Abstract
The high chroma of cellulosic ethanol production wastewater poses a serious environmental concern; however, color-causing compounds are still not fully clear. The characteristics of the color compounds and decolorization of biologically treated effluent by electro-catalytic oxidation were investigated in this study. Excitation-emission matrix (EEM), fourier transform infrared spectrometer (FTIR), UV-Vis spectra, and ultrafiltration (UF) fractionation were used to analyze color compounds. High chroma of wastewater largely comes from humic materials, which exhibited great fluorescence proportion (67.1 %) in the biologically treated effluent. Additionally, the color compounds were mainly distributed in the molecular weight fractions with 3–10 and 10–30 kDa, which contributed 53.5 and 34.6 % of the wastewater color, respectively. Further decolorization of biologically treated effluent by electro-catalytic oxidation was investigated, and 98.3 % of color removal accompanied with 97.3 % reduction of humic acid-like matter was achieved after 180 min. The results presented herein will facilitate the development of a well decolorization for cellulosic ethanol production wastewater and better understanding of the biological fermentation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulosic ethanol is considered a promising source of substitute energy because ethanol is the only kind of biomass-based liquid fuel which can be used for power fuel. Considering the abundance of lignocellulosic resources in the world, the production of fuel ethanol from cellulosic materials attracted more attention in recent years (Dwivedi et al. 2009, Mabee et al. 2011). However, substantial increases in cellulosic ethanol production will also result in large quantities of high-strength, high-color organic wastewater. Highly colored wastewater can have negative environmental impacts if discharged into surface water, which might reduce the penetration of solar energy into shallow waters, cause death and decay of aquatic plants, and also contribute to eutrophication (Wilkie et al. 2000).
The color of wastewater is usually hardly degraded by the conventional treatments and can even be deepened during anaerobic treatments, due to re-polymerization of color organic compounds (Pant and Adholeya 2007). However, characteristics of the color-causing compounds are still not fully clear. From the published paper, it can be suggested that the phenolics (tannic and humic acids) from the feedstock, melanoidins from Maillard reaction of sugars (carbohydrates) with proteins (amino groups), caramels from overheated sugars, and furfurals from acid hydrolysis can contribute to the color of the effluent (Kort 1979, Wilkie et al. 2000).
Generally, the characteristics of wastewater from the manufacture of ethanol depend on the raw materials and the processes used in fermentation and distillation, which include sugar crops, starch crops, and cellulosic materials (Wilkie et al. 2000). Currently, the melanoidin has been widely regarded as dark brown pigment of molasses wastewater (sugar-based crops) (Mohana et al. 2009, Satyawali and Balakrishnan 2008). Wine distillery wastewater was varied in color from yellow to dark red due to the phenolic compounds (starch-based crops) (Sales et al. 1987). Cellulosic feedstock typically contains a substantial amount of hemi-cellulose and lignin, which require a more substantial pretreatment before fermentation compared to the conventional sugar- and starch-based crops. It is possible that the color-causing compounds of wastewater from cellulosic feedstock compared to sugar-based and starch-based effluent might be different as well. Nevertheless, information describing the color-causing compounds of cellulosic ethanol production wastewater is extremely limited (Handelsman et al. 2013).
Color compound as dissolved organic matter may be a heterogeneous mixture of aromatic and aliphatic organic compound containing oxygen, nitrogen, and sulfur functional groups (e.g., phenolics, carbohydrates, amino groups, caramels, furfurals) (Chen et al. 2003, Kort 1979, Wilkie et al. 2000). Fluorescence excitation-emission matrices (EEMs) can be used to characterize the dissolved organic compounds in water and wastewater. The EEM peaks can be allocated to the different types of organic compounds according to Chen et al. (Chen et al. 2003). Handelsman et al. have compared the characterization of foulants in membrane filtration of molasses effluent (from cane and beet) and lignocellulosic effluent (from sugar cane mulch feedstock) with EEM (Handelsman et al. 2013), however, without use of quantitative analytical approach of fluorescence regional integration (FRI) to analyze the EEM fluorescence spectroscopy. More advanced characterization techniques such as molecular weight distributions, UV absorption spectra, Fourier transform infrared (FTIR) spectroscopy, and ultrafiltration (UF) fractionation can be used to characterize the dissolved organic matter (Chen et al. 2003).
In addition, a range of biological and physic-chemical methods have been investigated for decolorization of stillage effluents (bacteria and fungi, flocculation and coagulation, oxidation process, membrane treatment) (Mohana et al. 2009, Satyawali and Balakrishnan 2008, Wilkie et al. 2000). As one of effective methods for decolorization of nonbiodegradable organic compounds, electro-catalytic oxidation process was called “environment friendly technology” as its simplicity, easy control, and no secondary pollution (Feng et al. 2004, Martinez-Huitle and Ferro 2006). Electro-oxidation process was used for decolorization of distillery wastewater, which contains a dark-brown pigment known as melanoidin (Arulmathi et al. 2015, Manisankar et al. 2003, Piya-Areetham et al. 2006). However, application of this process in cellulosic ethanol production wastewater has not been found.
This study employed EEM fluorescence spectroscopy, FTIR spectrometer, UV-Vis spectra, and UF fractionation to analyze the color compounds in wastewater from cellulosic ethanol production. It is proposed that the color in the effluent of cellulosic ethanol wastewater (corn stover feedstock) comes from humic acid-like matter. To better understand the relationship between residual colors persistent in cellulosic ethanol wastewater and humic-acid like matter, electro-catalytic oxidation process was applied to study decolorization of biologically treated effluent. The results of the present study will help to facilitate the development of a well decolorization for cellulosic ethanol production wastewater.
Material and methods
Wastewater samples
The wastewater samples were collected from a combined system composed of a continuous stirred tank reactor (CSTR), expanded granular sludge bed (EGSB) reactor, and sequencing batch reactor (SBR) fabricated in our lab (schematic representation as described in previous study (Shan et al. 2015)). The effluent of combined system was characterized as follows: chemical oxygen demand (COD) 644 mg/L, biochemical oxygen demand (BOD) 98 mg/L, pH 8.92, total nitrogen 37.1 mg/L, total phosphorus 11.8 mg/L, and a high color (2926 Pt-Co). The samples were filtered through 0.45-μm membrane filters before analyzing and stored at 4 °C.
Electro-catalytic oxidation of biologically treated effluent
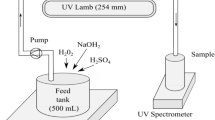
Electro-catalytic oxidation of combined system effluent was carried out in the electrolysis cell with 150-mL glass beaker. The operating conditions were Ti-based SnO2 (Ti/SnO2) electrode with 40 mm × 60 mm as the anode, stainless steel electrode in the same size as cathode, and the distance of electrodes as 1 cm. A DC potentiostat was used as the power supply for organic degradation studies. Electrolysis was performed under galvanostatic control. Solution samples were took out from the cell after electrolysis for 30, 60, 90, 120, and 180 min, respectively, and filtered through 0.45-μm membrane filters before analyzing.
Analytical methods
Dissolved organic carbon (DOC) concentration was measured using a total organic carbon analyzer (TOC-5000A, Shimadzu, Japan). COD was determined according to the procedures described in Standard Methods (APHA 1995). UV light absorption at 254 nm (UVA254) and UV-Vis spectra were determined using a variable-wavelength spectrophotometer (UV-2550, Shimadzu, Japan) and a quartz cuvette with a 1-cm path length. Color was measured with a Hach DR/3900 spectrophotometer at 465 nm in platinum cobalt (Pt-Co) units. All the above analysis was conducted in triplicates. Fluorescence EEM measurements were conducted using a luminescence spectrometer (FP 6500, JASCO, Japan). The spectrometer displayed a maximum emission intensity of 1000 arbitrary units (AU), and excitation and emission slits were both maintained at 5-nm band-pass, and the scanning speed was set at 2000 nm/min for all the measurements. To obtain fluorescence EEMs, emission wavelengths were incrementally increased from 220 to 600 nm at 2-nm steps; for each emission wavelength, the excitation wavelengths from 220 to 450 nm were detected at 0.5-nm steps. A quantitative analytical approach of fluorescence regional integration (FRI) was used to analyze the EEM fluorescence spectroscopy (Chen et al. 2003).
Functional group characteristics of biologically treated effluent of cellulosic ethanol stillage were characterized using a FTIR spectrometer (Spectrum One B, PerkinElmer, USA). KBr pellets containing 0.25 % of the sample were prepared, and the instrument was set up to scan from 4000 to 400 cm-1 averaging 10 scans at 1-cm-1 interval with a resolution of 4 cm-1.
UF fractionation experiments were conducted to determine the apparent molecular weight (MW) distribution using an ultrafiltration cell (Amicon 8400, Millipore Corp., USA). Nitrogen gas at a constant pressure of 0.03 MPa was utilized to drive feed solution through the membrane. Ultrafiltration membrane offering an appropriate series of molecular weight (100, 30, 10, and 3 kDa) was used. Sample was fractionated according to the procedures of previous reported (Shin et al. 1999), and color fractions with different nominal molecular size were classified as: > 100, 30–100, 10–30, 3–10, < 3 kDa.
Results and discussion
Fluorescence EEM characteristics of cellulosic ethanol wastewater
EEM fluorescence spectroscopy has been widely used to characterize dissolved organic matter in water and wastewater, and EEMs were delineated into five regions using consistent excitation and emission wavelength boundaries (Chen et al. 2003): simple aromatic proteins such as tyrosine (regions I and II), fulvic acid-like materials (region III), soluble microbial byproduct-like materials (region IV), and humic acid-like organics (region V). The organic compounds in the cellulosic ethanol wastewater are shown as one distinctive and intense peak in region IV (soluble microbial byproduct-like material, 275–280 nm/304–314 nm); weaker peaks are apparent in the regions for simple aromatic proteins I (225 nm/308 nm) and humic acid-like organics V (320/404 nm) (Fig. 1a), which similar to the standard humic acid characteristic (Fig. 1c). The biological treatment led to an apparent decrease in the intensity of all the peaks: the soluble microbial byproduct-like peak and simple aromatic proteins peak almost disappeared (Fig. 1b), while the humic acid-like peak was still presented in the EEM fluorescence spectra for the biologically treated effluent, which color was high to 2926 Pt-Co. It is likely that the presence of humic acid-like matter caused the color in the effluent. This finding on color source of cellulosic ethanol wastewater (corn stover feedstock) is not fully discussed previously. Only Handelsman et al. reported that large drop in humic and fulvic acid-like regions by ultrafiltration contributes to color of lignocellulosic effluent (sugar cane mulch feedstock) (Handelsman et al. 2013). Moreover, humic substances reported to present in stillage from wood-ethanol (cellulosic materials) process (Callander et al. 1986) would support the relationship between the color and humic acid-like organics of this kind of wastewater.
These changes were quantitatively analyzed using the fluorescence regional integration (FRI) technique integrated the volume beneath the EEM regions with the method Chen described (Chen et al. 2003). Each region proportion was calculated based on the total volume beneath the EEM regions. The results multiplied by corresponding dilution times were showed in Fig. 2.
The region proportion of the raw wastewater: The region V (humic acid-like matter) exhibited the greatest proportion (55.5 %). The proportion of the region IV (soluble microbial byproduct-like materials) and region III (fulvic acid-like materials) were 16.4 and 19.6 %, respectively. Regions I and II (simple aromatic proteins) made up the remaining 8.5 %. The biological treatment achieved a high fluorescent organics removal efficiency of 72.5 %. However, the fluorescence of the region V still exhibited great proportion (67.1 %) in the biologically treated effluent (Fig. 2). The results further confirmed that the color of biologically treated effluent was primarily due to the presence of humic acid-like matter.
UV-vis spectra analysis of cellulosic ethanol wastewater
Specific ultraviolet absorbance (SUVA) developed by Edzwald and co-workers (Edzwald and Tobiason 1999) has been widely used as an indicator of the nature organic matter (NOM). The high SUVA value (absorbance at 254 nm per unit DOC in mg/L) indicated the presence of mostly aquatic humics, strongly hydrophobic and high molecular mass organic content of the water. The SUVA value of the raw wastewater was 0.9 (L/mg · m). Following the treatments, the SUVA value of the biologically treated effluent was increased to 4.0 (L/mg · m) (Table 1), resulting in mostly aquatic humics (Edzwald and Tobiason 1999). These results further support the presence of humic acid-like matter in the biologically treated effluent.
The value of E2/E4 (the ratio of absorbance at 260 and 400 nm) characterizes the degradation of the phenolic/quinoid core of humic substances to simpler carboxylic aromatic compounds (Lipski et al. 1999, Visser 1984). The increasing E2/E4 ratio from raw wastewater (4.8) to biologically treated effluent (5.4) (Table 1) indicated a degradation of long-wavelength absorbing chromophores: quinoid and semiquinoid arrangements and aromatic and/or heterocyclic zwitteranions, etc. (Lipski et al. 1999). The values of the E4/E6 (the ratio of absorbance at 465 and 665 nm) of the raw wastewater and biologically treated effluent were 4.5 and 10.0, respectively (Table 1). The E4/E6 ratio is related to the particle sizes and molecular weights of HA fractions. Following the treatments, the E4/E6 ratio in biologically treated effluent was increased reflecting a decreasing molecular weights of the HA fractions (Chen et al. 1977).
FTIR spectra analysis of biologically treated effluent
The FTIR spectra of the organic compounds in biologically treated effluent were illustrated in Fig. 3, and the main absorption bands and corresponding assignments were similar to humic acid (González-Pérez et al. 2008, Inbar et al. 1992, Tatzber et al. 2007). The main absorbance bands are the following: a broad band at around 3400 cm-1 (H-bonded OH groups), a slight shoulder at 3050 cm-1 (aromatic C-H stretching), a sharp peak at 2921 cm-1 and a shoulder at 2876 cm-1 (aliphatic C-H stretching), a shoulder or peak at 1697 cm-1 (C = O stretching of COOH, C = O of ketonic carbonyl), a broad peak at 1622 cm-1 (aromatic C = C, COO-hydrogen-bonded C = O), a peak at 1452 cm-1 (aliphatic C-H, aromatic ring stretching), a broad peak at 1400–1300 cm-1 (aliphatic C-H groups), a broad peak centered at 1210 cm-1 (C-O stretching and OH bending of COOH groups), and peaks in the 1160–1110 cm-1 region (aromatic ring bending, symmetric bonding of aliphatic CH2, OH or C-O stretch of various groups) (Fig. 3). The FTIR data further confirmed and complemented the previous data suggesting that the humic acid-like matter was present in the biologically treated effluent.
Molecule weight (MW) distribution in color
Due to the polydisperse character of HA composed of a heterogeneous mixture of chemically dissimilar subunits with different molecular weights, a close relationship could be expected between the properties of the molecular size fractions and the color of biologically treated effluent. As described in Fig. 4, the apparent molecular weight distribution of biologically treated effluent was obtained by the ultrafiltration fractionation method. It was observed that color was mainly distributed in the MW fractions with 3–30 kDa. The color ratio of the fractions with molecules of 3–10 and 10–30 kDa were 53.5 and 34.6 %, respectively. Other fractions made up the remaining 11.9 %. This finding was different from Handelsman’ results: low molecular weight acids, humic compounds, and neutral compounds (<350 Da) were the most prominent peak in lignocellulosic effluent (sugar cane mulch feedstock), while high molecular weight humic compounds (500–20,000 Da) were not prominent. (Handelsman et al. 2013). However, results in this study could be supported by the similar molecular size distribution of humic acid after photocatalytic treatment expressed in terms of color (Sen Kavurmaci and Bekbolet 2013). The properties of the molecular size fractions could be related to the enrichment with acidic groups in lower molecular size fractions and the dense aromatic macromolecular character in larger molecular size fractions (Ghabbour and Davies 2001).
Decolorization of biologically treated effluent by electro-catalytic oxidation
To better understand the residual colors persistent in cellulosic ethanol wastewater, biologically treated effluent decolorization by electro-catalytic oxidation process was investigated. Previous research by our group has shown that the rate of organic materials removal increased as the current density; however, the increase in current density results in increase of energy consumption (Wei et al. 2011). Therefore, we synthetically considered the effective removal of the color and organic materials, enhance mineralization current efficiency, and reduce energy consumption, the current density of 20 mA cm-2 was selected in this study. As described in Fig. 5a, residual color decreased continually with an increase in degradation time, and color removal was relatively higher at the initial stage of 90 min than after further oxidation. 86.5 % color removal was rapidly achieved at 90 min, while 11.8 % of color removal was slowly obtained between 90 min and 180 min. Finally, 98.3 % of color removal was obtained after 180 min, which is better than those of previous studies regarding the lignocellulosic effluent by aerobic treatment and coagulation via alum dosing (89 %) (Kavanagh et al. 2011), photocatalysis (77-91.5 %) (RamMohan et al. 2013), and ultrafiltration membrane (75, 98 %) (Handelsman et al. 2013, Luiz et al. 2015), though membrane fouling and the high cost of membranes were not considered (Meng et al. 2009). The results presented herein showed that the inherent advantage of electro-catalytic oxidation: environmental compatibility, due to the fact that it uses a clean reagent (the electron), more efficient and compact (Feng et al. 2004, Martinez-Huitle and Ferro 2006). The concentration of color materials decreased exponentially with reaction time, and the degradation rate could be expressed by the following equation:
where c was the value of color compounds at the reaction time t, c 0 was the initial value, and k was the reaction rate constant. The degradation of color compounds was in accordance with pseudo-first-order kinetics as shown in Fig. 5b. The reaction rate constant k is found to be 2.3 × 10−2 min−1 at the current density of 20 mA cm-2 with regression coefficient R 2 of 0.9995.
The trend of color removal efficiency during degradation time indicated that for the present electrodes, there exists catalytic selectivity for the oxidation of electrochemical degradation intermediates (Feng and Li 2003). The initial reaction at rapid period (stage I) is the oxidation of humic acid-like matter to colorless compounds. The current efficiency decreases rapidly in this period, and the main reaction in slow period (stage II) is the oxidation of the formed colorless compounds in stage I. Obviously, they are hard for electro-oxidation than original color compounds.
Similar trends for reduction in COD during the electro-catalytic oxidation was observed, 56.6 % COD removal was achieved after 90 min, while 77.3 % COD removal was obtained after 180 min. This was likely that the initial reaction at rapid period (stage I) is the oxidation of organic materials, which might be the oxidation of humic acid-like matter and further oxidation to a series of intermediate products with the aromatic ring opening. The current efficiency decreases rapidly in this period, and the main reaction in slow period (stage II) is the oxidation of the intermediates formed in stage I, which are hard for electro-oxidation than original organic materials.
Reduction of fluorescence of humic acid-like matter after electro-catalytic oxidation
The electro-catalytic oxidation led to a gradual loss of fluorescence for humic acid-like matter during the degradation time (Fig. 6). These changes were quantitatively analyzed using the FRI technique which integrates the volume under the EEM regions (Chen et al. 2003). The fluorescence of the region V exhibited great proportion (67.1 %) in the biologically treated effluent, and this large proportion was nearly no change after electro-catalytic oxidation. The loss of fluorescence was relatively higher at the initial stage of 90 min than after further oxidation. The electro-catalytic oxidation reduced the fluorescence of the region V by 94.9 % after 90 min (Fig. 7) and achieved 97.3 % reduction of humic acid-like matter after 180 min. The loss of fluorescence of humic acid-like peak (Region V) corresponded closely to the trend for decolorization for the electro-catalytic oxidation (Fig. 5). The results further confirmed that the color removal was strongly correlated with the presence of humic acid-like matter.
With the results presented in this study, the color-causing compounds largely come from humic acid-like matter, which can be effectively removed by electro-catalytic oxidation. In addition, more work is required in optimizing the effect of electro-catalytic oxidation. Future work will include types of electrodes, pH of the solution, current density, dilution ratio, statistical analysis, and modeling.
Conclusions
The results presented herein confirmed that the color materials of the cellulosic ethanol wastewater largely come from humic materials, and the molecular weight distribution influences the color of biologically treated effluent of cellulosic ethanol production wastewater. The color compounds were mainly distributed in the molecular weight fractions with 3–30 kDa. Decolorization of biologically treated effluent by electro-catalytic oxidation was investigated, and 98.3 % of color removal was achieved after 180 min. The present study will facilitate development of a better decolorization for cellulosic ethanol production wastewater and significant for cellulosic ethanol production.
References
APHA (1995) Standard Methods for the examination of water and wastewater. American Public Health Association, Washington
Arulmathi P, Elangovan G, Sivakumar K (2015) Removal of colour and COD of distillery effluent by electrochemical-oxidation method. Res J Chem Environ 19:6
Callander IJ, Clark TA, McFarlane PN, Mackie KL (1986) Anaerobic digestion of stillage from a pilot scale wood‐to‐ethanol process I. Stillage characterisation. Environ Technol Lett 7:325–334
Chen Y, Senesi N, Schnitzer M (1977) Information provided on humic substances by E4/E6 ratios. Soil Sci Soc Am J 41:352–358
Chen W, Westerhoff P, Leenheer JA, Booksh K (2003) Fluorescence excitation—emission matrix regional integration to quantify spectra for dissolved organic matter. Environ Sci Technol 37:5701–5710
Dwivedi P, Alavalapati JRR, Lal P (2009) Cellulosic ethanol production in the United States: conversion technologies, current production status, economics, and emerging developments. Energy Sustain Dev 13:174–182
Edzwald JK, Tobiason JE (1999) Enhanced coagulation: US requirements and a broader view. Wat Set Tech 40:63–70
Feng YJ, Li XY (2003) Electro-catalytic oxidation of phenol on several metal-oxide electrodes in aqueous solution. Water Res 37:2399–2407
Feng YJ, Cui YH, Snu LX, Liu JF, Cai WM (2004) Development of electro-chemical technology and high efficiency catalytic electrode for wastewater treatment. J Harbin Inst Technol 4:011
Ghabbour EA, Davies G (eds) (2001) Humic substances: structures, models and functions. Royal Society of Chemistry, Cambridge, UK
González-Pérez M, Vidal Torrado P, Colnago LA, Martin-Neto L, Otero XL, Milori DMBP, Gomes FH (2008) 13C NMR and FTIR spectroscopy characterization of humic acids in spodosols under tropical rain forest in southeastern Brazil. Geoderma 146:425–433
Handelsman T, Nguyen T, Vukas M, Barton G, Coster H, Roddick F, Kavanagh J (2013) Characterisation of foulants in membrane filtration of biorefinery effluents. Desalin Water Treat 51:1563–1570
Inbar Y, Hadar Y, Chen Y (1992) Characterization of humic substances formed during the composting of solid wastes from wineries. Sci total environ 113:35–48
Kavanagh J, Sritharan S, Riad M, Kollaras A (2011) Lignocellulosic wastewater treatment. Chemeca 2011: Engineering a Better World, Sydney Hilton Hotel, NSW, Australia, 1431–1441
Kort MJ (1979) Colour in the sugar industry. In: de Birch GG, Parker KJ (Eds) Science and Technology. Applied Science, London, 97–130.
Lipski M, Sławiński J, Zych D (1999) Changes in the luminescent properties of humic acids induced by UV radiation. J Fluoresc 9:133–138
Luiz A, Handelsman T, Barton G, Coster H, Kavanagh J (2015) Membrane treatment options for wastewater from cellulosic ethanol biorefineries. Desalin Water Treat 53:1547–1558
Mabee W, McFarlane P, Saddler J (2011) Biomass availability for lignocellulosic ethanol production. Biomass bioenerg 35:4519–4529
Manisankar P, Viswanathan S, Rani C (2003) Electrochemical treatment of distillery effluent using catalytic anodes. Green chem 5:270–274
Martinez-Huitle CA, Ferro S (2006) Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem Soc rev 35:1324–40
Meng F, Chae SR, Drews A, Kraume M, Shin HS, Yang F (2009) Recent advances in membrane bioreactors (MBRs): membrane fouling and membrane material. Water Res 43:1489–512
Mohana S, Acharya BK, Madamwar D (2009) Distillery spent wash: treatment technologies and potential applications. J Hazard Mater 163:12–25
Pant D, Adholeya A (2007) Biological approaches for treatment of distillery wastewater: a review. Bioresource Technol 98:2321–2334
Piya-Areetham P, Shenchunthichai K, Hunsom M (2006) Application of electrooxidation process for treating concentrated wastewater from distillery industry with a voluminous electrode. Water Res 40:2857–2864
RamMohan G, Goswami T, Tian Z, Ingram L, Pullammanappallil P (2013) Photocatalytic treatment for final polishing of wastewater from a cellulosic ethanol process. J Adv Oxid Technol 16:244–251
Sales D, Valcárcel M, Pérez L, Martinez‐Ossa E (1987) Activated sludge treatment of wine‐distillery wastewaters. J Chem Technol Biot 40:85–99
Satyawali Y, Balakrishnan M (2008) Wastewater treatment in molasses-based alcohol distilleries for COD and color removal: a review. J environ manage 86:481–97
Sen Kavurmaci S, Bekbolet M (2013) Photocatalytic degradation of humic acid in the presence of montmorillonite. Appl Clay Sci 75–76:60–66
Shan L, Yu Y, Zhu Z, Zhao W, Wang H, Ambuchi JJ, Feng Y (2015) Microbial community analysis in a combined anaerobic and aerobic digestion system for treatment of cellulosic ethanol production wastewater. Environ Sci Pollut R 22:17789–17798
Shin H-S, Monsallier JM, Choppin GR (1999) Spectroscopic and chemical characterizations of molecular size fractionated humic acid. Talanta 50:641–647
Tatzber M, Stemmer M, Spiegel H, Katzlberger C, Haberhauer G, Mentler A, Gerzabek MH (2007) FTIR‐spectroscopic characterization of humic acids and humin fractions obtained by advanced NaOH, Na4P2O7, and Na2CO3 extraction procedures. J Plant Nutr Soil Sc 170:522–529
Visser S (1984) Seasonal changes in the concentration and colour of humic substances in some aquatic environments. Freshwater biol 14:79–87
Wei J, Feng Y, Sun X, Liu J, Zhu L (2011) Effectiveness and pathways of electrochemical degradation of pretilachlor herbicides. J Hazard Mater 189:84–91
Wilkie AC, Riedesel KJ, Owens JM (2000) Stillage characterization and anaerobic treatment of ethanol stillage from conventional and cellulosic feedstocks. Biomass bioenerg 19:63–102
Acknowledgments
This research was supported by the National Scientific Fund for Distinguished Young Scholars (51125033). The research also got the support from Key Science and Technology Program of the Heilongjiang Province (WB10A401) and the Heilongjiang Postdoctoral Fund (LBH-Z12132) in China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Shan, L., Liu, J., Yu, Y. et al. Characterization of persistent colors and decolorization of effluent from biologically treated cellulosic ethanol production wastewater. Environ Sci Pollut Res 23, 10215–10222 (2016). https://doi.org/10.1007/s11356-016-6220-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6220-5