scale. Rev Invest Mar 26(3):185–192
Hartmann M, Dentini M, Ingar Draget K, Skjåk-Bræk G (2006) Enzymatic modification of alginates with the mannuronan C-5epimerase AlgE4 enhances their solubility at low pH. Carbohydr Polym 63(2):257–262
Smidsrød O, Christensen BE (1991) Molecular structure and physical behavior of seaweed colloids as compared with microbial polysaccharides. In: Guiry MD, Blunden G (eds) Seaweeds resources in europe: uses and potential. Wiley, West Sussex, pp 185–217
Draget KI, Moe ST, Skjak-Bræk G, Smidsrød O (2006) Alginates. In: Stephen AM, Phillips GO, Williams PA (eds) Food polysaccharides and their applications (Chapter 9). CRC Press/Taylor & Francis Group, Boca Raton, pp 289–334
Rioux LE, Turgeon SL, Beaulieu M (2007) Characterization of polysaccharides extracted from brown seaweeds. Carbohydr Polym 69(3):530–537
Smidsrød O, Draget KI (1996) Chemistry and physical properties of alginates. Carbohydr Eur 14:6–13
Moral CK, Dogan O, Sanin FD (2013) Comparison of chemical fractionation method and H-1-NMR spectroscopy in measuring the monomer block distribution of algal alginates. J Polym Eng 33(3):239–246
Salomonsen T, Jensen HM, Stenbæk D, Engelsen SB (2008) Chemometric prediction of alginate monomer composition: a comparative spectroscopic study using IR, Raman, NIR and NMR. Carbohydr Polym 72(4):730–739
Grant GT, Morris ER, Rees DA, Smith PJC, Thom D (1973) Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett 32(1):195–198
Braccini I, Pérez S (2001) Molecular basis of Ca2+-induced gelation in alginates and pectins: the egg-box model revisited. Biomacromolecules 2(4):1089–1096
Plazinski W (2011) Molecular basis of calcium binding by polyguluronate chains. Revising the egg-box model. J Comput Chem 32(14):2988–2995
Mørch ÝA, Donati I, Strand BL (2006) Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 7(5):1471–1480
Smidsrød O, Haug A (1972) Properties of poly(1,4-hexuronates) in the gel state. II. Comparison of gels of different chemical composition. Acta Chem Scand 26:79–88
Haug A, Smidsrød O (1965) The effect of divalent metals on the properties of alginate solutions. II. Comparison of different metal ions. Acta Chem Scand 69(2):341–351
Smidsrød O, Haug A (1968) Dependence upon uronic acid composition of some ion-exchange properties of alginate. Acta Chem Scand 22(6):1989–1997
Draget KI, Strand B, Hartmann M, Valla S, Smidsrød O, Skjåk-Bræk G (2000) Ionic and acid gel formation of epimerised alginates; the effect of AlgE4. Int J Biol Macromol 27(2):117–122
Mancini M, Moresi M, Rancini R (1999) Mechanical properties of alginate gels: empirical characterisation. J Food Eng 39(4):369–378
Skjåk-Bræk G, Grasdalen H, Smidsrød O (1989) Inhomogeneous polysaccharide ionic gels. Carbohydr Polym 10(1):31–54
Draget KI, Simensen MK, Onsøyen E, Smidsrød O (1993) Gel strength of Ca-limited alginate gels made in situ. Hydrobiologia 260-261(1):563–565
Storz H, Zimmermann U, Zimmermann H, Kulicke W-M (2009) Viscoelastic properties of ultra-high viscosity alginates. Rheol Acta 49(2):155–167
Senuma Y, Lowe C, Zweifel Y, Hilborn JG, Marison I (2000) Alginate hydrogel microspheres and microcapsules prepared by spinning disk atomization. Biotechnol Bioeng 67(5):616–622
Moe ST, Draget KI, Skjåk-Braek G, Smidsrød O (1995) Alginates. In: Stephen AM (ed) Food polysaccharides and their applications. Marcel Dekker, New York, pp 245–286
Draget KI, Taylor C (2011) Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll 25(2):251–256
Ertesvåg H (2015) Alginate-modifying enzymes: biological roles and biotechnological uses. Front Microbiol 6:523. article 523
Pawar SN, Edgar KJ (2012) Alginate derivatization: a review of chemistry, properties and applications. Biomaterials 33(11):3279–3305
d’Ayala GG, Malinconico M, Laurienzo P (2008) Marine derived polysaccharides for biomedical applications: chemical modification approaches. Molecules 13(9):2069–2106
Stanford ECC (1883) On algin: a new substance obtained from some of the commoner species of marine algae. Chem News 47:254–257
Stanford ECC (1881) Improvements in the manufacture of useful products from seaweeds. British Patent No 142
Smith AM, Miri T (2010) Alginates in foods. In: Norton IT, Spyropoulos F, Cox P (eds) Practical food rheology: an interpretive approach. Wiley-Blackwell, Oxford, pp 113–132
Szekalska M, PuciBowska A, SzymaNska E, Ciosek P, Winnicka K (2016) Alginate: current use and future perspectives in pharmaceutical and biomedical applications. Int J Polym Sci 2016:1–17. article ID 7697031
McHugh DJ (2003) A guide to the seaweed industry. FAO fisheries technical paper no. 441. FAO, Rome
Bixler HJ, Porse H (2011) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23(3):321–335
Kraan S (2012) Algal polysaccharides, novel applications and outlook. In: Chang C-F (ed) Carbohydrates: comprehensive studies on glycobiology and glycotechnology. InTech, Maastricht, pp 489–532
Rioux LE, Turgeon SL (2015) Seaweed carbohydrates. In: Tiwari BK, Troy DJ (eds) Seaweed sustainability: food and non-food applications. Academic, London, pp 141–192
Remminghorst U, Rehm BHA (2006) Bacterial alginates: from biosynthesis to applications. Biotechnol Lett 28(21):1701–1712
Rehm BHA, Valla S (1997) Bacterial alginates: biosynthesis and applications. Appl Microbiol Biotechnol 48(3):281–288
Hay ID, Rehman ZU, Moradali MF, Wang Y, Rehm BHA (2013) Microbial alginate production, modification and its applications. Microb Biotechnol 6:637–650
Schiener P, Black KD, Stanley MS, Green DH (2015) The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J Appl Phycol 27(1):363–373
Westermeier R, Murúa P, Patiño DJ, Muñoz L, Ruiz A, Müller DG (2012) Variations of chemical composition and energy content in natural and genetically defined cultivars of Macrocystis from Chile. J Appl Phycol 24:1191–1201
Critchley AT, Ohno M, Largo DB (2006) The seaweed resources of the world. A CD-rom project. Expert Centre for Taxonomic Identification (ETI), Amsterdam
Zemke-White WL, Ohno M (1999) World seaweed utilisation: an end-of-century summary. J Appl Phycol 11:369–376
Graham L, Graham J, Wilcox L (2009) Algae, 2nd edn. Pearson Benjamin Cummings, San Francisco
Barsanti L, Gualtieri P (2014) Algae: anatomy, biochemistry, and biotechnology. CRC Press, Taylor & Francis Group, Boca Raton
Algaebase (2017) World-wide electronic publication. National University of Ireland, Galway. http://www.algaebase.org. Accessed 8 Jan 2017
Synytsya A, Čopíková J, Kim WJ, Park YI (2015) Cell wall polysaccharides of marine algae. In: Kim S-K (ed) Handbook of marine biotechnology. Springer, Berlin/Heidelberg, pp 543–590
Kloareg B, Quatrano RS (1988) Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr Mar Biol Annu Rev 26:259–315
Venegas M, Matsuhiro B, Edding ME (1993) Alginate composition of Lessonia trabeculata (Phaeophyta: Laminariales) growing in exposed and sheltered habitats. Bot Mar 36(1):47–51
Craigie JS, Morris ER, Rees DA, Thom D (1984) Alginate block structure in phaeophyceae from Nova Scotia: variation with species, environment and tissue-type. Carbohydr Polym 4(4):237–252
Black WAP (1950) The seasonal variation in weight and chemical composition of the common British Laminariaceae. J Mar Biol Assoc UK 29:45–72
Kelly BJ, Brown MT (2000) Variations in the alginate content and composition of Durvillaea antarctica and D. willana from southern New Zealand. J Appl Phycol 12(3/5):317–324
Andresen IL, Skipnes O, Smidsrød O, Ostgaard K, Hemmer P (1977) Some biological functions of matrix components in benthic algae in relation to their chemistry and the composition of seawater. Cell Chem Technol 48:361–381
Hernández-Carmona G (1985) Variación estacional del contenido de alginatos en tres especies de feofitas de Baja California Sur. Invest Marinas CICIMAR 2:29–45
Manns D, Nielsen MM, Bruhn A, Saake B, Meyer AS (2017) Compositional variations of brown seaweeds Laminaria digitata and Saccharina latissima in Danish waters. J Appl Phycol 29:1493–1506. in press
Minghou J, Yujun W, Zuhong X, Yucai G (1984) Studies on the M:G ratios in alginate. In: Eleventh international seaweed symposium. Dr W. Junk Publishers, Dordrecht, pp 554–556
Honya M, Kinoshita T, Ishikawa M, Mori H, Nisizawa K (1993) Monthly determination of alginate, M/G ratio, mannitol, and minerals in cultivated Laminaria japonica. Nippon Suisan Gakk 59(2):295–299
Chandía N (2001) Alginic acids in Lessonia trabeculata: characterization by formic acid hydrolysis and FT-IR spectroscopy. Carbohydr Polym 46(1):81–87
Lorbeer AJ, Charoensiddhi S, Lahnstein J, Lars C, Franco CMM, Bulone V, Zhang W (2017) Sequential extraction and characterization of fucoidans and alginates from Ecklonia radiata, Macrocystis pyrifera, Durvillaea potatorum, and Seirococcus axillaris. J Appl Phycol 29:1515–1526. in press
Panikkar R, Brasch DJ (1996) Composition and block structure of alginates from New Zealand brown seaweeds. Carbohydr Res 293(1):119–132
Obluchinskaya ED, Voskoboinikov GM, Galynkin VA (2002) Contents of alginic acid and fuccidan in Fucus algae of the Barents Sea. Appl Biochem Microbiol 38(2):186–188
Nai-yu Z, Yan-xia Z, Xiao F, Li-jun H (1994) Effects of composition and structure of alginates on adsorption of divalent metals. Chin J Oceanol Limnol 12(1):78–83
Indergaard M, Skjak-Braek G, Jensen A (1990) Studies on the influence of nutrients on the composition and structure of alginate in Laminaria saccharina (L.) Lamour. (Laminariales, Phaeophyceae). Bot Mar 33:277–288
Storz H, Müller KJ, Ehrhart F, Gómez I, Shirley SG, Gessner P, Zimmermann G, Weyand E, Sukhorukov VL, Forst T, Weber MM, Zimmermann H, Kulicke W-M, Zimmermann U (2009) Physicochemical features of ultra-high viscosity alginates. Carbohydr Res 344(8):985–995
Yuguchi Y, Urakawa H, Kajiwara K, Draget KI, Stokke BT (2000) Small-angle X-ray scattering and rheological characterization of alginate gels. 2. Time-resolved studies on ionotropic gels. J Mol Struct 554(1):21–34
Rosell K-G, Srivastava LM (1984) Seasonal variation in the chemical constituents of the brown algae Macrocystis integrifolia and Nereocystis luetkeana. Can J Bot 62(11):2229–2236
Franklin LA, Forster RM (1997) The changing irradiance environment: consequences for marine macrophyte physiology, productivity and ecology. Eur J Phycol 32:207–232
Harrison PJ, Hurd CL (2001) Nutrient physiology of seaweeds: application of concepts to aquaculture. Cah Biol Mar 42:71–82
Davison IR (1991) Environmental effects on algal photosynthesis: temperature. J Phycol 27:2–8
Cheshire AC, Hallam ND (1985) The environmental role of alginates in Durvillaea potatorum (Fucales, Phaeophyta). Phycologia 24(2):147–153
FMC (2016) FMC Biopolymer FMC Health & Nutrition – Pharmaceutical FMC Corporation http://www.fmcbiopolymer.com. Accessed 25 Dec 2016
Hernández-Carmona G, Freile-Pelegrín Y, Hernández-Garibay E (2013) Conventional and alternative technologies for the extraction of algal polysaccharides. In: Dominguez H (ed) Functional ingredients from algae for foods and nutraceuticals. Woodhead Publishing Limited, Cambridge, pp 475–516
McHugh DJ (1987) Production, properties and uses of alginates. In: DJ MH (ed) Production and utilization of products from commercial seaweeds. FAO fisheries technical paper, 288. Food and Agriculture Organization of the United Nations (FAO), Fishery and Aquaculture Economics and Policy Division, Rome
Hernández-Carmona G, McHugh DJ, Arvizu-Higuera DL, Rodríguez-Montesinos YE (2002) Pilot plant scale extraction of alginates from Macrocystis pyrifera. 4. Conversion of alginic acid to sodium alginate, drying and milling. J Appl Phycol 14(6):445–451
Hernández-Carmona G, McHugh DJ, López-Gutiérrez F (1999) Pilot plant scale extraction of alginates from Macrocystis pyrifera. 2. Studies on extraction conditions and methods of separating the alkaline-insoluble residue. J Appl Phycol 11(6):493–502
Gomez CG, Pérez Lambrecht MV, Lozano JE, Rinaudo M, Villar MA (2009) Influence of the extraction–purification conditions on final properties of alginates obtained from brown algae (Macrocystis pyrifera). Int J Biol Macromol 44(4):365–371
Hallmann A (2007) Algal transgenics and biotechnology. Transgenic Plant J 1(1):81–98
White WL (2015) World seaweed utilization. In: Tiwari BK, Troy DJ (eds) Seaweed sustainability: food and non-food applications. Academic Press/Elsevier, Oxford, pp 7–25
Vásquez JA (2016) The brown seaweeds fishery in Chile. In: Mikkola H (ed) Fisheries and aquaculture in the modern world. InTechOpen, Rijeka, pp 123–141
Lane CE, Mayes C, Druehl LD, Saunders GW (2006) A multi-gene molecular investigation of the kelp (Laminariales, Phaeophyceae) supports substantial taxonomic re-organization. J Phycol 42(2):493–512
Tellier F, Tapia J, Faugeron S, Destombe C, Valero M (2011) The Lessonia nigrescens species complex (Laminariales, Phaeophyceae) shows strict parapatry and complete reproductive isolation in a secondary contact zone. J Phycol 47(4):894–903
González A, Beltrán J, Hiriart-Bertrand L, Flores V, de Reviers B, Correa JA, Santelices B (2012) Identification of cryptic species in the Lessonia nigrescens complex (Phaeophyceae, Laminariales). J Phycol 48(5):1153–1165
Tellier F, Alonso Vega JM, Broitman BR, Vasquez JA, Valero M, Faugeron S (2011) The importance of having two species instead of one in kelp management: the Lessonia nigrescens species complex. Cah Biol Mar 52(4):455–465
Demes KW, Graham MH, Suskiewicz TS (2009) Phenotypic plasticity reconciles incongruous molecular and morphological taxonomies: the giant kelp, Macrocystis (Laminariales, Phaeophyceae), is a monospecific genus. J Phycol 45(6):1266–1269
Graham MH, Vasquez JA, Buschmann AH (2007) Global ecology of the giant kelp Macrocystis: from ecotypes to ecosystems. Oceanogr Mar Biol Annu Rev 45:39–88
Kirkman H, Kendrick GA (1997) Ecological significance and commercial harvesting of drifting and beach-cast macro-algae and seagrasses in Australia: a review. J Appl Phycol 9(4):311–326
Anderson RJ, Simons RH, Jarman NG (1989) Commercial seaweeds in southern Africa: a review of utilization and research. S Afr J Mar Sci 8(1):277–299
Morrissey J, Kraan S, Guiry MD (2001) A guide to commercially important seaweeds on the Irish coast. Bord Iascaigh Mhara/Irish Sea Fisheries Board, Dublin
Schiel DR, Nelson WA (1990) The harvesting of macroalgae in New Zealand. Hydrobiologia 204(205):25–33
Briand X (1991) Seaweed harvesting in Europe. In: Guiry MD, Blunden G (eds) Seaweeds resources in Europe: uses and potential. Wiley, West Sussex, pp 259–308
Davoult D, Engel CR, Arzel P, Knoch D, Laurans M (2011) Environmental factors and commercial harvesting: exploring possible links behind the decline of the kelp Laminaria digitata in Brittany, France. Cah Biol Mar 52(4):429–434
Lorentsen S-H, Sjøtun K, Gremillet D (2010) Multi-trophic consequences of kelp harvest. Biol Conserv 143(9):2054–2062
Vásquez JA, Piaget N, Vega JMA (2012) The Lessonia nigrescens fishery in northern Chile: “how you harvest is more important than how much you harvest”. J Appl Phycol 24(3):417–426
Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29(4):436–459
Chapman ARO (1995) Functional ecology of fucoid algae: twenty-three years of progress. Phycologia 34(1):1–32
Vea J, Ask E (2011) Creating a sustainable commercial harvest of Laminaria hyperborea, in Norway. J Appl Phycol 23(3):489–494
Frangoudes K (2011) Seaweeds fisheries management in France, Japan, Chile and Norway. Cah Biol Mar 52(4):517–525
Wernberg T, Russell BD, Thomsen MS, Gurgel CFD, Bradshaw CJA, Poloczanska ES, Connell SD (2011) Seaweed communities in retreat from ocean warming. Curr Biol 21:1828–1832
Filbee-Dexter K, Feehan CJ, Scheibling RE (2016) Large-scale degradation of a kelp ecosystem in an ocean warming hotspot. Mar Ecol Prog Ser 543:141–152
Chen J (2009) Laminaria japonica. In: Crespi V, New M (eds) Cultured aquatic species information programme. FAO Fisheries and Aquaculture Department, Rome
Pang SJ, Li X, Chopin T (2015) Undaria pinnatifida ((Harvey) Suringar, 1873). In: Cultured aquatic species information programme. FAO Fisheries and Aquaculture Department, Rome
FAO (2016) The state of world fisheries and aquaculture: contributing to food security and nutrition for all. Food and Agriculture Organization of the United Nations (FAO), Rome
Buschmann AH, Prescott S, Potin P, Faugeron S, Vásquez JA, Camus C, Infante J, Hernández-González MC, Gutíerrez A, Varela DA (2014) The status of kelp exploitation and marine agronomy, with emphasis on Macrocystis pyrifera, in Chile. In: Nathalie B (ed) Advances in botanical research, vol 71, (Chapter Six). Elsevier/Academic, London, pp 161–188
Peteiro C, Sánchez N, Martínez B (2016) Mariculture of the Asian kelp Undaria pinnatifida and the native kelp Saccharina lattisima along the Atlantic coast of southern Europe: an overview. Algal Res 15:9–23
Chopin T, Robinson S, Reid G, Ridler N (2013) Prospects for Integrated Multi-Trophic Aquaculture (IMTA) in the open ocean. Bull Aquacul Assoc Canada 111(2):28–35
Rebours C, Marinho-Soriano E, Zertuche-González J, Hayashi L, Vásquez J, Kradolfer P, Soriano G, Ugarte R, Abreu M, Bay-Larsen I, Hovelsrud G, Rødven R, Robledo D (2014) Seaweeds: an opportunity for wealth and sustainable livelihood for coastal communities. J Appl Phycol 26(5):1939–1951
Cottier-Cook EJ, Nagabhatla N, Badis Y, Campbell M, Chopin T, Dai W, Fang J, He P, Hewitt CL, Kim GH, Huo Y, Jiang Z, Kema G, Li X, Liu F, Liu H, Liu Y, Lu Q, Luo Q, Mao Y, Msuya FE, Rebours C, Shen H, Stentiford GD, Yarish C, Wu H, Yang X, Zhang J, Zhou Y, Gachon CMM (2016) Safeguarding the future of the global seaweed aquaculture industry. United Nations University, Institute for Water, Environment and Health (UNU-INWEH) & Scottish Association for Marine Science (SAMS), Hamilton
Kanda T (1936) On the gametophytes of some japanese species of Laminariales. Sci Pap Inst Algol Res, Fac Sci, Hokkaido Imp Univ 1(2):221–260
Schiel DR, Foster MS (2015) The biology and ecology of giant kelp forests. University of California Press, Oakland
Peteiro C (2015) Open-sea cultivation of commercial kelps in the Atlantic coast of southern Europe. Ph.D. thesis, King Juan Carlos University (URJC), Madrid
Perez R, Kaas R, Barbaroux O (1984) Culture expérimentale de l’algue Undaria pinnatifida sur les côtes de France. Sci Pêche 343:3–15
Barrento S, Camus C, Sousa-Pinto I, Buschmann AH (2016) Germplasm banking of the giant kelp: our biological insurance in a changing environment. Algal Res 13:134–140
Lüning K (1990) Seaweeds: their environment, biogeography and ecophysiology. Wiley, New York
Breeman AM (1988) Relative importance of temperature and other factors in determining geographic boundaries of seaweeds: experimental and phenological evidence. Helgoländer Meeresunters 42(2):199–241
van den Hoek C (1982) The distribution of benthic marine algae in relation to the temperature regulation of their life histories. Biol J Linnean Soc 18:81–144
Lüning K (1982) Seasonality of larger brown algae and its possible regulation by the environment. In: Srivastava LM (ed) Synthetic and degradative processes in marine macrophytes. Walter de Gruyter, Berlin, pp 47–67
Hurd CL (2000) Water motion, marine macroalgal physiology and production. J Phycol 36(3):453–472
Saito Y (1975) Undaria. In: Tokida J, Hirose H (eds) Advance in phycology in Japan. Dr. W. Junk, Hague, pp 304–320
Kawashima S (1984) Kombu cultivations in Japan for human foodstuff. Jpn J Phycol 32:379–394
Chen J (2004) Laminaria japonica (Areschoug, 1851). In: Cultured aquatic species information programme. FAO Fisheries and Aquaculture Department, Rome
Kain (Jones) JM (1991) Cultivation of attached seaweeds. In: Guiry MD, Blunden G (eds) Seaweeds resources in Europe: uses and potential. Wiley, West Sussex, pp 309–378
Brinkhuis BH, Levine HG, Schlenk CG, Tobin S (1987) Laminaria cultivation in the far east and north America. In: Bird KT, Benson PH (eds) Seaweed cultivation for renewable resources. Developments in aquaculture and fisheries science. Elsevier, Amsterdam, pp 107–146
Druehl LD, Baird R, Lindwall A, Lloyd KE, Pakula S (1988) Longline cultivation of some Laminariaceae in British Columbia, Canada. Aquacult Fish Manag 19:253–263
Pérez-Cirera JL, Salinas JM, Cremades J, Bárbara I, Granja A, Veiga AJ, Fuertes C (1997) Cultivo de Undaria pinnatifida (Laminariales, Phaeophyta) en Galicia. NACC Biol 7:3–28
Perez R, Kaas R, Defend JF, Le Bayon N, Planchon G (1991) Production de gamétophytes en “free living” pour la culture de l’algue brune Laminaria japonica (Aresch.) Aqua Revue 37:30–37
Ritschard RL (1992) Marine algae as a CO2 sink. Water Air Soil Pollut 64(1/2):289–303
Chung I, Beardall J, Mehta S, Sahoo D, Stojkovic S (2011) Using marine macroalgae for carbon sequestration: a critical appraisal. J Appl Phycol 23(5):877–886
Gao K, McKinley KR (1994) Use of macroalgae for marine biomass production and CO2 remediation: a review. J Appl Phycol 6:45–60
Muraoka D (2004) Seaweed resources as a source of carbon fixation. Bull Fish Res Agen (Jpn) 1:59–64
Kim JK, Kraemer GP, Yarish C (2015) Use of sugar kelp aquaculture in Long Island Sound and the Bronx River Estuary for nutrient extraction. Mar Ecol Prog Ser 531:155–166
Handå A, Forbord S, Wang X, Broch OJ, Dahle SW, Størseth TR, Reitan KI, Olsen Y, Skjermo J (2013) Seasonal- and depth-dependent growth of cultivated kelp (Saccharina latissima) in close proximity to salmon (Salmo salar) aquaculture in Norway. Aquaculture 414(415):191–201
Sanderson JC, Dring MJ, Davidson K, Kelly MS (2012) Culture, yield and bioremediation potential of Palmaria palmata (Linnaeus) Weber & Mohr and Saccharina latissima (Linnaeus) C.E.Lane, C.Mayes, Druehl & G.W.Saunders adjacent to fish farm cages in north west Scotland. Aquaculture 354:128–135
Reid GK, Chopin T, Robinson SMC, Azevedo P, Quinton M, Belyea E (2013) Weight ratios of the kelps, Alaria esculenta and Saccharina latissima, required to sequester dissolved inorganic nutrients and supply oxygen for Atlantic salmon, Salmo salar, in Integrated Multi-Trophic Aquaculture systems. Aquaculture 408(409):34–46
Chopin T, MacDonald B, Robinson S, Cross S, Pearce C, Knowler D, Noce A, Reid G, Cooper A, Speare D, Burridge L, Crawford C, Sawhney M, Ang KP, Backman C, Hutchinson M (2013) The canadian integrated multi-trophic aquaculture network (CIMTAN)-a network for a new era of ecosystem responsible aquaculture. Fisheries 38(7):297–308
Neori A (2007) Essential role of seaweed cultivation in integrated multi-trophic aquaculture farms for global expansion of mariculture: an analysis. J Appl Phycol 20(5):117–120
Neori A, Chopin T, Troell M, Buschmann AH, Kraemer GP, Halling C, Shpigel M, Yarish C (2004) Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modem mariculture. Aquaculture 231(1/4):361–391
Buschmann AH, Hernández-González MC, Astudillo C, de la Fuente L, Gutierrez A, Aroca G (2005) Seaweed cultivation, product development and integrated aquaculture studies in Chile. World Aquac 36(3):51–53
Buschmann AH, Stead RA, Hernández-González MC, Pereda SV (2013) Un análisis crítico sobre el uso de macroalgas como base para una acuicultura sustentable. Rev Chil Hist Nat 86:251–264
Chopin T, Robinson SMC, Troell M, Neori A, Buschmann AH, Fang JG (2008) Multitrophic integration for sustainable marine aquaculture. In: Jørgensen SE, Fath BD (eds) Ecological engineering, encyclopedia of ecology, vol 3. Elsevier, Oxford, pp 2463–2475
Camus C, Infante J, Buschmann AH (2017) Overview of 3 year precommercial seafarming of Macrocystis pyrifera along the Chilean coast. Rev Aquac (in press)
Edwards M, Watson L (2011) Cultivating Laminaria digitata. aquaculture explained, no. 26. Bord Iascaigh Mhara, Dublin
Tamigneaux E, Licois A, Bourdages D, Leblanc MJ (2013) Protocoles pour la culture de la laminaire à long stipe (Saccharina longicruris) et de la laminaire sucrée (Saccharina latissima) dans le contexte du Québec, vol Guide No. 13-01. Les publications MERINOV. Centre d’Innovation de l’aquaculture et des pêches du Québec, Québec
Westermeier R, Patino D, Piel MI, Maier I, Mueller DG (2006) A new approach to kelp mariculture in Chile: production of free-floating sporophyte seedlings from gametophyte cultures of Lessonia trabeculata and Macrocystis pyrifera. Aquac Res 37(2):164–171
Arbona JF, Molla M (2006) Cultivation of brown seaweed Alaria esculenta. Aquaculture explained. Bord Iascaigh Mhara, Dublin
Peteiro C, Freire Ó (2011) Effect of water motion on the cultivation of the commercial seaweed Undaria pinnatifida in a coastal bay of Galicia, Northwest Spain. Aquaculture 314(1/4):269–276
Camus C, Buschmann AH (2017) Macrocystis pyrifera aquafarming: Production optimization of rope-seeded juvenile sporophytes. Aquaculture 468:107–114
Wang W, Sun X, Wang G, Xu P, Wang X, Lin Z, Wang F (2010) Effect of blue light on indoor seedling culture of Saccharina japonica (Phaeophyta). J Appl Phycol 22:737–744
Zhang QS, Cong YZ, Qu SC, Luo SJ, Li XJ, Tang XX (2008) A simple and highly efficient method for the cryopreservation of Laminaria japonica (Phaeophyceae) germplasm. Eur J Phycol 42(2):209–213
Pang SJ, Lüning K (2004) Breaking seasonal limitation: year-round sporogenesis in the brown alga Laminaria saccharina by blocking the transport of puntative sporulation inhibitors. Aquaculture 240:531–541
Forbord S, Skjermo J, Arff J, Handå A, Reitan KI, Bjerregaard R, Lüning K (2012) Development of Saccharina latissima (Phaeophyceae) kelp hatcheries with year-round production of zoospores and juvenile sporophytes on culture ropes for kelp aquaculture. J Appl Phycol 24(3):393–399
Peteiro C, Cortés B, Arroyo NL, García-Tasende M, Vergés A, Martínez B (2016) Creación de bancos de germoplasma o “semillas” con algas laminarias para su conservación, restauración ecológica y/o cultivo comercial. Ipac Revista de acuicultura 105:15–19
Vasquez X, Gutierrez A, Buschmann AH, Flores R, Farias D, Leal P (2014) Evaluation of repopulation techniques for the giant kelp Macrocystis pyrifera (Laminariales). Bot Mar 57(2):123–130
Correa JA, Lagos NA, Medina MH, Castilla JC, Cerda M, Ramirez M, Martinez E, Faugeron S, Andrade S, Pinto R, Contreras L (2006) Experimental transplants of the large kelp Lessonia nigrescens (Phaeophyceae) in high-energy wave exposed rocky intertidal habitats of northern Chile: experimental, restoration and management applications. J Exp Mar Biol Ecol 335(1):13–18
Carney LT, Waaland JR, Klinger T, Ewing K (2005) Restoration of the bull kelp Nereocystis luetkeana in nearshore rocky habitats. Mar Ecol Prog Ser 302:49–61
Terawaki T, Hasegawa H, Arai S, Ohno M (2001) Management-free techniques for restoration of Eisenia and Ecklonia beds along the central Pacific coast of Japan. J Appl Phycol 13(1):13–17
Peteiro C, Sánchez N, Dueñas-Liaño C, Martínez B (2014) Open-sea cultivation by transplanting young fronds of the kelp Saccharina latissima. J Appl Phycol 26(1):519–528
Kawashima S (1993) Cultivation of the brown alga, Laminaria ‘kombu’. In: Ohno M, Critchley AT (eds) Seaweed cultivation and marine ranching, vol vol 4. Japan International Cooperation Agency (JICA), Jokosuka, pp 25–40
Tseng CK (1987) Laminaria mariculture in China. In: Doty MS, Caddy JF, Santelices B (eds) Case studies of seven commercial seaweed resources. FAO fisheries technical paper no. 281, Rome, pp 239–263. Electronic edition. Available at: http://www.fao.org/docrep/X5819E/x5819e09.htm#laminaria%20mariculture%20in%20china
Nanba N, Fujiwara T, Kuwano K, Ishikawa Y, Ogawa H, Kado R (2011) Effect of water flow velocity on growth and morphology of cultured Undaria pinnatifida sporophytes (Laminariales, Phaeophyceae) in Okirai Bay on the Sanriku coast, Northeast Japan. J Appl Phycol 23(6):1023–1030
Peteiro C, Freire Ó (2013) Biomass yield and morphological features of the seaweed Saccharina latissima cultivated at two different sites in a coastal bay in the Atlantic coast of Spain. J Appl Phycol 25(1):205–213
Acknowledgements
We would like to acknowledge the assistance of Dr. A. Secilla in the elaboration of the figures. The English language has been edited by American Journal Experts (AJE).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Peteiro, C. (2018). Alginate Production from Marine Macroalgae, with Emphasis on Kelp Farming. In: Rehm, B., Moradali, M. (eds) Alginates and Their Biomedical Applications. Springer Series in Biomaterials Science and Engineering, vol 11. Springer, Singapore. https://doi.org/10.1007/978-981-10-6910-9_2
Download citation
DOI: https://doi.org/10.1007/978-981-10-6910-9_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6909-3
Online ISBN: 978-981-10-6910-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)