Abstract
To purify cellulosic ethanol wastewater (CEW) and obtain algae simultaneously, culturing microalgae using CEW as the medium is a potentially promising technology. In this study, mutation breeding of Scenedesmus sp. induced by ultraviolet (UV) and ethyl methanesulfonate (EMS) was performed, and three mutant strains were screened using undiluted CEW. Among them, the mutant MU1 showed a high biomass yield of 1075 mg L−1 and biomass productivity of 39.81 mg L−1 day−1, and the lipid and protein content of MU1 can reach up to 21.4 and 41.9% (dry weight of biomass), respectively. Meanwhile, the chemical oxygen demand (COD), total ammonia nitrogen (TAN), total phosphorus (TP) and chroma (CHR) removal percentages in CEW reached to 33.5, 71.2, 46.4 and 17.8%, respectively. These results indicated that MU1 is suitable for purifying CEW and producing biomass simultaneously, providing a prospective way for CEW treatment and low-cost microalgae cultivation.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Compared with oil crops and food waste oil, microalgae can grow faster, require less land, and contain richer biomass, which makes microalgae prospective raw materials for producing biodiesel, bioethanol, feed and food products (Amini et al. 2016). However, the high cost of microalgal cultivation prevents microalgal biomass from being used in a large scale (Slade and Bauen 2013). To solve the problem, various technologies are being developed to reduce the cost of microalgae cultivation. For instance, Baer et al. (2016) found mixed light consisting of red, green and blue could improve microalgae productivity, and de Mooij et al. (2016) indicated microalgae perform photosynthesis at a high efficiency under low light conditions. Moreover, anaerobically digested piggery wastewater was used by Jia et al. (2016) as a cheap culture medium for microalgal growth, and Yang et al. (2015) found that the appropriate proportion between anaerobic digested starch wastewater and alcohol wastewater could improve microalgal growth. Among these technologies, cultivating microalgae in cheap wastewater may be a better choice to reduce the cost of microalgae cultivation and reuse the wastewater simultaneously.
The cellulosic ethanol production has received much attention around the world and large amounts of cellulosic ethanol wastewater (CEW) are produced during the production process (Mabee et al. 2011). It is predicted that 7.95 × 106 m3 of cellulosic biofuels will be produced by 2022 in USA, and 1.59 × 107 m3 of CEW will be accompanied by the generation of cellulosic ethanol (Dwivedi et al. 2009). CEW is composed of many compounds, e.g., humic acids, furfurals, phenolic compounds, aldehydes and aliphatic organic compounds, generated from different procedures of cellulosic ethanol production, which are difficult to be utilized by microorganism and might lead to high chroma (CHR) in CEW (Shan et al. 2016). Thus, dealing with so much CEW will be a new challenge in the future. Although various methods such as catalytic oxidation reaction, anaerobic fermentation, and membrane filtration have been used to treat CEW, the concentrations of nutrients in CEW were still very high (Zhao 2013). Taking into account the advantages of culturing microalgae in wastewater, incubating microalgae in the treated CEW can not only remove the nutrients in CEW, but also reduce the cost of culturing microalgae. The purpose of purifying CEW and obtaining microalgae simultaneously can be achieved.
Over the past 50 years the unicellular green algae Scenedesmus sp. has been used in wastewater treatment (Hintz et al. 1966; Garcia-Moscoso et al. 2013; Guerrero-Cabrera et al. 2014; Duong et al. 2015; Leite et al. 2016). However, in most studies, the chemical oxygen demand (COD), total ammonia nitrogen (TAN) and total phosphorus (TP) in wastewater were much lower than those of CEW (Li et al. 2011; Cabanelas et al. 2013; Chiu et al. 2015). As a kind of highly concentrated wastewater, the concentrations of COD, TAN, and TP in CEW were up to 5749.0, 865.5 and 12.24 mg L−1, respectively, in this study. Such high-concentration organic constituents will inhibit the growth of microalgae in CEW. Till now, to our knowledge, little research on culturing microalgae in CEW has ever been reported. Herein, developing the practical technology for cultivating microalgae in CEW is necessary and imperative.
In this study, the mutant strains with high tolerance to CEW were first screened. Ultraviolet light (UV) and ethyl methanesulfonate (EMS) were used as physical mutagen and chemical mutagen to improve the tolerance of Scenedesmus sp. (Fu et al. 2014; Zhang et al. 2016). Moreover, the growth characteristics and stability of mutants as well as the nutrients removal efficiency in CEW were also investigated. The results showed that the mutant was suitable for purifying highly concentrated CEW and producing biomass simultaneously, which has the practical potential in CEW treatment and raw material supply for biodiesel production.
Materials and methods
Microalgae strain and cultivation conditions
Scenedesmus sp. (FACHB-489) was obtained from the Freshwater Algae Culture Collection at the Institute of Hydrobiology (Wuhan, China). The initial strain was temporarily incubated in sterile BG11 medium (Luo et al., 2016). The cultivation conditions were 46 μmol photons m−2 s−1, pH 7.5 ± 0.5, and 25 ± 1 °C.
Source of wastewater
The CEW treated by anaerobic digestion was collected from Tianguan Fuel Ethanol Company (Nanyang, China). The constituents of CEW was analyzed by GC-MS, and the major constituents are shown in the supplementary material (Fig. S1 and Table S1). In the study, the suspended solids in CEW were simply filtered by gauze of eight layers, and the COD, TAN, TP, CHR and pH in CEW are shown in Table 1.
Mutation procedures and screening mutants
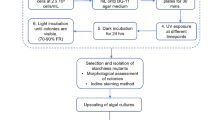
The procedures of mutation and screening as well as culturing are presented in Fig. 1.
UV mutation procedure: The suspension liquid with an algae density of 400 cells mL−1 was plated onto sterile BG11 medium solidified with 1.5% agar in Petri dishes. Then, the initial strain was irradiated by a 20 W UV lamp (GPH436, Jie Kang UV Lamp Co., Ltd., China; λ = 254 nm) at a distance of 35 cm for different time intervals (30, 60, 100, 120, 180, 240 and 270 s). After that, the initial strain was kept in darkness for 24 h, followed by incubation at 46 μmol photons m−2 s−1 and 25 ± 1 °C for 2 weeks. The optimal irradiation time was determined based on the cell percent mortality calculated by the observed quantities of single colonies on the plates. Each treatment was performed in triplicate. The cell percent mortality was calculated as follows:
where N 0 and N are the observed quantities of single colonies on the plates from experimental group and control group, respectively.
EMS mutation procedure: The suspension (2 mL) with an algae density of 2.2 × 107 cells mL−1 was harvested by centrifugation at 3007×g for 5 min and the algal pellet was incubated for 2 h in 2 mL of variously concentrated EMS (0.05, 0.1, 0.2, 0.3 and 0.4 M) prepared in phosphate buffer (pH 7.0). Another group was also treated the same way and the algal pellet was inoculated in 2 mL of 0.2 M EMS for different time intervals (0.5, 1, 2, 3 and 4 h). Thereafter, all the treated groups were first washed with sodium thiosulfate solution (5%, w/v), and then with BG11 medium for three times to remove the residual EMS. The algae from all treatments were inoculated in sterile BG11 media solidified with 1.5% agar in Petri dishes and then kept in darkness for 24 h, followed by being incubated under 46 μmol photons m−2 s−1 and 25 ± 1 °C for 2 weeks. The optimal EMS dose and treatment time were determined according to the quantity of single colonies observed on dishes.
Screening mutants: In this process, BG11 medium was replaced by undiluted CEW solidified with 1.5% agar. The dishes plated with algae were irradiated by a UV lamp of 20 W at the distance of 35 cm under the condition of the optimal irradiation time. Simultaneously, another group was treated under the optimal condition of EMS dose and treatment time. Subsequently, the selected mutant strains were inoculated into sterile undiluted CEW solidified with 1.5% agar for three times to ensure the growth stability of mutant strains.
Culturing mutants in undiluted CEW
The mutated strains were grown in 250-mL Erlenmeyer flasks with undiluted CEW. The cultivation conditions were as follows: inoculum density 0.3 g L−1 (dry weight, DW), 63 μmol photons m−2 s−1, pH 8.0 ± 0.5, and 25 ± 2 °C. The optical density (OD) of the suspension was monitored every 3 days. Each experimental group was performed in triplicate and the undiluted CEW without microalgae was used as the control group.
Growth characteristics of microalgae
The microalgae densities were monitored using the OD of the suspension as indicator, and the OD was determined with undiluted CEW as reference solution by a spectrophotometer (UV-2102PC, Shanghai Instrument Co., Ltd., China) at 680-nm wavelength. The specific growth rate (μ) was calculated according to Eq. (2).
where N 2 and N 1 are microalgal densities at time t 2 and t 1 , respectively.
All strains were harvested by centrifugation (1925×g, 20 min) after 27 days of growth and the dry weight of microalgae was measured gravimetrically with control groups as reference.
Analysis of COD, TP, TAN and CHR in CEW
The COD and TP in CEW were analyzed by a multi-parameter water analysis instrument (5B-3B (V8), Beijing LianHua Technology Co., Ltd., China) with undiluted CEW as reference solution. The TAN in CEW was measured by Nessler Reagent Spectrophotometry (HJ535-2009) with undiluted CEW as reference solution (Wu et al. 2016). Meanwhile, CHR in CEW was determined by a water chroma analysis instrument (XZ-S, Shanghai HaiZheng Electronic Technology Company, China). Thus, the nutrient removal percentages (CODrp, TPrp, TANrp, CHRrp) were calculated according to Eqs. (3)–(6):
where COD1, TP1, TAN1 and CHR1 and COD2, TP2, TAN2 and CHR2 are the COD, TP, TAN, and CHR in CEW before and after growth of microalgae.
Total lipid extraction
The total lipids in microalgae were measured gravimetrically using a modified method according to the Ryckebosch et al. (2012). One hundred-milligram dry algae powder was added into a 10 mL centrifuge tube, followed by 2 mL distilled water. Then, the tube containing algae and distilled water was heated in a water bath at 70 °C for 15 min and then frozen immediately to −20 °C for 30 min to disrupt cells of microalgae. The step of heating and cooling was repeated four times, after which 4 mL solvent mixtures of chloroform: methanol (2:1, v/v) were added and then 2 mL distilled water was subsequently added for another 10 min. The suspensions were centrifuged at 1925×g for 10 min; then, the chloroform extracts were collected and the algae residual was extracted for another four times. All the chloroform extracts were combined and dried to constant weight. The total lipid content was expressed as a dry weight percentage (% of biomass DW).
Fatty acid composition
Fatty acid (FA) was first converted into fatty acid methyl esters (FAMEs) using 2% H2SO4–MeOH solution and then analyzed by gas chromatography-mass spectrometry (GC-MS) with FID detector (Shimadzu, GC-2010, Japan) using a Supelco SP-2340 fused silica capillary column (30 m × 0.25 mm × 0.2 μm) (Wu et al. 2011). The content of FA constituents was expressed as a weight percentage (% of total FAs).
Analysis of protein
The protein content in microalgae was determined by the Kjeldahl Method (Wang et al. 2016). Then, 0.4 g dry algae powder was used for nitrogen determination. The protein content was calculated according to Eq. (7).
where V 1 and V 2 are the volume (mL) of titration solution (HCl) used for sample groups and the volume (mL) of titration solution used for blank group. N is the concentration (mol L−1) of HCl and m is the sample weight (g). F is the nitrogen to protein conversion factor (F = 6.25) and the constant of 0.0140 is the weight of nitrogen corresponding to 1 mL of 1 M HCl.
All the data are expressed as the mean ± standard deviation. The results were analyzed by one-way ANOVA and two-tailed t test. p < 0.01 indicates a statistically significant difference. The error bars in all figures show the standard deviation.
Results
Comparisons of mutagenesis efficiency on Scenedesmus sp. between UV and EMS
The CEW mainly consists of phenol, ketone, aldehyde, benzene and heterocyclic compounds (Table S1) which are commonly toxic to most of microorganisms. It was observed that the original wild-type Scenedesmus sp. (FACHB-489) could not grow well in CEW due to the inhibitation effect of harmful constituents in CEW. To improve the resistance to CEW, UV and EMS were used as the mutagens and the mutagenesis efficiencies of UV and EMS were investigated.
The percent mortality of the microalgae cells increased from 6 to 100% as the UV radiation time increased from 30 to 270 s (Fig. 2a). When the radiation time was 60 s, the percent mortality sharply increased to 44%, which was six times more than that at 30 s exposure. Furthermore, the extension of irradiation time resulted in an obvious increase of percent mortality. When the irradiation time was 120 s, the percent mortality was 72%. When the irradiation time surpassed 180 s, the percent mortality was above 90%, which means that most algae cells were killed by UV. As shown in Fig. 2b, as the EMS concentration increased, the percent mortality of Scenedesmus sp. increased gradually. It was observed that when the treatment time was fixed to 2 h, at a low EMS concentration of 0.2 M, the percent mortality was about 75%. When the concentrations of EMS were increased to 0.3 and 0.4 M, the percent mortality increased to 82 and 90%. Similarly, under the fixed EMS concentration of 0.2 M, the percent mortality of Scenedesmus sp. cells also increased with the prolonging of treatment time (Fig. 2c). When the treatment time was less than 2 h, the percent mortality was lower than 75%. A higher percent mortality of 85 and 95% was obtained with treatment times of 3 and 4 h.
Mutant clones were observed on plates containing undiluted CEW solidified with 1.5% agar under the condition of UV irradiation after 13 days of growth. Fifteen large and deep green strains named MU1~MU15 were first selected and then transferred onto the new CEW solidified with 1.5% agar for three times. It was found that only MU1, MU2, and MU15 were able to form clones steadily (Fig. 3a). Moreover, the morphology of the three mutant strains was different from that of initial wild-type strain (Fig. 3b). Unfortunately, no clones were observed under the condition of EMS treatment.
Growth characteristics and stability of Scenedesmus sp.
The growth characteristics of initial algae and mutants in CEW are shown in Fig. 4. Almost no growth of cells was observed for initial algae throughout the cultivation of 27 days. In comparison, the cell concentrations of three mutant strains increased greatly at the end of cultivation, and the precipitated microalgae can be easily observed. The biomass yields, biomass productivities and specific growth rates of all strains are shown in Table 2. The maximum biomass yield of 1074.8 mg L−1 was obtained from MU1, showing a 423.8% increase than that of the initial strain. A 259.1 and 326.7% increase in biomass yields of MU2 and MU15 was also obtained. The biomass productivity which was closely related to biomass yield and cultivation time showed a similar trend as biomass yield. Meanwhile, a low specific growth rate of 0.0042 day−1 was obtained from the initial strain. However, the specific growth rates of MU1, MU2 and MU15 increased significantly compared to that of initial strain, and they were 0.060, 0.045 and 0.0685 day−1, respectively.
To investigate the growth stability of three mutant strains, the biomass yields, lipid contents, and protein contents in three mutant strains were determined after serial subculture for nine times in undiluted CEW (Fig. 5). The biomass yields in MU1, MU2 and MU15 decreased slightly after nine times of subculture (Fig. 5a) and the protein contents in MU1, MU2 and MU15 also showed a slight decrease (Fig. 5b). The lipid contents in MU1, MU2 and MU15 were 21.45, 23.75 and 18.55%, respectively, at the end of subculture (Fig. 5c), which were slightly lower than that before subculture.
a The biomass yield (n = 3, t test: t(4) = 2.731, 2.416 and 2.654, p = 0.052, 0.073, and 0.057 for MU1, MU2 and MU15, respectively). b Protein content (n = 3, t test: t(4) = 1.967, 2.656 and 2.328, p = 0.121, 0.057 and 0.080 for MU1, MU2 and MU15, respectively). c Lipid content (n = 3, t test: t(4) = 1.559, 2.303 and 1.7955, p = 0.194, 0.083 and 0.147 for MU1, MU2 and MU15, respectively) in mutant strains before and after serial subcultures for nine times (pH 8.0, 63 μmol photons m−2 s−1, 25 °C and inoculum density 0.3 g L−1)
Protein and lipid content and fatty acids composition
The protein contents and protein yields in all strains are shown in Table 3. The protein contents in all algae were in the range of 25~45%. The maximum protein content (41.9%) and protein yield (450.3 mg L−1) were obtained from MU1, an increase of 3 and 441% compared to the initial strain. In MU2 and MU15, protein content decreased by 3 and 12% compared with the initial strain. However, the protein yields in MU2 (290.3 mg L−1) and MU15 (311.7 mg L−1) increased by 249 and 274%.
The total lipid contents in microalgae were measured and expressed as a weight percentage of dry algae (Table 3). Fifty percent more lipid than the initial strain was produced by MU2, showing the maximum lipid content (23.8%). Compared with the initial strain, a 35 and 17% increased lipid content was obtained in MU1 and MU 15, respectively.
The lipid yields in all algae are shown in Table 3. MU1 showed the maximum lipid yield of 230.0 mg L−1, increasing by 606% compared with the initial strain. Lipid yields in MU2 and MU15 also increased by 438 and 400% compared with the initial strain.
The fatty acids (FAs) composition in all algae is shown in Table 4. The FAs mainly consisted of palmitic acid (C16:0), octadecenoic acid (C18:1n9), linoleic acid (C18:2n6), and linolenic acid (C18:3n3). For each strain, the total contents of C16 and C18 were above 88% (of total FAs). In addition, polyunsaturated fatty acids (PUFAs) and monounsaturated fatty acids (MUFAs) are two important indexes for biodiesel production. The content of PUFAs in initial strain (44.63%) was slightly lower than that in MU1 (46.21%) and a little higher than in MU2 (43.00%) and MU15 (43.22%). The MUFAs contents in MU1, MU2 and MU15 were 33.33, 34.17 and 31.64%, respectively, which were slightly higher than the 31.58% in the initial strain.
Nutrients removal
Purification efficiency on CEW by microalgae at the end of fermentation was measured and the nutrients removal percentage in CEW was calculated. As shown in Fig. 6, little nutrient in CEW was removed by the wild-type initial strain. However, significant nutrient removal percentages, particularly TANrp in CEW by the three mutant strains can be observed. The maximum CODrp of 38.9% in CEW was obtained from MU2, which was 2892% higher than for the initial strain. In addition, 2677 and 2477% increased CODrp by MU1 and MU15 over initial strain were obtained. As shown in Fig. 6, the maximum TANrp and TPrp with a value of 71.6 and 47.1% were obtained from MU1. No significant difference with TANrp among three mutant strains can be found, while TPrp by MU1 was 1863, 76 and 26% higher than that of initial strain, MU2 and MU15, respectively. The CHRrp in CEW was also investigated in this study. Using distilled water as the reference, MU1 showed the maximum CHRrp (17.8%), which was 4350, 80 and 9% higher than initial strain, MU2 and MU15, respectively.
Discussion
Comparisons of mutagenesis efficiency on Scenedesmus sp. between UV and EMS
UV irradiation can cause the polymerization between two DNA pyrimidines, which will inhibit the DNA double helix from unwinding and then interfere with DNA replication. As the radiation time ranged from 30 to 60 s, the percent mortality of microalgae increased sharply, which suggested that the sensitive region of cell mutation was between 30 and 60 s. The results indicated that UV radiation is an efficient mutagenesis method for breeding of Scenedesmus sp. It has been reported that low percent mortality might lead to reverse mutation in subculture, and a percent mortality range of 75~90% might have a positive impact on mutagenesis for cells (Liu et al. 2015). Therefore, 120 s was selected to be the optimal radiation time in the study.
EMS treatment can lead to mismatches among basic pairs by alkylation reaction between active alkyl of EMS and DNA hydrogen atom. With the increase of EMS concentration and treatment time, the percent mortality of Scenedesmus sp. showed a similar trend to that of UV irradiation (Fig. 2b, c). Thus, considering the combined effects of EMS concentration and treatment time, 0.2~0.3 M EMS and 2~3 h of treatment time were chosen as the optimal mutagenic conditions.
MU1, MU2 and MU15 could grow steadily in CEW solidified with 1.5% agar for three times (Fig. 2a), suggesting the metabolic pathway of the three mutant algae might have been improved by UV. Moreover, the shapes of mutants were observed like an ellipse and the sizes of mutants were almost half of that of initial strain, which may increase the specific surface areas of mutant strains. The lack of microalgae being observed after EMS treatment may be due to the difference of mutagenesis mechanism between UV and EMS mutagenesis. The results indicated that UV mutagenesis is more suitable to breed Scenedesmus sp.
The growth characteristics and stability
The poor growth of the wild-type initial algae in CEW was probably due to the inhibition effect of toxic constituents in CEW. In addition, it has been reported that the optimal ratio of nitrogen to phosphorus (N/P ratio) for microalgae biomass production was 8~12, and more than 22 of N/P ratio will have a negative influence on microalgal growth (Huo et al. 2012; Cabanelas et al. 2013; Chiu et al. 2015; Rasdi and Qin 2015). The N/P ratio was 70:1 in CEW, which may be another reason for the poor growth of the initial algae. However, all of the mutant strains grew well, suggesting that the tolerance of all mutant strains to CEW had been improved. In addition, the possible increased specific surface area of the mutants which equals the surface area of microalgae divided by the volume of microalgae, can also result in an increase of nutrient absorption (Fig. 3b). This might enhance the cell growth of the mutant strains as well. As for the growth stability of the three mutant strains, the decreases in biomass yield and lipid and protein content were insignificant, which was similar to the previous research (Zhang et al. 2016). These results showed that all the mutant strains can grow steadily in CEW-based medium. Among them, strain MU1 was more suitable for CEW medium and has potential to purify CEW and to be used as the raw materials of biomass production.
The protein and lipid content and fatty acids composition
The protein content in all microalgae was comparable to previous reports (Guerrero-Cabrera et al. 2014; Duong et al. 2015) and the protein contents in all strains were also similar. This indicated that UV irradiation had little effect on protein content in the microalgae. However, it can be seen that the protein yields in the three mutant strains were much higher than that of the initial strain, which was mainly due to the high biomass yields.
It is known that lipid content is an important indicator for biodiesel production. To improve the lipid content, it has been reported that nitrogen starvation strategy can be adopted (Xu et al. 2015). Thus, the excess nitrogen in CEW might inhibit lipid accumulation in the microalgae. Fortunately, we found the lipid content (10~35%) in this study was comparable to previous results (Xu et al. 2015; Gour et al. 2016; Zhan et al. 2016). It can be inferred that the metabolic mechanism related to lipid accumulation in mutants might have improved. The lipid content and yield showed a similar trend to the protein content and yield. Since the lipid content changed little, the high lipid yield mainly benefited from the higher biomass yield. The FA compositions in all algae were different in different degrees, probably due to the different adaptability in the wastewater (Han et al. 2016). PUFAs can result in oxidation and MUFAs could enhance the low-temperature liquidity. Both of them can improve biodiesel quality (Knothe 2008; Gour et al. 2016). Moreover, the total content of C16 and C18 in all strains were above 88%. This suggests that all strains could be used for biodiesel production from the point of their FAs composition.
Nutrients removal
Nutrients removal in CEW was closely related to the growth of the microalgae, indicating the cultivation conditions, such as pH, temperature and light intensity have effects on nutrients removal (Di Pippo et al. 2012). In this study, the initial COD in CEW was 5749.0 mg L−1. Thus, 1% of CODrp corresponds to COD decrement of 57.5 mg L−1, suggesting that although the CODrp was lower, the absorbed COD by three mutant strains was actually higher than those in most reports (Cabanelas et al. 2013; Chiu et al. 2015). At the end of cultivation, the COD in CEW decreased greatly to 3514 mg L−1. Wen et al. (2016) treated anaerobically digested swine wastewater with an initial COD of 4800 mg L−1 using two strains of photosynthetic bacteria and COD decrement in wastewater reached 3998 mg L−1 which was much higher the 2235 mg L−1 in this study. The reason may be that initial high nutrient concentrations could result in high osmotic pressure and high concentration of toxic substances in wastewater, which were harmful for microalgae to grow. Since the value of COD was still high, different methods might need to be combined with algal cultivation to further decrease the COD (Zhao 2013).
Nitrogen and phosphorus removal percentages are important indicators in wastewater purification. The content of nitrogen was significantly higher than that of phosphorus and the main form of nitrogen was ammonia nitrogen in CEW (Table 1). It was reported that more than 450 mg L−1 of TAN is commonly toxic to algae (Jia et al. 2016). Therefore, TANrp can be regarded as an important indicator for the purification of CEW. In addition, high chroma caused by humic acid-like matters in CEW also result in a low purification efficiency in CEW, and the deep color of CEW has a significantly negative effect on the utilization of light by microalgae (Shan et al. 2016). In the study, although the TAN and CHR in CEW reached up to 865.5 mg L−1 and 4242 times, respectively, higher TANrp can be obtained. Moreover, effects of illumination and TP content on microalgal yield were further investigated. It was found the biomass yields of the mutant strains were increased when using diluted CEW as the medium. Meanwhile, when the irradiance was increased to 100 μmol photons m−2 s−1, the biomass yield of these mutant strains was also increased to some extent. However, only less than 10% of increase can be obtained. Interestingly, when the TP content in CEW was increased, the biomass yield of these mutants increased. This result indicated that the N/P ratio in CEW plays a key role. It has been reported that Scenedesmus requires an N/P ratio of ~ 30 to grow without limitation (Lavoie and de la Noüe 1985). Therefore, an appropriate increase of TP content in CEW may be an effective way to increase the microalgae yield.
In general, MU1 compared with MU2 and MU15, showed rapid growth and superior nutrients removal in CEW. It has practical potential for CEW treatment and low-cost cultivation. MU1 can be used as good material for biodiesel production due to its higher FAs of C16 and C18 (> 88%). Therefore, it is suitable for purifying CEW and producing biomass simultaneously. Future work will focus on further increasing the biomass yield in MU1 and improving the purification efficiency on wastewater by MU1.
Conclusions
UV mutation breeding was an efficient method to improve the tolerance of Scenedesmus sp. to CEW. The mutant strain MU1 showed a rapid growth in CEW and a good stability in producing biomass with the highest biomass yield of 1074.8 mg L−1. Moreover, compared with initial wild-type strain, a 441% increase of protein and 606% increase of lipid yields was obtained from MU1. Along with the growth of MU1, the COD, TAN, TP, and CHR in CEW were decreased significantly. The results showed that MU1 is suitable for purifying CEW and producing biomass simultaneously.
References
Amini H, Wang L, Shahbazi A (2016) Effects of harvesting cell density, medium depth and environmental factors on biomass and lipid productivities of Chlorella vulgaris grown in swine wastewater. Chem Eng Sci 152:403–412
Baer S, Heining M, Schwerna P, Buchholz R, Hübner H (2016) Optimization of spectral light quality for growth and product formation in different microalgae using a continuous photobioreactor. Algal Res 14:109–115
Cabanelas ITD, Ruiz J, Arbib Z, Chinalia FA, Garrido-Pérez C, Rogalla F, Nascimento IA, Perales JA (2013) Comparing the use of different domestic wastewaters for coupling microalgal production and nutrient removal. Bioresour Technol 131:429–436
Chiu S, Kao C, Chen T, Chang Y, Kuo C, Lin C (2015) Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour Technol 184:179–189
de Mooij T, de Vries G, Latsos C, Wijffels RH, Janssen M (2016) Impact of light color on photobioreactor productivity. Algal Res 15:32–42
Di Pippo F, Ellwood NTW, Guzzon A, Siliato L, Micheletti E, De Philippis R, Albertano PB (2012) Effect of light and temperature on biomass, photosynthesis and capsular polysaccharides in cultured phototrophic biofilms. J Appl Phycol 24:211–220
Duong VT, Ahmed F, Thomas-Hall SR, Quigley S, Nowak E, Schenk PM (2015) High protein- and high lipid-producing microalgae from northern Australia as potential feedstock for animal feed and biodiesel. Front Bioeng Biotech 3:53
Dwivedi P, Alavalapati JRR, Lal P (2009) Cellulosic ethanol production in the United States: conversion technologies, current production status, economics, and emerging developments. Energy Sust Dev 13:174–182
Fu F, Sui Z, Zhou W, Wang J, Chang L, Ci S (2014) UV-irradiation mutation of tetraspores of Gracilariopsis lemaneiformis and screening of thermotolerant strains. J Appl Phycol 26:647–656
Garcia-Moscoso JL, Obeid W, Kumar S, Hatcher PG (2013) Flash hydrolysis of microalgae (Scenedesmus sp.) for protein extraction and production of biofuels intermediates. J Supercrit Fluid 82:183–190
Gour RS, Chawla A, Singh H, Chauhan RS, Kant A (2016) Characterization and screening of native Scenedesmus sp. isolates suitable for biofuel feedstock. PLoS One 11(5):e0155321
Guerrero-Cabrera L, Rueda JA, García-Lozano H, Navarro AK (2014) Cultivation of Monoraphidium sp., Chlorella sp. and Scenedesmus sp. algae in batch culture using Nile tilapia effluent. Bioresour Technol 161:455–460
Han S, Jin W, Tu R, Abomohra AE, Wang Z (2016) Optimization of aeration for biodiesel production by Scenedesmus obliquus grown in municipal wastewater. Bioprocess Biosyst Eng 39:1073–1079
Hintz HF, Heitman H, Weir WC, Torrell DT, Myers JH (1966) Nutritive value of algae grown on sewage. J Anim Sci 25:675–681
Huo S, Wang Z, Zhu S, Zhou W, Dong R, Yuan Z (2012) Cultivation of Chlorella zofingiensis in bench-scale outdoor ponds by regulation of pH using dairy wastewater in winter, South China. Bioresour Technol 121:76–82
Jia Q, Xiang W, Yang F, Hu Q, Tang M, Chen C, Wang G, Dai S, Wu H, Wu H (2016) Low-cost cultivation of Scenedesmus sp. with filtered anaerobically digested piggery wastewater: biofuel production and pollutant remediation. J Appl Phycol 28:727–736
Knothe G (2008) “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energ Fuel 22:1358–1364
Lavoie A, de la Noüe J (1985) Hyperconcentrated culture of Scenedesmus obliquus: a new approach for wastewater tertiary treatment? Water Res 19:1437–1442
Leite GB, Paranjape K, Hallenbeck PC (2016) Breakfast of champions: fast lipid accumulation by cultures of Chlorella and Scenedesmus induced by xylose. Algal Res 16:338–348
Li Y, Chen YF, Chen P, Min M, Zhou W, Martinez B, Zhu J, Ruan R (2011) Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour Technol 102:5138–5144
Liu S, Zhao Y, Liu L, Ao X, Ma L, Wu M, Ma F (2015) Improving cell growth and lipid accumulation in green microalgae Chlorella sp. via UV irradiation. Appl Biochem Biotech 175:3507–3518
Luo L, He H, C2 Y, Wen S, Zeng G, Wu M, Zhou Z, Lou W (2016) Nutrient removal and lipid production by Coelastrella sp. in anaerobically and aerobically treated swine wastewater. Bioresour Technol 216:135–141
Mabee WE, McFarlane PN, Saddler JN (2011) Biomass availability for lignocellulosic ethanol production. Biomass Bioenergy 35:4519–4529
Rasdi NW, Qin JG (2015) Effect of N:P ratio on growth and chemical composition of Nannochloropsis oculata and Tisochrysis lutea. J Appl Phycol 27:2221–2230
Ryckebosch E, Muylaert K, Foubert I (2012) Optimization of an analytical procedure for extraction of lipids from microalgae. J Am Oil Chem Soc 89:189–198
Shan L, Liu J, Yu Y, Ambuchi JJ, Feng Y (2016) Characterization of persistent colors and decolorization of effluent from biologically treated cellulosic ethanol production wastewater. Environ Sci Pollut Res 23:10215–10222
Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 53:29–38
Wang H, Pampati N, McCormick WM, Bhattacharyya L (2016) Protein nitrogen determination by Kjeldahl digestion and ion chromatography. J Pharm Sci 105:1851–1857
Wen S, Liu H, He H, Luo L, Li X, Zeng G, Zhou Z, Lou W, Yang C (2016) Treatment of anaerobically digested swine wastewater by Rhodobacter blasticus and Rhodobacter capsulatus. Bioresour Technol 222:33–38
Wu HL, Hu ZB, Chai XS, Wang C, Yang OM (2016) Rapid detection of ammonia nitrogen in water with dual-wavelength spectroscopy. Spectrosc Spectr Anal 36:1396–1399
Wu X, Tong Y, Shankar K, Baumgardner JN, Kang J, Badeaux J, Badger TM, Ronis MJ (2011) Lipid fatty acid profile analyses in liver and serum in rats with nonalcoholic steatohepatitis using improved gas chromatography−mass spectrometry methodology. J Agric Food Chem 59:747–754
Xu X, Shen Y, Chen J (2015) Cultivation of Scenedesmus dimorphus for C/N/P removal and lipid production. Electron J Biotechnol 18:46–50
Yang L, Tan X, Li D, Chu H, Zhou X, Zhang Y, Yu H (2015) Nutrients removal and lipids production by Chlorella pyrenoidosa cultivation using anaerobic digested starch wastewater and alcohol wastewater. Bioresour Technol 181:54–61
Zhan J, Zhang Q, Qin M, Hong Y (2016) Selection and characterization of eight freshwater green algae strains for synchronous water purification and lipid production. Front Env Sci Eng 10:548–558
Zhang Y, He M, Zou S, Fei C, Yan Y, Zheng S, Rajper AA, Wang C (2016) Breeding of high biomass and lipid producing Desmodesmus sp. by ethylmethane sulfonate-induced mutation. Bioresour Technol 207:268–275
Zhao YB (2013) Study on treatment of cellulose fuel ethanol wastewater and application. Adv Mater Res 777:365–369
Acknowledgements
The authors gratefully acknowledge the financial support from the Natural Science Foundation of Henan Province of China (162300410247); the Foundation and Frontier Technology Research Project of Henan Province of China (162300410007), the Sino-Thai project of National Natural Science Foundation of China (51561145015), and the Key project of Natural Science Foundation of Guangdong Province (No. 2015A030311022).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(PDF 258 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Chang, C., Bai, J. et al. Mutants of Scenedesmus sp. for purifying highly concentrated cellulosic ethanol wastewater and producing biomass simultaneously. J Appl Phycol 30, 969–978 (2018). https://doi.org/10.1007/s10811-017-1311-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1311-3