Abstract
Seaweed extracts (SWE) are widely used to improve plant growth, fruit quality, and stress tolerance. However, the functional link between the complex composition of algal-based products and their mechanisms of action has been only marginally addressed. A greenhouse experiment was performed on Microtom tomato plants in order to evaluate the effect of two Ascophyllum nodosum-based algal derivatives, Rygex (R) and Super Fifty (SF), on a tomato exposed to salinity (0, 42.5, and 85 mM NaCl) and normal and reduced nutrient availability (100 and 70% of the standard regimen). Bioactive compounds, with possible beneficial effects on growth and stress adaptation, were characterized via gas chromatography-mass spectrometry analysis (GC-MS). Enhanced growth of 13% was observed with Super Fifty treatment under a full-strength nutritional regimen, independent of the salinity treatment. Although Rygex and Super Fifty treatments did not significantly enhance plant growth and yield under salt treatment, they enhanced the accumulation of minerals, antioxidants, and essential amino acids in tomato fruits, with an overall improvement in nutritional value. Overall, SWE may affect and ameliorate different aspects of nutrition and stress tolerance and thus contribute to the sustainability of agricultural systems. Elucidating the link between bioactive compounds in SWE and plant responses will be critical to characterizing the mechanism of action of SWE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reducing the use of chemical fertilizers in agriculture is essential to minimize the environmental impact and improve the sustainability of agricultural systems (du Jardin 2015; Van Oosten et al. 2017). In response to this need, biostimulants are gaining an increasing market share since they possess several properties that comply well with sustainability principles (Calvo et al. 2014). Agricultural biostimulants include a diverse array of compounds, substances, and other natural products that are applied to plants or soils to regulate and enhance crop’s physiological processes (http://www.biostimulants.eu/2011/10/biostimulants-definition-agreed). Biostimulants are products containing substances and/or microorganisms that, when sprayed onto the plant canopy, supplied via irrigation or given as liquid/solid fertilizers and stimulate physiological and molecular processes that ameliorate both nutrient uptake and nutrient use efficiency, stress tolerance, and crop quality (du Jardin 2012; Bulgari et al. 2015; Van Oosten et al. 2017). Despite their extensive use in agriculture, biostimulants have been mostly tested with respect to crop yield and quality aspects (du Jardin 2015), whereas their potential role in biotic and abiotic stress tolerance is still emerging. Considering that climate change is predicted to exacerbate the outbreak of pests and diseases (Rosenzweig et al. 2001), as well as crop exposure to extreme temperatures, drought, and soil salinization (Ahuja et al. 2010), the contribution of biostimulants to the global crop yield as stress protectants and general growth enhancers may become increasingly important.
Various biostimulants have been used to increase plant tolerance to salt stress, such as protein hydrolysates, glycine betaine, bacteria, mycorrhizal fungi, and algal extracts (Plaut et al. 2013; du Jardin 2015). The effects of single molecules (duJardin 2012; Calvo et al. 2014; Halpern et al. 2015) on plant growth and stress tolerance have been clearly demonstrated, while complex mixtures (Rayorath et al. 2008; Battacharyya et al. 2015) or living microorganisms (Ahmad et al. 2008; Siddiqui et al. 2008) represent a more complex and challenging system to study. The effects of biostimulants have generally been attributed to the presence of phytohormones and a range of organic molecules (Battacharyya et al. 2015) acting as compatible solutes, including betaines (Blunden et al. 2009). Mixtures rich in carbohydrates and amino acids (Khan et al. 2009) as well as bioactive secondary metabolites such as vitamins and their precursors (Berlyn and Russo 1990; Blunden et al. 1985) also may play a role. Stress tolerance may be augmented through high concentrations of phenolic compounds with antioxidant properties that protect against stress-induced reactive oxygen species (ROS) (Laetitia et al. 2010). Brown seaweeds such as Ecklonia maxima contain phytohormones including auxins, cytokinins, gibberellins, abscisic acid, and brassinosteroids (Stirk et al. 2014). These can act as single molecules or in combinations that contribute to plant growth and development and stress adaptation (Rouphael et al. 2017). These components may exhibit a synergistic effect although many of the single molecules present in algal extracts and their modes of action remain unknown (Vernieri et al. 2005; Wally et al. 2012). Brown seaweeds (Phaeophyta) are commonly used in commercial extracts for agriculture and Ascophyllum nodosum, Ecklonia maxima, Macrocystis pyrifera, and Durvillea potatorum are the most frequently used by the biostimulant industry (Khan et al. 2009; Lötze and Hoffman 2016). Ascophyllum nodosum extracts are a common component in commercial formulations, and their application has been proven to significantly increase yield, biometric characteristics, and the quality of several crops (Abdel-Mawgoud et al. 2010; Mattner et al. 2013; Dogra and Mandradia 2014; Ali et al. 2016). Ascophyllum-based algal derivatives may facilitate stress adaptation because of their high concentration of betaines that may serve as compatible solutes under osmotic stress (Khan et al. 2009). Most brown seaweed extracts also contain mannitol, which may also facilitate osmotic adaptation (Battacharyya et al. 2015). However, the diverse and complex nature of these formulations makes it difficult to establish a univocal cause of the various biostimulant effects observed. Dissecting the mechanisms of action of biostimulants is necessary to define leverage points that can be used to improve their effects and to define technical guidelines to improve the efficacy and reliability of commercial products.
In this context, the goal of this experimental work was to profile the function of two A. nodosum-based algal derivatives on a tomato crop exposed to increasing salinity and nutritional deficiency. In addition to assessing the protective effects of these commercial products on yield and qualitative parameters, these findings help to establish a functional link between the main constituents of these algal derivatives and plant responses to salinity.
Material and methods
Growth conditions and water relations
A greenhouse experiment was carried out at the experimental station of the University of Naples Federico II, Southern Italy (lat. 43° 31′ N, long. 14° 58′ E; alt. 60 m above sea level) on Microtom tomato plants (Solanum lycopersicum L.). The experimental design was a split plot in which were factorially combined two nutrient concentrations, three levels of salinity, and three algal applications, with five plants for each experimental unit in three replicates (total = 270 plants). Tomato seeds were germinated in peat in May 2014 and grown until the third to fourth true leaf stage before transplanting. Plants were transplanted in 10-cm plastic pots containing pure peat moss (100%) and drip fertigated from 35 days after sowing (DAS). The irrigation water had pH and electrical conductivity (EC) of 7.3 and 0.58 dS m−1, respectively. A basic nutrient solution (BNS) was used for all treatments: (mM) N03− = 1.93, P2O5 = 2.53, K2O = 7.64, MgO = 1.48 and (μM) B = 37, Cu EDTA = 0.84, Fe DTPA = 10, Mn EDTA = 3.45, Mo = 2.08, Zn EDTA = 0.83, having an EC of 1.60 dS m−1. The BNS was given at two different concentrations (100 and 70% BNS) and combined with three NaCl levels of 0, 42.5, and 85 mM NaCl (S0, S1, and S2, respectively). The nutrient solution was pumped from independent 100-L tanks through a drip irrigation system, with one emitter per plant (2 L h−1). Three fertigations were applied per day, each of 1–3-min duration. At transplanting (35 DAS), the rhizosphere was treated with two A. nodosum extracts: Rygex (R) (Agriges S.R.L., Benevento, Italy) and Super Fifty (SF) (BioAtlantis Ltd., Kerry, Ireland). The two seaweed extracts (SWE), in liquid formulation, were diluted with deionized water by 1:400 for Rygex and 1:500 for Super Fifty. The dilutions were applied at the substrate at a dose of 200 mL per pot. Control plants were treated with only 200 mL of tap water. The SWE application was repeated every 2 weeks until the end of the cultivation cycle. During the cultivation cycle, the total water potential was measured at 75 DAS, on young fully expanded leaves, in the sealed chamber of a “dew-point” psychrometer (WP4, Decagon Devices, USA).
Biometric measurements
At 90 DAS, plants were harvested and separated into leaves, stems, roots, and fruits for the fresh biomass determination, and their tissues were dried to constant weight in a forced-air oven at 80 °C for 72 h for the dry biomass determination. The final plant height, number of leaves and fruits per plant, and plant leaf area were also measured. The leaf area was measured with a Li-Cor 3000 area meter (Li-Cor, USA).
GC-MS analysis of algal extracts
Derivatization of metabolites and GC-MS analysis was performed as described in Lisec et al. (2006), using half of the volume of the solutions for derivatization. Chromatograms and mass spectra were evaluated by Chroma TOF 4.2 (Leco, USA) and TagFinder 4.0 (Luedemann et al. 2012). Analytes were manually identified using TagFinder by comparing to the reference library mass spectra and retention indices in the Golm Metabolome Database (Kopka et al. 2004). The amount of metabolites was analyzed as relative metabolite abundance calculated by normalization of signal intensity to that of 13C-sorbitol, which was added as an internal standard normalization (Obata et al. 2013). The concentrations of different metabolites were normalized based on algal extract proline concentration, assayed according to Woodrow et al. (2017), allowing the conversion of the results.
Mineral analysis of plant tissue
Ion measurements were performed according to a procedure described by Carillo et al. (2011) with few modifications. Powdered dried material, 100 mg per sample, was suspended in 10 mL of Milli-Q grade water (Milli-Q PLUS, Millipore, USA) and subjected to four freeze–thaw cycles by freezing in liquid nitrogen and thawing at 40 °C. Samples were centrifuged at 34,000×g for 10 min, and the clear supernatants were analyzed by ion exchange chromatography using a DX500 apparatus (Dionex, Switzerland) with an IONPACATC1 anion trap column (Dionex), an IONPAC-AG11 guard column (Dionex), and an analytical IONPAC-AS11 4-mm column (Dionex), fitted with an ASRSII 4-mm suppressor for anions (Dionex), an IONPAC-CTC cation trap column (Dionex), an IONPAC-CG12A guard column (Dionex), and an analytical IONPAC-CS12A 4-mm column (Dionex), fitted with a CSRS4-mm suppressor for cations (Dionex), with detection by a CD20 conductivity detector (Dionex), according to the manufacturer’s instructions.
Amino acids and antioxidants
Primary amino acids and proline extraction and determination were performed according to Woodrow et al. (2017). Ascorbic acid (ASCAc), dehydroascorbic acid (DHA), and reduced and oxidized glutathione (GSH and GSSG) were extracted as described by Annunziata et al. (2012) and Woodrow et al. (2012) and determined according to Queval and Noctor (2007).
Statistical analysis
Data were analyzed by ANOVA, and least significant different (LSD) multiple range comparison tests were used to determine differences between means (P ≤ 0.05).
Results
Algal extract composition
GC-MS analysis of the two SWE revealed the presence of a large amount of bioactive compounds with significant differences between the two commercial formulations. Among the identified metabolites, the most relevant were low molecular weight soluble molecules. High concentrations of proline, branched chain amino acids (valine, betaines, polyols, and isoleucine), and polyol sugars (mannitol and sorbitol) were detected (Fig. 1). The actual concentration of these molecules as stress protectants was quantified by multiplying raw data from GC-MS analysis by the actual biostimulant quantity applied to the plant as indicated by the manufacturer (i.e., concentrations in the nutrient solution adjusted for the number of treatments received by the plants). R-treated plants received approximately four times more proline and γ- aminobutyric acid compared to SF-treated plants (Fig. 1a). In contrast, mannitol and sorbitol were applied to the plants only via Super Fifty treatment (Fig. 1a). These molecules were virtually absent in the Rygex nutrient solution which, in contrast, delivered to the plants a much larger amount of essential amino acids (Fig. 1b).
The ion content, also normalized by the product dosage, was different for the two SWEs. Super Fifty treatment delivered more potassium and magnesium, approximately four times more than Rygex (Fig. 1c). While the Rygex treatment supplied substantially more Ca2+, about seven times higher than Super Fifty (Fig. 1c).
Biomass and water potential
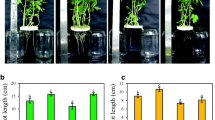
The biostimulant treatment with both products did not have any significant effect on fruit biomass (Table 1). A significant interaction between biostimulant application and the nutritional regimen was only found with respect to plant height and above ground fresh biomass (Figs. 2 and 3). Under full strength nutrient solution (100% BNS), the aboveground fresh biomass and plant height increased by 13 and 16%, respectively (Figs. 2 and 3), while Rygex did not affect these parameters in response to the variables tested.
Effect of two seaweed extracts, Rygex (R) and Super Fifty (SF), on plant height at different nutrient concentrations (100 and 70% of the tomato nutritional requirements). Control plants (C) were treated with only water. Different letters indicate significant differences at P ≤ 0.05. Vertical bars indicate means, n = 5, ± SE
Effect of two seaweed extracts, Rygex (R) and Super Fifty (SF), on the above ground biomass at different nutrient concentrations (100 and 70% of the tomato nutritional requirements). Control plants (C) were treated with only water. Different letters indicate significant differences at P ≤ 0.05. Vertical bars indicate means, n = 5, ± SE
S1 and S2 NaCl levels reduced the average fresh biomass by 16 and 30%, respectively. Salinization also resulted in a decrease of fruit production of 32% for the S1 treatment and of 45% for the S2. Tomato yield was not affected by the reduced strength of the nutrient solution (70% BNS), and no significant differences were observed in terms of fruit fresh weight between 100 and 70% BNS. Rygex applications reduced the fruit fresh weight in all treatments (Table 1). While water potential was significantly reduced by increasing salinity, it was higher with SWE treatments and in particular by Rygex application (Fig. 4).
Effect of increasing salinity S0, S1, and S2 (0, 42.5, and 85 mM NaCl, respectively) and two seaweed extracts, Rygex (R) and Super Fifty (SF), on the total water potential. Control plants (C) were treated with only water. Different letters indicate significant differences at P ≤ 0.05. Vertical bars indicate means, n = 5, ± SE
Mineral analysis of plant tissues
Salinization increased the overall Na+ and Cl− content in all organs (data not shown). However, a different accumulation pattern was observed in fruits. Fruit ion content was significantly altered by application of both algal extracts when compared to control plants. The Ca2+ content of fruits increased by 31 and 22% for Rygex and Super Fifty, respectively (Fig. 5a); K+ increased by 17 and 45% for Rygex and Super Fifty, respectively (Fig. 5b); Mg2+ increased by approximately 32% for both seaweed treatments (Fig. 5c). In low nutrient conditions (70% BNS), the Ca2+ content of fruits, negatively affected in control plants, was increased by 66% under both SWE (Fig. 6).
Effect of two seaweed extracts, Rygex (R) and Super Fifty (SF), on the Ca2+ fruit accumulation at different nutrient concentrations (100 and 70% of the tomato nutritional requirements). Control plants (C) were treated with only water. Different letters indicate significant differences at P ≤ 0.05. Vertical bars indicate means, n = 3, ± SE
Salinity stress enhanced Na+ and Cl− accumulation and reduced K+ and NO3− in tomato leaves (Fig. 7). Under moderate salt stress, treatments with Super Fifty seemed to mitigate these effects increasing K+ content by 18 and 12% over that of Rygex treatments and control plants, respectively (Fig. 7a). The Cl− accumulation in leaves was reduced by the Rygex treatment at both salinity levels (Fig. 7c). While induction of salt stress reduced nitrate levels in controls, Super Fifty increased the NO3− content in unstressed plants as well as both levels of salt stress. Rygex treatment increased nitrate levels over controls in unstressed and low salinity conditions (Fig. 7d).
Effect of two seaweed extracts, Rygex (R) and Super Fifty (SF), on the Na+ (a), K+ (b), Cl− (c), and NO3− (d), leaves accumulation at increasing salinity S0, S1, and S2 (0, 42.5, and 85 mM NaCl, respectively). Control plants (C) were treated with only water. Different letters indicate significant differences at P ≤ 0.05. Vertical bars indicate means, n = 3, ± SE
Essential amino acids and antioxidant accumulation
Rygex applications led to a general increase in the fruit content of essential amino acids, independently of salinity and nutrient concentration (Fig. 8). In particular, among the nine essential amino acids, Rygex-treated plants showed increased levels for lysine (37%), threonine (7%), isoleucine (19%), methionine (9%), valine (20%), and phenylalanine (11%) over untreated controls. The amino acid content of Super Fifty-treated fruits showed no significant increases compared to the control. Independently of nutrient concentration, the fruit ascorbate content increased by 30% in untreated control plants under salt stress (Fig. 9). Rygex-treated plants showed an increase of 22% of ascorbate in S2 compared to S0 treatment. Plants treated with Super Fifty in salinity conditions (S1) had 39% more ascorbate than the respective S0 treatment (Fig. 9).
Effect of two seaweed extracts, Rygex (R) and Super Fifty (SF), on the ascorbate fruit content at increasing salinity: S0, S1, and S2 (0, 42.5, and 85 mM NaCl, respectively). Control plants (C) were treated with only water. Different letters indicate significant differences at P ≤ 0.05. Vertical bars indicate means, n = 3, ± SE
Discussion
Composition of algal-based biostimulants and their effects on plant growth
Ascophyllum nodosum is the most studied algal biostimulant, and the observed benefits to plant crops have been attributed to the supply of essential nutrients (Craigie 2011). Commercial extracts made from brown seaweeds contain a diverse range of inorganic and organic components. The inorganic components of A. nodosum extracts include nitrogen, phosphorous, potassium, calcium, iron, magnesium, zinc, sodium, and sulfur (Rayorath et al. 2009). They also contain compatible compounds such as betaines and amino acids (Blunden et al. 2009). All these chemical components may affect plant growth and confer tolerance to abiotic stresses (Khan et al. 2009; Van Oosten et al. 2017). Insufficient information on product composition and the amount of bioactive substances that are actually delivered to the plants is available, however. This is critical to establish a functional link between specific molecules and/or specific combinations of molecules that are delivered to the plant via bioeffector applications. Some initial attempts to link the complex composition of these products to a possible mechanism of action have been reported (Norrie and Keathley 2005; Craigie 2011; Sharma et al. 2011). To move forward our understanding of the effects of biostimulant applications to plants, in this paper, the composition of two algal-based products, their potential to enhance growth, yield, adaptation to salt stress, and likely mechanisms of action, were assessed (Figs. 1 and 2). Analysis of the GC-MS results revealed the presence of specific compounds known to be effective in the alleviation of abiotic stress and improving plant growth with clear differences: Rygex had higher concentrations of proline and GABA, whereas Super Fifty had higher concentrations of mannitol and sorbitol (Fig. 1). It has been shown that increased levels of compatible solutes (or osmoprotectants) through exogenous application to plants improves performance under stress conditions (Heuer 2003). Proline for instance contributes significantly to balancing the osmotic potential of the vacuole (Annunziata et al. 2017), stabilizing subcellular structures and buffering cellular redox potential under stress conditions (Ashraf and Foolad 2007). In many cases, exogenous applications of proline or glycine betaine enhanced drought/salt stress tolerance with varying effects on plants growth in different species including tomato (Heuer 2003; Okuma et al. 2004; Barbieri et al. 2011). Similarly, γ- aminobutyric acid (GABA) is accumulated at high levels in response to different environmental stresses, and this finding is consistent with the presumed role of GABA in stress mitigation against abiotic stresses (Kinnersley and Turano 2000; Renault et al. 2010; Woodrow et al. 2017). GABA exogenous treatment of tomato seedlings under chilling stress increases sugars and proline concentrations in comparison with non-treated plants, suggesting that GABA is able to enhance free radical scavenging activity and maintain membrane integrity (Malekzadeh et al. 2014). Exogenous or endogenous accumulation of proline or GABA, after relief from stress, can supply energy, carbon, and nitrogen to recover and repair stress-induced damages (Carillo 2018). Mannitol is also a well-known osmoprotectant, ROS scavenger, and a stabilizer of protein and membrane structure and acts to protect photosynthesis under abiotic stress (Seckin et al. 2009; Kaya et al. 2013). Similarly, sorbitol is known to serve as compatible solute that may act also as free radical scavenger (Cuin and Shabala 2007). Amino acids can directly or indirectly influence the physiological activities in plant growth and development. Exogenous application of amino acids has been reported to modulate growth, production, and quality of tomato fruits (Boras et al. 2011). An increase of essential amino acids, and in particular of branched chain amino acids (e.g., valine and isoleucine), can be very useful because these metabolites can function as osmolytes for osmotic adjustment and as alternative electron donors for the mitochondrial electron transport chain (Woodrow et al. 2017). In addition, GABA increases by means of a GABA shunt can provide NADH and succinate to the electron transport chain in the case of impaired respiration and ROS excess (Carillo 2018). Despite proven evidence from the literature for the role/effects of these metabolites delivered as single-molecules on plant growth and stress protection, there is a need for an in-depth analysis of the functional specificities that link plant responses with phenological stage, dosage, and molecule interactions within a specific seaweed extract and/or commercial formulation to growth enhancement and stress protection. Applications of A. nodosum extracts may promote growth and yield under field conditions (Ali et al. 2016) or inhibit root growth at high concentrations (Finnie and van Staden 1985). In our experimental conditions, feeding these molecules via SWE may have contributed to a general growth enhancement. A moderate increase (+ 13%) of plant biomass with the Super Fifty applications was observed (Fig. 3), although no specific effect on salt stress tolerance was found under the experimental conditions used in this study. It is worth noting, however, that both Rygex and Super Fifty increased tomato leaf water potentials indicating that SWE may have a potential as stress protectants under saline and/or drought stress (Fig. 4).
Algal-based biostimulants enhanced qualitative aspects of tomato fruits and reduced the accumulation of toxic ions under salt stress
Treatment with both SWE formulations increased the content of a number of ions required for adaptation to salinity. The Ca2+ content of fruits increased by 31 and 22% for Rygex and Super Fifty, respectively. Content of K+ increased by 17% in Rygex-treated plants and by 45% in the Super Fifty treatment. Treatments with both SWEs increased Mg2+ content by 32%. At reduced nutrient concentrations, Ca2+ increased by 66% for both SWE applications compared to the control (Fig. 5). SWE applications (Kappaphycus alvarezii) stimulated mineral nutrient uptake in tomato with increased accumulation of both macronutrients (N, P, K, Ca, S) and micronutrients (Mg, Zn, Mn, Fe) and a subsequent increase in yield of tomato fruits (+ 60.89%) compared to control plants (Zodape et al. 2011). Under salinity, modifications in the leaf mineral contents can be mainly ascribed to competition between Na+ and K+ and between Cl− and NO3− (Grattan and Grieve 1999). Hyperaccumulation of Na+ disturbs essential cellular mechanisms such as protein synthesis, enzyme activity, and photosynthesis (Hasegawa 2013), and its exclusion paired with potassium uptake is crucial in adaptation to high salinity (Hasegawa et al. 2000). The algal extracts seem to improve this adaptive mechanism with a reduced Na+ cellular influx and consequent enhanced K+ accumulation in leaves of the Super Fifty-treated plants under moderate salt stress (Fig. 7). K+ is involved in different cellular functions, such as activation of enzymatic reactions, charge balancing, and osmoregulation (Wakeel et al. 2011). Therefore, the control of K+ homeostasis is fundamental in salinity tolerance. Salt stress generally reduced the NO3− leaf contents (Wang et al. 2012), and this effect could be mainly attributed to the competition with Cl−. The Cl−/NO3− balance was instead modified by the Rygex treatment, with a significant Cl− reduction in the leaf tissues and higher NO3− accumulation compared to control plants.
The GC-MS analysis of the two algal extracts revealed a higher proline content in Rygex, whereas Super Fifty had higher concentrations of polyols. This could explain the partial osmoprotectant behavior observed in the two algal extracts and the better exclusion of toxic Cl− ions in Rygex-treated plants. This was also observed in previous experiments with exogenous applications of proline in tomato plants (Heuer 2003). Although the accumulation of osmolytes is most effective in cellular osmotic adjustment (Chen and Murata 2002), proline and GABA are also substrate for mitochondrial respiration and redox control and are involved in the nitrogen balance, storage, and transport (Hasegawa et al. 2000; Bouché et al. 2004). GC-MS analysis on Super Fifty showed a larger amount of potassium than Rygex, resulting in a larger K+ availability in the growth media. Adequate potassium in the soil leads to a more efficient exclusion of sodium in higher plants and thus contributes to tolerance (Bonhert and Shen 1999). While both SWE formulations did not enhance growth under salt stress (Heuer 2003), they did reduce the impact on water potential through different effectors. Rygex contributed more proline, while Super Fifty contributed more polyols and K+. These results can contribute to novel formulations for the improvement of osmotic stress tolerance.
Both SWE formulations enhanced the ascorbate content of fruits, even in the presence of salt stress. Lola-luz et al. (2013) evaluated the effect of three commercial extracts of A. nodosum on phytochemical content and yield in cabbage. They observed higher phenolic and flavonoid content for the seaweed-treated plants; however, no statistically significant increases in yield with any of the seaweed extracts was found. Manna et al. (2012) obtained a significant increase in the fruit ascorbic acid content of pepper treated with an A. nodosum extract. Fan et al. (2011) obtained an increased phenolic and flavonoid content in spinach treated with an A. nodosum extract. These results found similar increases in ascorbic acid for tomato (Fig. 9) and demonstrate that these effects also extend to fruit quality of plants subjected to salt stress.
Conclusions
In the experimental conditions reported here, only moderate differences with both algal extracts and stress tolerance were observed. It was observed that plants had improved water relations under stress treatment and a number of fruit quality traits. Rygex and Super Fifty possess compositions that reduce the negative effects of water potential imbalance in the plant and enhance fruit quality. With the increased use of algal extracts as biostimulants in agriculture, it is essential to understand which active ingredients and their functional combinations in these formulations elicit beneficial responses for plant growth, yield, and stress adaptation.
References
Abdel-Mawgoud AMR, Tantaway AS, Magda MH, Hoda AM (2010) Seaweed extract improves growth, yield and quality of different watermelon hybrids. Res J Agric Biol Sci 6:161–168
Ahmad I, Pichtel J, Hayat S (2008) Plant-bacteria interactions. Strategies and techniques to promote plant growth. Wiley-VCH, Weinheim
Ahuja I, de Vos CHR, Bones AM, Hall RD (2010) Plant molecular stress responses face climate change. Trends Plant Sci 15:664–674
Ali N, Farrell A, Ramsubhag A, Jayaraman J (2016) The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J Appl Phycol 28:1353–1362
Annunziata MG, Ciarmiello LF, Woodrow P, Maximova E, Fuggi A, Carillo P (2017) Durum wheat roots adapt to salinity remodeling the cellular content of nitrogen metabolites and sucrose. Front Plant Sci 7:02035. https://doi.org/10.3389/fpls.2016.02035
Annunziata MG, Attico A, Woodrow P, Oliva MA, Fuggi A, Carillo P (2012) An improved fluorimetric HPLC method for quantifying tocopherols in Brassica rapa L. subsp. sylvestris after harvest. J Food Compos Anal 27:145–150
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Env Exp Bot 59:206–216
Barbieri G, Bottino A, Di Stasio E, Vallone S, Maggio A (2011) Proline and light as quality enhancers of rocket (Eruca sativa miller) grown under saline conditions. Sci Hortic 128:393–400
Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B (2015) Seaweed extracts as biostimulants in horticulture. Sci Hortic 196:39–48
Berlyn GP, Russo RO (1990) The use of organic biostimulants to promote root growth. Bioregul. in Fruit Prod., May 29–June 2, 2005, Leuven, pp: 243–248
Blunden G, Currie M, Mathe J, Hohmann J, Critchley AT (2009) Variations in betaine yields from marine algal species commonly used in the preparation of seaweed extracts used in agriculture (abstract). Phycologist 76:14
Blunden G, Gordon SM, Smith BE, Fletcher RL (1985) Quaternary ammonium compounds in species of the Fucaceae (Phaeophyceae) from Britain. Br Phycol J 20:105–108
Bonhert HJ, Shen B (1999) Transformation and compatible solutes. Sci Hort 78:237–260
Boras M, Zidan R, Halloum W (2011) Effect of amino acids on growth, production and quality of tomato in plastic greenhouse. Tishreen Univ J Res Sci Studies-Biol Sci Series 33(5):229–238
Bouché N, Fait A, Zik M, Fromm H (2004) The root-specific glutamate decarboxylase (GAD1) is essential for sustaining GABA levels in Arabidopsis. Plant Mol Biol 55:315–325
Bulgari R, Cocetta G, Trivellini A, Vernieri A, Ferrante A (2015) Biostimulants and crop responses: a review. Biol Agric Hortic 31:1–17
Calvo P, Nelson L, Kloepper JW (2014) Agricultural uses of plant biostimulants. Plant Soil 383:3–41
Carillo P (2018) GABA shunt in durum wheat. Front Plant Sci 9:100. https://doi.org/10.3389/fpls.2018.00100
Carillo P, Parisi D, Woodrow P, Pontecorvo G, Massaro G, Annunziata MG, Fuggi A, Sulpice R (2011) Salt-induced accumulation of glycine betaine is inhibited by high light in durum wheat. Funct Plant Biol 38:139–150
Chen T, Murata N (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5:250–257
Craigie JS (2011) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol 23:371–393
Cuin TA, Shabala S (2007) Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ 30:875–885
Dogra BS, Mandradia RK (2014) Effect of seaweed extract on growth and yield of onion. Int J Farm Sci 2:59–64
du Jardin P (2012) The science of biostimulants. A Bibliographic Analysis. (Final report for EU). Contract 30-CEO455515/00–96. p. 37
Du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic 196:3–14
Fan D, Hodges DM, Zhang J, Kirby CW, Ji X, Locke SJ, Critchley AT Prithiviraj B (2011) Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem 124:195–202
Finnie JF, van Staden J (1985) Effect of seaweed concentrate and applied hormones on in vitro cultured tomato roots. J Plant Physiol 120:215–222
Grattan SR, Grieve CM (1999) Mineral nutrient acquisition and response by plants grown in saline environments. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel Dekker, New York, pp 203–229
Halpern M, Bar-Tal A, Ofek M, Minz D, Muller T, Yermiyahu U (2015) The use of biostimulants for enhancing nutrient uptake. Adv Agron 130:141–174
Hasegawa PM (2013) Sodium (NaCl) homeostasis and salt tolerance of plants. Environ Exp Bot 92:19–31
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Heuer B (2003) Influence of exogenous application of proline and glycinebetaine on growth of salt-stressed tomato plants. Plant Sci 165:693–699
Kaya C, Sonmez O, Aydemira S, Ashraf M, Dikilitas M (2013) Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L). J Plant Interac 8:234–241
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, Critchley AT, Craigie JS, Norrie J, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J plant growth Regul 28: 386–399. Kinnersley AM, Turano FJ (2000) gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci 19:479–509
Kopka J, Fernie A, Weckwerth W, Gibon Y, Stitt M (2004) Metabolite profiling in plant biology: platforms and destinations. Genome Biol 5(6):109
Laetitia A, Fauchon M, Blanc N, Hauchard D, ArGall E (2010) Phenolic compounds in the brown seaweed Ascophyllum nodosum: distribution and radical-scavenging activities. Phytochem Anal 21:399–405
Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1:387–396
Lola-Luz T, Hennequart F, Gaffney M (2013) Enhancement of phenolic and flavonoids compounds in cabbage (Brassica oleraceae var italica) following application of commercial seaweed extracts of the brown seaweed, Ascophyllum nodosum. Agric Food Sci 22:288–295
Lötze E, Hoffman EW (2016) Nutrient composition and content of various biological active compounds of three south African-based commercial seaweed biostimulants. J Appl Phycol 28:1379–1386
Luedemann A, von Malotky L, Erban A, Kopka J (2012) TagFinder: preprocessing software for the fingerprinting and the profiling of gas chromatography-mass spectrometry based metabolome analyses. Meth Mol Biol 860:255–286
Malekzadeh P, Khara J, Heydari R (2014) Alleviating effects of exogenous gamma-aminobutiric acid on tomato seedling under chilling stress. Physiol Mol Biol Plants 20:133–137
Manna D, Sarkar A, Maity TK (2012) Impact of biozyme on growth, yield and quality of chilli (Capsicum annuum L.). J Crop Weed 8:40–43
Mattner SW, Wite D, Riches DA, Porter IJ, Arioli T (2013) The effect of kelp extract on seedling establishment of broccoli on contrasting soil types in southern Victoria, Australia. Biol Agric Hortic 29:258–270
Norrie J, Keathley JP (2005) Benefits of Ascophyllum nodosum marine-plant extract applications to Thompson seedless grape production. Acta Hortic 727:243–224
Obata T, Schoenefeld S, Krahnert I, Bergmann S, Scheffel A, Fernie AR (2013) Gas-chromatography mass-spectrometry (GC-MS) based metabolite profiling reveals mannitol as a major storage carbohydrate in the coccolithophorid alga Emiliania huxleyi. Metabolites 3:168–184
Okuma E, Murakami Y, Shimoishi Y, Tada M, Murata Y (2004) Effects of exogenous application of proline and betaine on the growth of tobacco cultured cells under saline conditions. Soil Sci Plant Nutr 50:1301–1305
Plaut Z, Edelstein ZM, Ben-Hur M (2013) Overcoming salinity barriers to crop production using traditional methods. Crit Rev Plant Sci 32:250–291
Queval G, Noctor G (2007) A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: application to redox profiling during Arabidopsis rosette development. Anal Biochem 363:58–69
Rayorath P, Benkel B, Hodges DM, Allan-Wojtas P, MacKinnon S, Critchley AT, Prithiviraj B (2009) Lipophilic components of the brown seaweed, Ascophyllum nodosum, enhance freezing tolerance in Arabidopsis thaliana. Planta 230:135–147
Rayorath P, Narayanan JM, Farid A, Khan W, Palanisamy R, Hankins S, Critchley AT, Prithiviraj B (2008) Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. Using a model plant, Arabidopsis thaliana (L.) Heynh. J Appl Phycol 20:423–429
Renault H, Roussel V, El Amrani A, Arzel M, Renault D, Bouchereau A, Deleu C (2010) The Arabidopsis pop2-1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol 10:20–20
Rosenzweig C, Iglesias A, Yang XB, Epstein PR, Chivian E (2001) Climate change and extreme weather events—implications for food production, plant diseases, and pests. Global Change Human Health 2:90–104
Rouphael Y, De Micco V, Arena C, Raimondi G, Colla G, De Pascale S (2017) Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange, and leaf anatomy of zucchini squash grown under saline conditions. J Appl Phycol 29:459–470
Seckin B, Sekmen AH, Turkan I (2009) An enhancing effect of exogenous mannitol on the antioxidant enzyme activities in roots of wheat under salt stress. J Plant Growth Regul 28:12–20
Sharma HSS, Lyons G, Mc Roberts C, Mc Call D, Carmichael E, Andrews F, Swan R, McCormack R, Mellon R (2011) Biostimulant activity of brown seaweed species from Strangford lough: compositional analyses of polysaccharides and bioassay of extracts using mung bean (Vigno mungo L.) and Pak Choi (Brassica rapa chinensis L.) J Appl Phycol 24:1081–1091
Siddiqui ZA, Akhtar MS, Futai K (2008) Mycorrhizae: sustainable agriculture and forestry. Springer, Berlin
Stirk W, Tarkowská D, Turecová V, Strnad M, Staden J (2014) Abscisic acid, gibberellins and brassinosteroids in Kelpak, a commercial seaweed extract made from Ecklonia maxima. J Appl Phycol 26:561–567
Van Oosten MJ, Pepe O, De Pascale S, Silletti S, Maggio A (2017) The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem Biol Techn Agric 4(1):5
Vernieri P, Borghesi E, Ferrante A, Magnani G (2005) Application of biostimulants in floating system for improving rocket quality. J Food Agric Environ 3:86–88
Wakeel A, Farooq M, Qadir M, Schubert S (2011) Potassium substitution by sodium in plants. Crit Rev Plant Sci 30:401–413
Wally OSD, Critchley AT, Hiltz D, Craigie JS, Han X, Zaharia LI, Abrams SR, Prithiviraj B (2012) Regulation of phytohormone biosynthesis and accumulation in Arabidopsis following treatment with commercial extract from the marine macroalga Ascophyllum nodosum. J Plant Growth Regul 32:324–339
Wang H, Zhang M, Guo R, Shi D, Liu B, Lin X, Yang C (2012) Effects of salt stress on ion balance and nitrogen metabolism of old and young leaves in rice (Oryza sativa L.) BMC Plant Biol 12:194
Woodrow P, Fuggi A, Pontecorvo G, Kafantaris I, Annunziata MG, Massaro G, Carillo P (2012) cDNA cloning and differential expression patterns of ascorbate peroxidase during post-harvest in Brassica rapa L. Mol Biol Rep 39:7843–7853
Woodrow P, Ciarmiello LF, Annunziata MG, Pacifico S, Iannuzzi F, Mirto A, D’Amelia L, Dell’Aversana E, Piccolella S, Fuggi A, Carillo P (2017) Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol Plant 159:290–312
Zodape ST, Gupta A, Bhandari SC et al (2011) Foliar application of seaweed sap as biostimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill.). J Sci Ind Res 70:215–219
Acknowledgements
The authors gratefully thank Dr. Saleh Alseekh and Prof. Dr. Alisdair R. Fernie of Max Planck Institut für Molekulare Pflanzen physiologie, Golm-Potsdam, Germany, for the help with the analyses of the algal extracts. This work was supported in part by the EU Project BIOFECTOR Plant Growth-Promoting Bio-Effectors (#FP7-KBBE-2012-6 Grant Agreement 312117).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Stasio, E., Van Oosten, M.J., Silletti, S. et al. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J Appl Phycol 30, 2675–2686 (2018). https://doi.org/10.1007/s10811-018-1439-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1439-9