Abstract
Because of their high lipid content, microalgae are regarded as a potentially competitive source for biofuels. However, one of the main biotechnological challenges in algae-based biofuels is that cell division is arrested under conditions which promote lipid accumulation, resulting in reduced overall lipid yield. In this study, sethoxydim-resistant mutants of the promising thermotolerant green microalga Micractinium sp., which has a relatively fast growth rate and tolerance to outdoor high temperatures, were generated by UV-C mutagenesis for increased cellular triacylglycerol (TAG) without compromising on growth rate. Two Micractinium mutants were isolated (designated as Mut 3 and Mut 4) that displayed significant increase in TAG cell content up to 71% and 84%, with decreased chlorophyll content by 37% and 38%, respectively, compared with the wild-type cells. Final TAG culture productivity of Mut 3 and Mut 4 were also greater, reaching up to 0.61 ± 0.01 and 0.62 ± 0.02 g L−1, respectively, compared with the wild-type culture of 0.40 ± 0.03 g L−1. The mutants were stable without using antibiotics or any other chemicals during the experiments and preservation. These results highly nominate the mutants of Micractinium sp. isolated in this work as potential microalgae candidates to serve as a feedstock for sustainable and cost-effective biofuel production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae are increasingly considered as promising sources of biofuel to supplant fossil fuels. Under optimal growth conditions, microalgae produce fatty acids mainly for esterification into glycerol-based membrane lipids, which constitute about 5–25% of their dry cell weight (Viso and Marty 1993; Abomohra et al. 2013). Fatty acids are synthesized by a set of repeated enzymatic reactions, initiated by the formation of malonyl CoA from acetyl CoA, catalyzed by the acetyl CoA carboxylase (ACCase). This step as well as the subsequent ones is inhibited by specific inhibitors such as triclosan (Heath et al. 1999), quizalofop (Burton et al. 1989), sethoxydim and haloxyfop (Burton et al. 1987), and cerulenin (Chaturvedi and Fujita 2006) that act as efficient herbicides. Previous works with algae, fungi, and bacteria have shown that mutants conferring resistance to lethal levels of these herbicides produced elevated levels of fatty acids (Chaturvedi et al. 2004; Chaturvedi and Fujita 2006; Liu et al. 2017).
Under stressful environmental conditions, especially nitrogen starvation, many algal species change their lipid profile to form neutral lipids in the chloroplast then exported to the cytoplasm where they are attached to the glycerol backbone in the form of triacylglycerols (TAGs) that are accumulated in storage droplets (Solovchenko 2012). Under such conditions, total lipid content of certain microalgae can increase up to about 50% and above (Hu et al. 2008). Despite that some stress conditions may lead to increasing lipid production in algae, however, these conditions also result in the halt of cell division, which eventually does not lead to an overall increase in lipid productivity.
However, under periods of nutrient starvation, total rates of lipid production are reduced because higher cellular content of lipids is offset by arresting cell division (Breuer et al. 2012). Therefore, using existing technologies with the most appropriate algal species, it is possible to either grow microalgae under optimum conditions to promote a high growth rate, or to grow microalgae under stressful conditions to promote an elevated cellular TAG content; however, it is not possible to do both (Yu et al. 2009; Abu-Ghosh et al. 2015). In fact, in most cases, biomass productivity and lipid content are inversely related (Francisco et al. 2010). Moreover, the production of TAG or any other valuable chemicals from microalgae is performed by a two-stage cultivation strategy (Yen et al. 2013), first stage to accumulate biomass and the second stage is applying stress conditions on microalgae, which is considered to be the most energy-consuming step in the production process (Morales et al. 2019). Therefore, there is a need to develop stable microalgal strains that are capable of maintaining a lipid-rich phenotype under growth conditions that allow a fast cell division, which would significantly enhance the economic feasibility of microalgae-based biofuels.
Cellular TAG content and culture TAG productivity are the most important parameters for cost-effective production of biodiesel (Griffiths and Harrison 2009). As a part of that, many efforts have been spent to generate mutant cells that accumulate large amounts of TAG, mainly by creating transgenic strains via synthetic biology. This approach is based on a thorough study of the organism’s genome, biochemical pathways, and control mechanisms, and is dependent on an efficient transformation and a precise protocol. And yet, there are still unresolved mechanisms that prevent the achievement of the desired phenotype. The approach undertaken in this study, the “rapid breeding”, requires a large population of genetic variants, which is obtained easily by random mutagenesis of a large population, and efficient selection/screening methods. One notable advantage of that approach is that it can produce the desired phenotype with no prior knowledge of the corresponded genes in the target organisms. Second and most important advantage of that approach is that in many cases, a phenotype can be achieved via a combination of yet unknown genetic factors. Third advantage of that approach is its rapidity—a matter of weeks—and its suitability to micro-organisms.
Recently, it was reported that algal breeding through random mutagenesis, followed by selection over lethal levels of different inhibitors of fatty acid synthesis, resulted in the isolation of some fatty acid overproducers that were superior over the wild-type cells (Chaturvedi et al. 2006; Liu et al. 2017; Moha-León et al. 2018).
Thermotolerant microalgae are excellent candidates for biotechnology and mass production since they grow well outdoors under high light and can tolerate moderately high temperatures (Patel et al. 2019). However, the use of thermotolerant species in biofuel production was poorly studied.
Recently, it was shown that the thermotolerant microalga Micractinium sp., which was isolated from the Syrian-African rift Valley, Israel (Adar et al. 2016), is a potential feedstock for biodiesel since it has fast growth rate of 0.263 day−1 (Adar et al. 2016), tolerates a wide range of temperatures (Sonmez et al. 2016), with an optimal growth temperature of 35–40 °C (Onay et al. 2014; Adar et al. 2016), and with the ability to grow under both heterotrophic and mixotrophic cultivation conditions (Engin et al. 2018). In addition, this algal species contains adequate amounts of TAG (Abu-Ghosh et al. 2018) with a matching fatty acid profile for biodiesel production (Shi et al. 2019).
In this work, we have generated sethoxydim-resistant mutants of the fast-growing thermotolerant microalga Micractinium sp., isolated from the Syrian-African rift Valley, Israel (Adar et al. 2016). These mutants showed dramatically greater TAG productivities than the wild-type during the exponential phase of cellular growth, without the need for a second stage of nutrient starvation.
Material and methods
Experimental strain
Axenic pre-cultures of the thermotolerant Micractinium sp. were grown on modified BG-11 medium containing in addition Na2SiO3.9H20 (58 mg L−1) as described in Watanabe et al. (2000) with final pH 7.4 in Erlenmeyer flasks capped with a silicone sponge under optimal conditions of continuous illumination of 250 μmol photons m−2 s−1 provided by EdiStar 120 W point-source light-emitting diodes (LEDs) at 30 °C and ambient CO2 concentrations, and were shaken at 150 rpm in a DFO-150 shaker incubator (MRC, Israel). The cultures were maintained in the log phase by dilution every 3 days to minimize self-shading according to Abu-Ghosh et al. (2018).
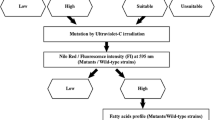
Mutagenesis and screening of mutants
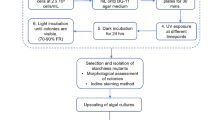
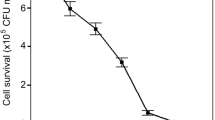
The mutagenesis device consisted of UV-C lamp (Philips TUV 30W G30 T8 lamp with 12W UV-C output at 253.7 nm) that is placed 23 cm away from the cells, in a sealed wooden box. In the first stage, the UV-C time exposure, which was able to kill 90% of the cells in the specified device (UV-C output; distance from the lamp and cell concentration), was determined. To that end, 9 sterile Petri dishes, each bearing 2 × 107Micractinium sp. cells, suspended in 20 mL distilled water, were exposed to UV-C radiation for 0 to 80 s in a 10 s increment. The exposed cells were then left in the darkness for 24 h to prevent photolyase activity and UV-C damage correction. Consequently, known volumes were drawn from each plate after resuspension and spread on triplicate agar plates with BG11 medium for colony counting following 10 days of incubation. According to the generated killing curve (exposure time vs. percent viable cells), the exact time needed to provide 90% killing was calculated. It was found that a 60 s exposure provides that level of killing. In the second stage, in order to determine sethoxydim lethal levels, 2 × 107Micractinium sp. cells were grown on BG11 agar plates with increasing levels of the acetyl CoA carboxylase inhibitor sethoxydim (Sigma-Aldrich) from 0 to 80 μM in 10 μM increment (Liu et al. 2017). After 10 days of incubation, 60 μM of sethoxydim was found to be the lowest lethal level for Micractinium sp. In the third stage, 18 Petri dishes, each bearing 2 × 107Micractinium sp. cells, suspended in 20 mL distilled water, were exposed to UV-C radiation for 60 s and left in the darkness for 24 h. Consequently, the cells were re-suspended, transferred to sterile 50-mL Falcon tubes and centrifuged at 5000×g for 8 min. The concentrated cells were then spread on BG11 agar plates bearing 60 μM sethoxydim. After 10 days, the surviving colonies were transferred to other BG11 agar plates without 60 μM sethoxydim for longer time preservation. Wild-type and mutant cell lines were then grown and kept in sethoxydim-free medium and were diluted weekly with the same medium for at least 5 weeks prior to the experiments.
Experimental conditions
Exponential cultures of the Micractinium wild-type and mutants were adjusted to a starting optical density of 0.02 at 750 nm (OD750) to reduce self-shading for reliability. Seventy-five milliliter of each culture was incubated in 150-mL Erlenmeyer flasks in a shaker incubator with a controlled temperature (for determination of optimal temperature, see Supplementary Fig. 1), and rotational speed of 150 rpm. Continuous saturating illumination intensity of 350 μmol photons m−2 s−1 was provided by a homemade panel of six EdiStar 120-W point-source LEDs (Edison, Karlsbad, Germany) at the top surface of the algal flasks during the cultivation period for 24 h a day without dark periods to shorten the experiment time. For inorganic source of carbon, sodium bicarbonate (1 g L−1) was added to the medium (White et al. 2013; Mokashi et al. 2016). Sterilized double distilled water was added to the cultures prior sampling to compensate for the minor water loss due to evaporation.
Determination of growth
Growth of Micractinium cell lines was monitored by measuring the change in the optical density of the cultures at 750 nm (OD750), cell count, and dry biomass.
All cultures were initiated with an OD750 value of 0.02, which was equivalent to 1 × 105 cells. Cell numbers were counted using a BD FACSCalibur flow cytometer (Breda, Netherlands). For dry biomass, 5 mL culture samples was filtered and collected on a mixed cellulose membrane (pore size, 0.45 μM), washed with deionized water twice, and dried overnight at 60 °C and were then immediately desiccated before weighing.
Quantification of neutral lipids
The neutral lipids in the algal cultures were determined using the standard Nile Red assay. Samples (2 mL) of algal culture were flash-frozen in liquid nitrogen and then were stored at − 80 °C prior to analysis. Nile Red (Sigma-Aldrich, USA) was dissolved in HPLC grade ethanol at a concentration of 500 μg mL−1, and was stored in complete darkness in a vial covered in aluminum foil to prevent photodegradation at 4 °C. In a black opaque 96-microwell plate, 185 μL of vortex-mixed algal samples was aliquoted into the wells in duplicates (multiplied by the 3 triplicates of each treatment, thus total of 6 replicates) along with triplicate blanks. Following the addition of the algal samples to the wells, the Nile Red suspension (final concentration 5%) and DMSO (final concentration 10%) were added to the treatment wells. The microwell plate was covered and incubated in darkness with gentle shaking for 10 min to allow dye penetration into the cells.
To analyze the development of fluorescence within the poststaining time, the fluorescence intensity of the stained mixture was recorded at regular intervals with a fluorescence spectrophotometer Synergy HT multimode plate reader (BioTek, USA) at excitation and emission wavelengths of 530 and 590 nm, respectively. For the TAG calibration curve, stock glyceryl trioleate (≥ 99% purity) was purchased from Sigma Aldrich (Rehovot, Israel) and then used as standard at different concentrations (5 to 2000 mg L−1) as previously described (Abu-Ghosh et al. 2018).
Quantification of total chlorophyll
Total chlorophyll content was measured by centrifuging 5 mL samples of each culture at 5000×g at 4 °C for 10 min. The pellets were suspended in 5 mL DMSO, vortexed thoroughly, and then incubated at 70 °C for 5 min in the dark until the sample turned white. After cooling, the extracts were centrifuged at 5000×g for 15 min and the supernatant pigment was then transferred into 1-cm quartz cuvettes and analyzed spectrophotometrically. The wavelengths 649, 665, and 480 nm were used to calculate the total chlorophyll concentration by the trichromatic equations (Wellburn 1994).
Microscopic analyses
Micractinium cells stained with Nile Red for10 min were photographed using a Leica SP8 confocal microscope equipped with Olympus DP10 digital camera, fitted with a 100-W High Pressure Mercury Burner with a lateral resolution down to 140 nm. Using CUDA-based processing, differential interference contrast (DIC), chlorophyll autofluorescence, and Nile Red epifluorescence (excitation 510–530 nm, emission 590 nm) images were obtained at × 1000 magnification with oil immersion.
Estimation of cell diameter
Cell diameter was determined by means of fluorescence-activated cell sorter (FACS, BD Fortessa, USA) using side scatter pulse width (SSC-W) data from cells against size calibration bead standards of polystyrene (Spherotech, USA). Briefly, six different bead standards ranging between 3 and 17 μM were used to plot a calibration standard curve of amplifier gains where the pulse width was independent of peak height and peak area. The curve was then fitted with a linear curve according to Sharpless and Melamed (1976), and was used to transform the median SSC-W data from a given microalgal cell population into a median cell diameter (Supplementary Fig. 2).
Statistical analysis
For each parameter, the average and standard deviation were calculated. The statistical significance of the results was evaluated using the Student’s paired t test.
Results
Successful isolation of stable mutant cell lines accumulates superior amounts of TAG without stress
Following UV-C mutagenesis and sethoxydim screening for survival, two cell lines were isolated, labeled as Mut 3 and Mut 4, and subsequently characterized along with the wild-type cells. To test whether the isolated mutant cell lines accumulate neutral lipids in relatively large quantities, we grew the three different phenotypes under optimal conditions and then examined the accumulation of lipid droplets with the aid of the Nile Red staining under the confocal microscope (Fig. 1). These images were taken 6 weeks after the mutant cell lines were isolated and maintained on sethoxydim-free medium. The images show that the cytoplasmic lipid droplets within the algal cells are more evident in Mut 3 and Mut 4 cell lines than in the wild-type cells, with higher accumulation in Mut 4. Notably, chlorophyll fluorescence in the two mutant cell lines was less than in the wild-type. Moreover, Mut 4 seems to have slightly bigger cell diameter than Mut 3 and the wild-type cells.
To confirm that the mutant cell lines accumulate more lipids than the wild-type cells of Micractinium sp., lipid content was measured by the Nile Red fluorescence analysis for each of the three phenotypes (Fig. 2). The accumulation of lipids in both mutant cell lines Mut 3 and Mut 4 was significantly (P < 0.001) increased up to 16 and 120%, respectively, in regard to the wild-type cells after 7 days of cultivation under optimal conditions (Fig. 2a). Interestingly, the percent of increase in lipid production changed with cell aging. The volumetric TAG content of Mut 3 and Mut 4 had a dramatic increase on day 10, reaching up to 71 and 84% increase, respectively. Obviously, lipid increase in the mutant cell lines, especially in Mut 4, was accompanied by the increase in granulation and cell size which were observed in the microscope images.
Triacylglycerol (TAG) and chlorophyll content of wild-type Micractinium sp. and two mutant cell lines Mut 3 and Mut 4. a Nile Red fluorescence intensity during mid- and late-exponential phase. b Cell content and culture of TAG. c Total chlorophyll content. Averages of six samples of two separate biological repetitions ± SD. *(P ≤ 0.05), **(P ≤ 0.01)
For precise TAG quantification in the three phenotypes, cell content and culture content of TAG were determined and presented in Fig. 2 b. TAG content here was determined after 10 days of cultivation. This was performed only because sufficient cell density was available for the analysis. The wild-type cells contained 32.9 ± 0.9 pg TAG cell−1, whereas Mut 3 and Mut 4 showed a superior cell content of TAG with 56.0 ± 2 and 60.7 ± 4 pg TAG cell−1, respectively. Most importantly, volumetric TAG content was also significantly higher (P < 0.001) in Mut 3 and Mut 4 up to 0.61 ± 0.01 and 0.62 ± 0.02 g L−1, respectively, ~ 50%greater than the wild-type culture (0.40 ± 0.03 g L−1).
On the other hand, per-biomass chlorophyll content was less in Mut 3 and Mut 4, with 7.0 ± 0.1 and 6.8 ± 0.2 mg Chl g−1 DW, respectively, compared with 9.4 ± 0.7 mg Chl g−1 DW of the wild-type (Fig. 2c).
Mutant cell lines have the same growth rate as wild-type, with a different prospective
Cell size of Micractinium sp. changes during growth when physiochemical alterations occur within the growth cycle (Adar et al. 2016). In the current work, average cell size of the three phenotypes during the mid and late exponential phase is presented in Fig. 3. The wild-type cells increased their size with cultivation time; however, no significant changes in size of Mut 3 and Mut 4 were observed. During mid-exponential phase, both mutants were bigger than the wild-type cells. After 10 days of cultivation, i.e., in the late-exponential phase, Mut 4 cells were slightly bigger (~ 12 μM) than both the wild-type and Mut 3 (~ 10 μM).
Comparative growth analysis of the wild-type and the mutants Mut 3 and Mut 4 cultures was performed to determine per-cell and per-biomass TAG productivity of the three cell lines. The growth kinetics of the wild-type and the two mutant of Micractinium sp. are presented in Fig. 4. Under optimal growth temperature of 35 °C, the growth of the three phenotypes as judged by turbidity was essentially identical, with initial OD750 of 0.02 and final of ~ 2.9 after 10 days of cultivation (Fig. 4a). Moreover, the growth of the mutant cell lines at 25 and 42 °C was not significantly different from that of the wild-type (Supplementary Fig. 1).
Growth kinetics of wild-type Micractinium sp. and two mutant cell lines Mut 3 and Mut 4 under growth temperature of 35 °C. a Optical density at 750 nm (OD750). b Cell number. c Cell dry weight. d Culture dry weight. Averages of six samples of two separate biological repetitions ± SD. *(P ≤ 0.05), **(P ≤ 0.01)
In addition to culture OD750, cell numbers were monitored during the mid- and late-exponential phase of growth (on days 7 and 10). After 7 days of cultivation, cell number of Mut 3 was close to that of wild-type (9.8 ± 0.4 × 106 and 9.6 ± 0.2 × 106 cells mL−1, respectively), whereas cell number of Mut 4 was lower (7.0 ± 0.3 × 106 cells mL−1) (Fig. 4b). When cultures aged, i.e., after 10 days, Mut 4 had a sharp increase in cell number to catch up with Mut 3, yet both mutants displayed slightly lower cell number (~ 10.2 × 106 cells mL−1) than the wild-type (12.0 ± 0.2 × 106 cells mL−1).
In another aspect, cell dry weight of Mut 4 was significantly (p < 0.001) the highest compared with M3 and the wild-type, with 93.8 ± 6.6, 63.2 ± 2.5 and 57.1 ± 1.7 pg, respectively, on day 7, and 106.8 ± 5.3, 90 ± 1.8 and 75.5 ± 2.3 pg, respectively, on day 10 (Fig. 4c). The increase in cell dry weight of Mut 4 compensated for the less cell number than the wild-type and Mut 4; therefore, the overall growth in terms of accumulation of culture biomass dry weight was almost similar in all cultures during the sampling points on day 7 (0.65 ± 0.1 vs. 0.62 ± 0.03 vs. 0.54 ± 0.02 mg mL−1, respectively) and on day 10 (1.1 ± 0.1 vs. 0.97 ± 0.1 vs. 0.9 ± 0.1 mg mL−1, respectively) (Fig. 4d).
Discussion
In the current work, UV-C mutagenesis followed by selection over lethal doses of the fatty acid biosynthesis inhibitor sethoxydim was used to generate stable microalgae mutants that contain higher TAG levels, while maintaining wild-type growth characteristics, from the thermotolerant microalga Micractinium species. After mutagenesis and screening for sethoxydim-resistant lines, the isolated colonies were examined for TAG content and yield. Compared with the wild-type cells, the Nile Red fluorescence intensity of two mutant cell lines (Mut 3 and Mut 4) was obviously stronger, while the autofluorescence (chlorophyll) was lesser (Fig. 1). This was an indication for a successful isolation of TAG overproducer mutants of Micractinium sp.
After 10 days of cultivation, per-cell and volumetric (culture) TAG contents were similar in both mutant cell lines, which were almost twofold higher than the wild-type cells (Fig. 2b). However, chlorophyll content (analysis) of the mutant cell lines was less than that of the wild-type cells (Fig. 2c), balancing between lipid accumulation and chlorophyll biosynthesis in the cells, since all cultures were grown under the same environmental conditions, including light intensity. For example, under nutrient starvation, the decrease in chlorophyll content, which usually intensified during long-term nitrogen starvation, has the possible function of facilitating TAG synthesis and accumulation as energy storage mechanism (Li et al. 2008; Sandmann et al. 2018).
TAG content of Mut 3 and Mut 4 (~ 62%) is much higher than that of the wild-type thermotolerant Micractinium sp. ME05 which was isolated from Central Antonia and showed fast growth rate but low lipid content of only 10.70% (Onay et al. 2014), and higher than the recently reported thermotolerant Micractinium sp. IC-76 (Piligaev et al. 2018) with maximum TAG of ~ 18.7%, or the mesophilic Micractinium inermum (up to 40%) (Shi et al. 2019). In terms of producing mutants of thermotolerant, Mut 3 and Mut 4 showed higher TAG content than the thermotolerant mutant of Chlamydomonas reinhardtii (Lee et al. 2014) that increased its lipid content from 6.4% (wild-type) to only 7.5% (mutant).
It is expected that a promising algal strain for biodiesel production should have a fast growth rate and high TAG cell content for a final high TAG yield, and perform well in downstream processes (Wijffels and Barbosa 2010). In this work, the growth rates and biomass productivities of the wild-type and the mutant cell lines of Micractinium sp. were similar during the cultivation period (Fig. 4a, d). However, after 10 days of incubation, cell and culture TAG contents of the mutant cell lines increased significantly (P ≤ 0.01) in comparison with the wild-type cells (Fig. 2a, b). These results are evidence that the increased TAG contents of the vegetative cells of Mut 3 and Mut 4 have no effect, as sorting criteria, on growth rate or biomass productivity. The fact that the mutants Mut 3 and Mut 4 actively and consistently grow and had higher lipid content than the wild-type Micractinium sp. and other mutants makes them the candidates for further improvement in photoautotrophic or mixotrophic lipid productivity, which may be achieved by more optimization of environmental factors or effective bioreactors.
Conclusions
Mutants of the thermotolerant fast-growing microalga Micractinium sp. were obtained by UV mutagenesis followed by the sethoxydim-resistance screening method. Two potent mutants were selected and showed significantly increased TAG synthesis and accumulation without compromising the growth rate. Mut 3 and Mut 4 cell lines had 71% and 84%, respectively, increase in TAG cell content compared with the wild-type, and ≥ 60% higher overall volumetric TAG content during the late-exponential phase in non-stress conditions. The isolated mutants were stable in regular medium without any special requirements, herbicides, or antibiotics. Therefore, Mut 3 and Mut 4 cell lines of Micractinium sp. should be considered as a feedstock for biodiesel production, since the increased TAG productivity shortens the batch runtime (no stage 2 of nitrogen starvation is needed) with respect to the wild-type, and thus they could make commercialization of algal-lipid biofuels more feasible.
References
Abomohra A, Wagner M, El-Sheekh M, Hanelt D (2013) Lipid and total fatty acid productivity in photoautotrophic fresh water microalgae: screening studies towards biodiesel production. J Appl Phycol 25:931–936
Abu-Ghosh S, Fixler D, Dubinsky Z, Iluz D (2015) Energy-input analysis of the life-cycle of microalgal cultivation systems and best scenario for oil-rich biomass production. Appl Energy 154:1082–1088
Abu-Ghosh S, Dubinsky Z, Banet G, Iluz D (2018) Optimizing photon dose and frequency to enhance lipid productivity of thermophilic algae for biofuel production. Bioresour Technol 260:374–379
Adar O, Kaplan-Levy RN, Banet G (2016) High temperature Chlorellaceae (Chlorophyta) strains from the Syrian-African Rift Valley: the effect of salinity and temperature on growth, morphology and sporulation mode. Eur J Phycol 51:1–14
Breuer G, Lamers PP, Martens D, Draaisma RB, Wijffels RH (2012) Triacylglycerol accumulation in microalgae. Bioresour Technol 124:217–226
Burton JD, Gronwald JW, Somers DA, Connelly JA, Gengenbach BG, Wyse DL (1987) Inhibition of plant acetyl-coenzyme A carboxylase by the herbicides sethoxydim and haloxyfop. Biochem Biophys Res Commun 148:1039–1044
Burton JD, Gronwald JW, Somers DA, Gengenbach BG, Wyse DL (1989) Inhibition of corn acetyl-CoA carboxylase by cyclohexanedione and aryloxyphenoxypropionate herbicides. Pestic Biochem Physiol 34:76–85
Chaturvedi R, Fujita Y (2006) Isolation of enhanced eicosapentaenoic acid producing mutants of Nannochloropsis oculata ST-6 using ethyl methane sulfonate induced mutagenesis techniques and their characterization at mRNA transcript level. Phycol Res 54:208–219
Chaturvedi R, Uppalapati SR, Alamsjah MA, Fujita Y (2004) Isolation of quizalofop-resistant mutants of Nannochloropsis oculata (Eustigmatophyceae) with high eicosapentaenoic acid following N-methyl-N-nitrosourea-induced random mutagenesis. J Appl Phycol 16:135–144
Engin IK, Cekmecelioglu D, Yücel AM, Oktem HA (2018) Evaluation of heterotrophic and mixotrophic cultivation of novel Micractinium sp. ME05 on vinasse and its scale up for biodiesel production. Bioresour Technol 251:128–134
Francisco ÉC, Neves DB, Jacob-Lopes E, Franco TT (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Heath RJ, Rubin JR, Holland DR, Zhang E, Snow ME, Rock CO (1999) Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J Biol Chem 274:11110–11114
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Lee B, Choi GG, Choi YE, Sung M, Park MS, Yang JW (2014) Enhancement of lipid productivity by ethyl methane sulfonate-mediated random mutagenesis and proteomic analysis in Chlamydomonas reinhardtii. Korean J Chem Eng 31:1036–1042
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Liu J, Pei G, Diao J, Chen Z, Liu L, Chen L, Zhang W (2017) Screening and transcriptomic analysis of Crypthecodinium cohnii mutants with high growth and lipid content using the acetyl-CoA carboxylase inhibitor sethoxydim. Appl Microbiol Biotechnol 101:6179–6191
Moha-León JD, Pérez-Legaspi IA, Ortega-Clemente LA, Rubio-Franchini I, Ríos-Leal E (2018) Improving the lipid content of Nannochloropsis oculata by a mutation-selection program using UV radiation and quizalofop. J Appl Phycol 31:191–199
Mokashi K, Shetty V, George SA, Sibi G (2016) Sodium bicarbonate as inorganic carbon source for higher biomass and lipid production integrated carbon capture in Chlorella vulgaris. Achiev Life Sci 10:111–117
Morales M, Collet P, Lardon L, Hélias A, Steyer J-P, Bernard O (2019) Life-cycle assessment of microalgal-based biofuel. In: Pandey A, Chang J-S, Soccol CR, Ricardo C, Lee D-J, Chisti Y (eds) Biomass, biofuels and biochemicals: biofuels from algae, 2nd edn. Elsevier, Amsterdam, pp 507–550
Onay M, Sonmez C, Oktem HA, Yucel AM (2014) Thermo-resistant green microalgae for effective biodiesel production: isolation and characterization of unialgal species from geothermal flora of Central Anatolia. Bioresour Technol 169:62–71
Patel A, Matsakas L, Rova U, Christakopoulos P (2019) A perspective on biotechnological applications of thermophilic microalgae and cyanobacteria. Bioresour Technol 278:424–434
Piligaev AV, Sorokina KN, Samoylova YV, Parmon VN (2018) Lipid production by microalga Micractinium sp. IC-76 in a flat panel photobioreactor and its transesterification with cross-linked enzyme aggregates of Burkholderia cepacia lipase. Energy Convers Manag 156:1–9
Sandmann M, Schafberg M, Lippold M, Sascha Rohn S (2018) Analysis of population structures of the microalga Acutodesmus obliquus during lipid production using multi-dimensional single-cell analysis. Sci Rep 8:6242
Sharpless TK, Melamed MR (1976) Estimation of cell-size from pulse shape in flow cytofluorometry. J Histochem Cytochem 24:257–264
Shi M, Wei H, Chen Q, Wang X, Zhou W, Liu J (2019) Exploring an isolate of the oleaginous alga Micractinium inermum for lipid production: molecular characterization and physiochemical analysis under multiple growth conditions. J Appl Phycol 31:1035–1046
Solovchenko A (2012) Physiological role of neutral lipid accumulation in eukaryotic microalgae under stresses. Russ J Plant Physiol 59:167–176
Sonmez C, Elcin E, Akin D, Oktem HA, Yucel M (2016) Evaluation of novel thermoresistant Micractinium and Scenedesmus sp. for efficient biomass and lipid production under different temperature and nutrient regimes. Bioresour Technol 211:422–428
Watanabe MM, Kawachi M, Hiroki M, Kasai F (2000) NIES Collection list of strains. Microalgae and Protozoa. Microbial culture collections, National Institute for Environmental Studies, 6 edn, Tsukuba, pp 159
Viso A-C, Marty J-C (1993) Fatty acids from 28 marine microalgae. Phytochem 34:1521–1533
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
White DA, Pagarette A, Rooks P, Ali ST (2013) The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures. J Appl Phycol 25:153–165
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329:796–799
Yen H-W, Hu I-C, Chen C-Y, Ho S-H, Lee D-J, Chang J-S (2013) Microalgae based biorefinery–from biofuels to natural products. Bioresour Technol 135:166–174
Yu ET, Zendejas FJ, Lane PD, Gaucher S, Simmons BA, Lane TW (2009) Triacylglycerol accumulation and profiling in the model diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum (Baccilariophyceae) during starvation. J Appl Phycol 21:669–681
Funding
This research was supported by a grant from the Ministry of National Infrastructure, Energy and Water Resources (No. 215–11–047).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The Author Said Abu-Ghosh dedicates this work to his parents.
Electronic supplementary material
ESM 1
(DOCX 421 kb)
Rights and permissions
About this article
Cite this article
Abu-Ghosh, S., Ronen, B., Feingold, D. et al. Sethoxydim-resistant mutants of the thermotolerant microalga Micractinium sp. accumulate significant amounts of triacylglycerol in non-stressful conditions. J Appl Phycol 31, 3433–3440 (2019). https://doi.org/10.1007/s10811-019-01859-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01859-w