Abstract
In coastal environments, acidification and eutrophication affect the physiology of marine macroalgae. We investigated the responses of Ulva pertusa Kjellman (Ulvales, Chlorophyta) under such conditions. Samples were cultured at two different pH settings (low, 7.5; high, 8.0) and at three different ammonium levels (low, 4; medium, 60; high, 120 μM NH4 +). Our objective was to analyze the influence that elevated CO2 and NH4 + might have on pH, oxygen evolution, rates of nutrient uptake, chlorophyll fluorescence, growth, and C/N ratio of that organism. Variability in pH value was enhanced under low pH/high NH4 + and was significantly different (p < 0.05) from changes measured when the high pH/low NH4 + combination was applied. Rates of NH4 + uptake and relative growth rates by U. pertusa were increased under low pH/high NH4 + conditions and that response was significantly different (p < 0.05) from the other treatments. The rate of photosynthetic oxygen evolution and chlorophyll fluorescence were increased under elevated NH4 + concentrations (p < 0.05). However, the C/N ratio of U. pertusa was not affected by higher concentrations of CO2 and NH4 + (p > 0.05). Our results indicated that the physiological reactions of this alga were heightened when exposed to either the elevated combination of CO2/NH4 + or even when only the level of NH4 + was raised. Although such excessive growth can lead to bloom formations in coastal areas, this species also has greater capacity for taking up nutrients and dissolved inorganic carbon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because continuous expansion of industrial activities since the Industrial Revolution has affected coastal environments, many coastal organisms are being challenged by anthropogenic changes such as acidification and eutrophication. The combination of these two environmental phenomena can have individual influences as well as synergistic interactions with marine species (Horta et al. 2012; Reymond et al. 2013). The effects of eutrophication have been studied in various marine organisms, but our current examination focused first on the influence of ocean acidification (OA). The Intergovernmental Panel on Climate Change (IPCC 2014) has projected that atmospheric CO2 concentrations will increase by 280 to 400 ppm. Caldeira and Wickett (2005) also have predicted decreases in pH values of 0.3 to 0.4 units by the year 2100 and 0.7 to 0.8 units by the year 2300. Ocean acidification presents a severe challenge to the physiology and ecology of marine ecosystems in coastal areas and can have a negative effect on the metabolism of marine organisms (Kroeker et al. 2013; Cornwall and Hurd 2015; Kram et al. 2016). However, some macroalgal species can also show a positive response to acidification (Hurd et al. 2009; Suárez-Álvarez et al. 2012; Sarker et al. 2013) because of their unique carbon-concentrating mechanisms (CCMs) (Raven et al. 2011). Such macroalgae use CCMs that acquire carbon either directly from bicarbonate or via the carbonic anhydrase enzyme (Raven 1997; Giordano et al. 2005). Those processes could enhance algal growth because of the energy saved from dissolved inorganic carbon (DIC) transport (Beardall et al. 1998; Beardall and Giordano 2002; Wu et al. 2008).
Cultural eutrophication is another serious environmental issue that is promoted by anthropogenic activities such as urbanization, inflow of waste water, and aquaculture. Excessive input of nutrients can lead to massive algal blooms and induce bacterial respiration. This active respiration can lead to a state of OA because of elevated CO2 levels in the water (Cai et al. 2011; Sunda and Cai 2012). Eutrophication also damages marine communities and reduces species diversity (Lohman and Priscu 1992; Valiela et al. 1997; de Faveri et al. 2015). Nevertheless, some photosynthetic macroalgae, e.g., Ulva spp., have a metabolic advantage under enriched nutrient conditions (Ye et al. 2011; Luo et al. 2012).
In terms of functional form and high rates of growth and nutrient uptake, Ulva spp. are opportunistic because of their thin sheet-like thallus (Littler 1980; Cohen and Fong 2006). Worldwide, green tides that result from excessive algal growth and blooms have a negative ecological impact because the decomposition of biomass into the water column decreases oxygen levels and affects the productivity and biodiverse of the local community (Valiela et al. 1997; Hiraoka et al. 2004; Morand and Merceron 2005; Wang et al. 2009). However, these Ulva spp. might be beneficial because they can function in bioremediation and as a component of Integrated Multi-Trophic Aquaculture systems (Bolton et al. 2009; Ben-Ari et al. 2014).
Numerous studies have investigated the physiological responses of Ulva sp. under different culture conditions that manipulate CO2 levels, nutrient concentrations, and temperature (Figueroa et al. 2014a, b; Stengel et al. 2014). However, little research has been done using U. pertusa and a combination of acidification and eutrophication conditions. Here, we examined the physiological responses of that species to elevated levels of CO2 and ammonium (NH4 +), as well as any possible interactions among different combinations of CO2 and NH4 + concentrations. We monitored changes in pH levels, oxygen evolution for photosynthesis, rates of NH4 + uptake to show the nutrient removal capacity, fresh weight for growth, chlorophyll fluorescence to show the physiological status during the experiments, and biochemical composition to reveal any change of C/N ratio.
Materials and methods
Experiment preparation
Samples of Ulva pertusa were collected at Namhae, Korea (34°56′N, 127°51′E) in April of 2016. At this sampling site, the ambient temperature was approximately 14.7 ± 0.1 °C and salinity was 28.2 ± 0.2‰, as measured with a YSI Pro 2030 (USA) meter. After transport to the laboratory, they were cleaned several times with filtered seawater (0.2 μm) to remove any dirty epiphytes. The samples were then maintained in a culture room in filtered seawater under conditions of 20 °C, 80 μmol photons m−2 s−1 (12:12 light/dark), and S = 28‰. They were acclimated for 3 days before the experiments began. For each treatment, samples (3 g each) were placed in 600-mL culture beakers, each containing 500 mL of filtered seawater. In all, four replicates were made for each of the treatments. The multi-factorial design comprised two pH levels (low, 7.5; high, 8.0) and three ammonium concentrations (low, 4 μM; medium, 60 μM; high, 120 μM). The lowest pH was selected based on an ocean prediction model (Caldeira and Wickett 2005) while the ambient pH reflected conditions at the sampling site. The minimum NH4 + level was based on the ambient concentration (3.62 ± 0.31 μM) at the sampling site, while the medium and high NH4 + levels had been determined in our preliminary studies (data not shown). These treatment combinations were designated as follows: LpHLA, low pH/low NH4 +; HpHLA, high pH/low NH4 +; LpHMA, low pH/medium NH4 +; HpHMA, high pH/medium NH4 +; LpHHA, low pH/high NH4 +; and HpHHA, high pH/high NH4 +. The cultures were carried out at constant temperature (20 °C) along a constant-temperature table, under LED fixtures that supplied a light intensity checked with a LI-250 light meter (LI-COR, USA). We set the intensity at 80 μmol photons m−2 s−1 to prevent photochemical stress due to acidification conditions (Liu et al. 2012). Filtered seawater in the low pH culture media was injected with pure CO2 gas from a tank. To support the low-, medium-, or high-NH4 + treatments, we added ammonium to the seawater to a final concentration of 4, 60, or 120 μM NH4Cl. The medium in each treatment was replaced every 2 days and nutrients were added daily to avoid any deficiencies. Total alkalinity (TA) of the seawater was determined according to the electrotitration method (Gran 1952), at a precision of ±4 μmol kg−1. The inorganic carbon concentration and pCO2 were calculated based on pH, TA, salinity, and temperature, using the CO2SYS software program (Lewis and Wallace 1998). Dissociation constants and KSO4 values were defined as described by Millero et al. (2006) and Dickson (1990) (Table 1).
Monitoring changes in pH, photosynthetic oxygen evolution, and rates of ammonium uptake
During the first 8 h of treatment, we measured changes in pH of the seawater medium and calculated the rates of oxygen evolution and NH4 + uptake. An Orion-250A pH meter (Thermoscientific, USA) was used to record pH levels at 2-h intervals for each treatment. Those values can serve as an indicator of physiological characteristics of algae under culture conditions (Maberly 1990; Murru and Sandgren 2004). Dark respiration by LpHHA and HpHLA samples were measured because we speculated that OA is mediated by photosynthesis. Changes in pH were also investigated in beakers containing culture media without specimens (blanks), under both low- and high-pH conditions.
Photosynthetic oxygen evolution rates (μmol O2 g−1 FW h−1) were also analyzed with a Clark-type microelectrode oxygen sensor (Unisense, Denmark). The microelectrode was calibrated with a solution of C6H7NaO6 (sodium ascorbate) and NaOH (sodium hydroxide) that detected response times of less than 1 s.
The rate of NH4 + uptake (V; μmol NH4 + g−1 FW h−1) was determined based on the average amount that was removed from the culture medium during the incubation period. The measurement method of NH4 + uptake rates were described by Parsons et al. (1984). The following equation was used in this calculation:
where S i is the initial concentration of NH4 +, S f is the final concentration after T hours of culture, vol is the volume of the culture medium, and W is the fresh weight of each algal specimen.
Growth rates, chlorophyll fluorescence, and C/N ratio
Algal growth rates, chlorophyll fluorescence, and C/N ratio were measured after 14 days of incubation. Changes in biomass were determined at the end of the experimental period, and the relative growth rate (RGR; % day−1) was calculated as follows (Yong et al. 2013):
where W 1 is the initial fresh weight and W 2 is the final fresh weight after T days of incubation.
Chlorophyll fluorescence was checked with a pulse amplitude modulation fluorometer (DIVING-PAM, Walz, Germany). The maximum quantum yield of Photosystem II was calculated as (Cosgrove and Borowitzka 2011):
where F v is variable fluorescence, F m is maximum fluorescence after dark-adaptation, F o is minimum fluorescence after dark-adaptation, and F v/F m is photosynthetic efficiency, as measured using a saturating pulse under dark-adaptation. Samples were kept in the dark for 15 min before chlorophyll fluorescence measurement.
Tissue carbon (C) and nitrogen (N) in the alga were analyzed after the experiment. Samples were dried at 60 °C for 48 h and then ground to powder with a Tissuelyser LT (Qiagen, Germany). Approximately 2 to 3 mg of ground tissues was used with an elemental analyzer (Flash 2000 Series; Thermo Fisher Scientific, USA). The C/N ratio was calculated on a molar basis.
Statistical analysis
Two-way analysis of variance (ANOVA) was conducted with all experimental data. The distributions of values obtained for pH, rate of photosynthetic oxygen evolution, NH4 + uptake rate, relative growth rate, chlorophyll fluorescence, and C/N ratio were tested for normality and homogeneity before the statistical analysis began. Tukey’s tests were used to compare among treatments. Differences were considered statistically significant at p <0.05. All analyses were performed with the SPSS Program (version 23.0).
Results
Change in pH, photosynthetic oxygen evolution, and ammonium uptake rates
Initial pH values in the culture beakers were recorded before the experiments began. After 8 h of treatment, the maximum increase in pH was 2.76 ± 0.07 units, which occurred under the LpHHA treatment (Fig. 1), while the smallest rise, 2.12 ± 0.17 units, resulted from the HpHLA combination. For pH, responses to treatment, from highest to lowest, followed the order of LpHHA > LpHMA > LpHLA > HpHHA > HpHMA > HpHLA. When the same NH4 + concentration was examined across treatments, ΔpH in low-pH treatment beakers was significantly higher than that in the high-pH beakers (Table 2). At each pH level, the change in pH under high NH4 + was greater, but not significantly, than levels calculated under low or medium NH4 + conditions (Table 2). After 8 h of treatment, pH values were not significantly different among culture conditions but all were above 10.00 units (Fig. 1, Table 3). The pH did not change over time in cultures that had no specimen (i.e., blank treatment) and values ranged from 7.50 to 7.51 under low pH conditions and from 8.00 to 8.01 for high pH conditions.
Photosynthetic oxygen evolution rates during the 8-h experiments ranged from 42.39 ± 2.49 to 69.75 ± 4.25 μmol O2 g−1 FW h−1 (Fig. 2). When pH remained constant, photosynthetic oxygen evolution rates increased as the NH4 + concentration rose, and differences among treatments were significant. However, at individual NH4 + levels, evolution rates were not significantly different (Table 2).
Rates of NH4 + uptake by Ulva pertusa ranged from 0.08 ± 0.01 to 3.33 ± 0.10 μmol NH4 + g−1 FW h−1 (Fig. 3). Values were highest for the LpHHA samples and differences were significant among treatment combinations (p < 0.05). The lowest rate was 0.08 ± 0.01 μmol NH4 + g−1 FW h−1 for HpHLA samples and was similar to that calculated for the LpHLA combination (p > 0.05). When rates were compared among all treatments, values were higher under low pH conditions than under high pH treatment for all samples exposed to the HA status (p < 0.05). However, at the medium NH4 + concentration, differences in responses were not significant (p > 0.05). When NH4 + levels were elevated, uptake rates were increased at each pH value (Table 2). In particular, the rate was approximately 41 times faster for LpHHA than for HpHLA.
Growth rates, chlorophyll fluorescence, and C/N ratio
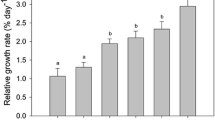
During the 14 days of observation, relative growth rates for U. pertusa ranged from 2.23 ± 0.13 to 4.27 ± 0.09% day−1 (Fig. 4), with values being lowest under HpHLA conditions. The highest rate was associated with the LpHHA treatment and was significantly different from rates calculated for the other treatments (p < 0.05). At a high NH4 + level, growth was more rapid under low pH conditions than under high pH conditions (p < 0.05). By contrast, low and medium levels of NH4 + did not have a significant effect on growth rates (p > 0.05). At each pH level, relative growth rates increased as the NH4 + concentration rose (Table 2).
Photosynthetic efficiencies, as measured by chlorophyll fluorescence, ranged from 0.54 ± 0.04 (HpHLA) to 0.67 ± 0.06 (LpHHA) after 14 days (Fig. 5). Significant differences were detected between the LpHLA and LpHHA conditions (Fig. 5, Table 2). Although F v/F m values were greater under high NH4 + than under other conditions, they were similar when the low NH4 + and medium NH4 + treatments were compared.
Neither the pH conditions generated by the addition of CO2 nor NH4 + concentrations affected the C/N ratios of U. pertusa (Table 2). Levels of C and N were 25.65 ± 0.65 to 27.26 ± 0.73% and 3.09 ± 0.04 to 3.15 ± 0.06%, respectively, and did not differ significantly among treatments (p > 0.05). The C/N ratio in tissues ranged from 8.24 ± 0.15 to 8.74 ± 0.22, but did not differ significantly among culture conditions (Table 4).
Discussion
The physiological responses of Ulva pertusa were influenced by pH and NH4 + status. This was demonstrated by the changes in pH, oxygen evolution, ammonium uptake, algal growth, chlorophyll fluorescence, and C/N ratio. The addition of CO2 gas to the culture medium, which was used to mimic OA, altered pH values because of chemical reactions in the solution. When that gas was dissolved, carbonic acid formed and then partitioned into bicarbonate and hydrogen ions (Falkowski and Raven 2007). Through this process, the dissolved hydrogen ions caused pH to decrease while the DIC concentration increased in that solution. We noted that changes in pH values were greater under low pH conditions than under the ambient pH treatment due to photosynthetic activity. This was a result of greater DIC available for photosynthesis, which enhances pH levels (Zhang et al. 2012). In response to higher CO2, many species down-regulate their CCM activity to save energy, thereby improving their rates of growth (Sarker et al. 2013).
Although our data showed that pH values under all of our culture conditions rose to almost 10.20 after 8 h, those differences were not significant. Zou (2014) has demonstrated that the pH compensation point of Ulva prolifera remains near pH 10.40 under experimental conditions and pH is not affected by different culturing treatments because U. prolifera has a similar extract HCO3 − capacity under a range of conditions. Maberly (1990) has reported that photosynthesis is limited at pH >9.0, which is contrary to our results. Under natural conditions, Ulva spp. utilize two means for taking up HCO3 − when pH levels are high. The first mechanism involves external carbonic anhydrase, which converts, through dehydration, HCO3 − to CO2 that is then transported into the cells (Beer and Israel 1990; Axelsson et al. 1995; Larsson et al. 1997; Björk et al. 2004). The second possible mechanism is direct uptake of HCO3 − via anion exchange protein under experimental conditions (Axelsson et al. 1995; Larsson et al. 1997; Björk et al. 2004). We suspect that U. pertusa uses both mechanisms for taking up HCO3 − under either low- or high-pH conditions in the laboratory (i.e., beaker cultivation).
We found that photosynthetic oxygen evolution rates for U. pertusa were influenced by elevated nutrient concentrations. Those rates improved under both low and high pH when NH4 + levels increased. At higher NH4 +, the difference in relative rates was significant between low NH4 + and medium NH4 + conditions but not between the LpHHA and HpHHA treatments. These results demonstrated that oxygen evolution by U. pertusa is more strongly modulated by a rise in NH4 + concentrations than by pH levels.
The greater rates of NH4 + uptake by U. pertusa in response to elevated CO2 and NH4 + levels or NH4 + alone noted here are similar to those reported for Porphyra leucosticte, Ulva rigida, Sargassum fusiforme, Gracilaria lemaneiformis, U. prolifera, Ulva linza, and Pyropia haitanensis (Mercado et al. 1999; Gordillo et al. 2001; Zou 2005; Xu et al. 2010; Luo et al. 2012; Xu and Gao 2012; Chen et al. 2016). For example, higher N concentrations lead to faster uptake by Ulva spp. (Luo et al. 2012). Elevated CO2 levels also enhance the uptake of N (Gordillo et al. 2001; Zou 2005; Liu and Zou 2015). When more nutrients are available, Ulva spp. can take up N more efficiently (Björnsäter and Wheeler 1990; Runcie et al. 2003; Pérez-Mayorga et al. 2011). The distinct morphological characteristics of Ulva spp. facilitate the opportunistic uptake of nutrients, which can then increase growth rates when NH4 + concentrations are higher (Duke et al. 1987; Arévalo et al. 2007). We also found that uptake rates were greatest in response to the interactive effect of LpHHA treatment. Therefore, future investigations should focus on aspects of metabolism, e.g., algal growth and nutrient uptake, in combination with CO2, NH4 +, and other environmental factors such as light intensity and temperature (Suárez-Álvarez et al. 2012). Our results indicated that relative growth rates of U. pertusa were faster when either nutrient levels alone or in combination with CO2 were increased. Both factors enhance seaweed growth and biomass accumulations in U. prolifera, U. linza, Hypnea spinella, Chondrus crispus, and P. haitanensis (Luo et al. 2012; Suárez-Álvarez et al. 2012; Xu and Gao 2012; Sarker et al. 2013; Chen et al. 2016). In our experiments, algal growth was maximized under LpHHA conditions and performance was significantly different when compared with results from the other treatments. Young and Gobler (2016) have also reported that the growth of Ulva spp. is promoted by elevated CO2 and nutrient concentrations. Because U. pertusa utilizes both CO2 and NH4 + for photosynthesis, that process can possibly be improved under OA and eutrophication conditions. In fact, such a scenario has been described with G. lemaneiformis and P. haitanensis (Xu et al. 2010; Chen et al. 2016). Therefore, we conclude that the interaction between CO2 and nutrient levels can have a dramatic impact on the growth of U. pertusa.
In addition to some changes in pH values, ammonium uptake rates, and relative growth rates, the photosynthetic efficiency (F v/F m) of U. pertusa was significantly affected by NH4 + concentrations. A previous study has found that photosynthetic efficiency is improved when nitrogen is more readily available (Zou and Gao 2014). Dawes and Koch (1990) have also shown that increased N concentrations can enhance chlorophyll fluorescence in algae. This is in contrast to a previous report that chlorophyll fluorescence is greater under higher CO2 concentrations (Chen et al. 2015). However, Liu et al. (2012) have found that elevated CO2 induces photochemical stress in U. prolifera under high-light conditions. Our results indicated that U. pertusa was not subjected to such stress because the experiments involved less intense lighting.
Regardless of treatment, the C/N ratio of U. pertusa was not significantly affected by elevated CO2 or NH4 + concentrations. Similar findings have been described for Porphyra leucosticta and Hypnea spinella (Mercado et al. 1999; Suárez-Álvarez et al. 2012). However, we calculated a C/N ratio of approximately 8:1, which is lower than the ratio of 10:1 that generally occurs when nitrogen supplies are abundant (Gómez-Pinchetti et al. 1998; Suárez-Álvarez et al. 2012). Chen and Johns (1991) have suggested that the C/N ratio is a good tool for assessing the physiological status of macroalgae. In fact, under our culture conditions, samples were able to maintain a good status throughout the experimental period.
Although U. pertusa is currently regarded as a harmful species in several coastal areas, our findings indicated that it can also provide a mitigating solution to problems associated with acidification and eutrophication because of its capacity to take up nutrients and DIC. Nevertheless, this study focused on short-term responses by U. pertusa and was conducted only on a small experimental scale. Therefore, further investigations should include long-term and large-scale examinations, such as mesocosm and field studies, so that researchers can obtain more practical information as they develop new strategies to deal with OA and eutrophication.
In summary, our data demonstrated that the physiological activities of U. pertusa are positively affected by OA and eutrophication. Increases in either CO2 levels or NH4 + concentrations had a strong impact on variations in pH, photosynthetic oxygen evolution rates, and chlorophyll fluorescence. In particular, the combination of elevated CO2 and NH4 + had the greatest influence on NH4 + uptake and algal growth. By comparison, rises in levels of CO2 and NH4 + did not affect the C/N ratio of this species.
References
Arévalo R, Pinedo S, Ballesteros E (2007) Changes in the composition and structure of Mediterranean rocky-shore communities following a gradient of nutrient enrichment: descriptive study and test of proposed methods to assess water quality regarding macroalgae. Mar Poll Bull 55:104–113
Axelsson L, Ryberg H, Beer S (1995) Two modes of bicarbonate utilization in the marine green macroalga Ulva lactuca. Plant Cell Environ 18:439–445
Beardall J, Giordano M (2002) Ecological implications of microalgal and cyanobacterial CO2 concentrating mechanisms, and their regulation. Funct Plant Biol 29:335–347
Beardall J, Beer S, Raven JA (1998) Biodiversity of marine plants in an era of climate change: some predictions based on physiological performance. Bot Mar 41:113–124
Beer S, Israel A (1990) Photosynthesis of Ulva fasciata. IV. pH, carbonic anhydrase and inorganic carbon conversions in the unstirred layer. Plant Cell Environ 13:555–560
Ben-Ari T, Neori A, Ben-Ezra D, Shauli L, Odintsov V, Shpigel M (2014) Management of Ulva lactuca as a biofilter of mariculture effluents in IMTA system. Aquaculture 434:493–498
Björk M, Axelsson L, Beer S (2004) Why is Ulva intestinalis the only macroalga inhabiting isolated rockpools along the Swedish Atlantic coast? Mar Ecol Prog Ser 284:109–116
Björnsäter BR, Wheeler PA (1990) Effect of nitrogen and phosphorus supply on growth and tissue composition of Ulva fenestrata and Enteromorpha intestinalis (Ulvales, Chlorophyta). J Phycol 26:603–611
Bolton JJ, Robertson-Andersson DV, Shuuluka D, Kandjengo L (2009) Growing Ulva (Chlorophyta) in integrated systems as a commercial crop for abalone feed in South Africa: a SWOT analysis. J Appl Phycol 21:575–583
Cai WJ, Hu X, Huang WJ, Murrell MC, Lehrter JC, Lohrenz SE, Chou WC, Zhai W, Hollibaugh JT, Wang Y, Zhao P, Guo X, Gundersen K, Dai M, Gong GC (2011) Acidification of subsurface coastal waters enhanced by eutrophication. Nat Geosci 4:766–770
Caldeira K, Wickett ME (2005) Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res 110:C09S04
Chen F, Johns MR (1991) Effect of C/N ratio and aeration on the fatty acid composition of heterotrophic Chlorella sorokiniana. J Appl Phycol 3:203–209
Chen B, Zou D, Jiang H (2015) Elevated CO2 exacerbates competition for growth and photosynthesis between Gracilaria lemaneiformis and Ulva lactuca. Aquaculture 443:49–55
Chen B, Zou D, Ma J (2016) Interactive effects of elevated CO2 and nitrogen–phosphorus supply on the physiological properties of Pyropia haitanensis (Bangiales, Rhodophyta). J Appl Phycol 28:1235–1243
Cohen RA, Fong P (2006) Using opportunistic green macroalgae as indicators of nitrogen supply and sources to estuaries. Ecol Appl 16:1405–1420
Cornwall CE, Hurd CL (2015) Experimental design in ocean acidification research: problems and solutions. ICES J Mar Sci 72. doi:10.1093/icesjms/fsv118
Cosgrove J, Borowitzka MA (2011) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Prásil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 1–17
Dawes CJ, Koch EW (1990) Physiological responses of the red algae Gracilaria verrucosa and G. tikvahiae before and after nutrient enrichment. Bull Mar Sci 46:335–344
de Faveri C, Schmidt ÉC, Simioni C, Martins CD, Bonomi-Barufi J, Horta PA, Bouzon ZL (2015) Effects of eutrophic seawater and temperature on the physiology and morphology of Hypnea musciformis JV Lamouroux (Gigartinales, Rhodophyta). Ecotoxicology 24:1040–1052
Dickson AG (1990) Standard potential of the reaction: AgCl (s) + 12H2 (g) = Ag (s) + HCl (aq), and the standard acidity constant of the ion HSO4 − in synthetic sea water from 273.15 to 318.15 K. J Chem Thermodyn 22:113–127
Duke CS, Litaker RW, Ramus J (1987) Seasonal variation in RuBPCase activity and N allocation in the chlorophyte seaweeds Ulva curvata (Kutz.) De Toni and Codium decorticatum (Woodw.) Howe. J Exp Mar Biol Ecol 112:145–164
Falkowski PG, Raven JA (2007) Aquatic photosynthesis. Princeton University Press, Princeton 484 pp
Figueroa FL, Barufi JB, Malta EJ, Conde-Álvarez R, Nitschke U, Arenas F, Mata M, Connan S, Abreu MH, Marquardt R, Vaz-Pinto F, Konotchick T, Celis-Plá PSM, Hermoso M, Ordoñez G, Ruiz E, Flores P, de los Rios J, Kirke D, Chow F, CAG N, Robledo D, Pérez-Ruzafa Á, Bañares-España E, Altamirano M, Jiménez C, Korbee N, Bischof K, Stengel DB (2014a) Short-term effects of increasing CO2, nitrate and temperature on three Mediterranean macroalgae: biochemical composition. Aquat Biol 22:177–193
Figueroa FL, Conde-Álvarez R, Barufi JB, Celis-Plá PSM, Flores P, Malta EJ, Stengel DB, Meyerhoff O, Pérez-Ruzafa Á (2014b) Continuous monitoring of in vivo chlorophyll a fluorescence in Ulva rigida (Chlorophyta) submitted to different CO2, nutrient and temperature regimes. Aquat Biol 22:195–212
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Gómez-Pinchetti JL, del Campo Fernández E, Díez PM, Reina GG (1998) Nitrogen availability influences the biochemical composition and photosynthesis of tank-cultivated Ulva rigida (Chlorophyta). J Appl Phycol 10:383–389
Gordillo FJ, Niell FX, Figueroa FL (2001) Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta 213:64–70
Gran G (1952) Determination of the equivalence point in potentiometric titrations of seawater with hydrochloric acid. Oceanol Acta 5:209–218
Hiraoka M, Shimada S, Uenosono M, Masuda M (2004) A new green-tide-forming alga, Ulva ohnoi Hiraoka et Shimada sp. nov. (Ulvales, Ulvophyceae) from Japan. Phycol Res 52:17–29
Horta PA, Vieira-Pinto T, Martins CDL, Sissini MN, Ramlov F, Lhullier C, Scherner F, Sanches PF, Farias JN, Bastos E, Bouzon JL, Munoz P, Valduga E, Arantes NP, Batista MB, Riul P, Almeida RS, Paes E, Fonseca A, Schenkel EP, Rorig L, Bouzon Z, Barufi JB, Colepicolo P, Yokoya N, Copertino MS, de Oliveira EC (2012) Evaluation of impacts of climate change and local stressors on the biotechnological potential of marine macroalgae: a brief theoretical discussion of likely scenarios. Rev Bras Farmacogn 22:768–774
Hurd CL, Hepburn CD, Currie KI, Raven JA, Hunter KA (2009) Testing the effects of ocean acidification on algal metabolism: consideration for experimental designs. J Phycol 45:1236–1251
IPCC (2014) Climate Change 2014: synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York
Kram SL, Price NN, Donham EM, Johnson MD, Kelly ELA, Hamilton SL, Smith JE (2016) Variable responses of temperate calcified and fleshy macroalgae to elevated pCO2 and warming. ICES J Mar Sci 73:693–703
Kroeker KJ, Gambi MC, Micheli F (2013) Community dynamics and ecosystem simplification in a high-CO2 ocean. Proc Natl Acad Sci 110:12721–12726
Larsson C, Axelsson L, Ryberg H, Beer S (1997) Photosynthetic carbon utilization by Enteromorpha intestinalis (Chlorophyta) from a Swedish rockpool. Eur J Phycol 32:49–54
Lewis E, Wallace DWR (1998) Program developed for CO2 system calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN, USA
Littler MM (1980) Morphological form and photosynthetic performances of marine macroalgae: tests of a functional/form hypothesis. Bot Mar 23:161–166
Liu C, Zou D (2015) Responses of elevated CO2 on photosynthesis and nitrogen metabolism in Ulva lactuca (Chlorophyta) at different temperature levels. Mar Biol Res 11:1043–1052
Liu Y, Xu J, Gao K (2012) CO2-driven seawater acidification increases photochemical stress in a green alga. Phycologia 51:562–566
Lohman K, Priscu JC (1992) Physiological indicator of nutrient deficiency in Cladophora (Chlorophyta) in the Clark Fork of the Columbia River, Montana. J Phycol 28:443–448
Luo MB, Liu F, Xu ZL (2012) Growth and nutrient uptake capacity of two co-occurring species, Ulva prolifera and Ulva linza. Aquat Bot 100:18–24
Maberly SC (1990) Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae. J Phycol 26:439–449
Mercado JM, Javier F, Gordillo L, Niell FX, Figueroa FL (1999) Effects of different levels of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta. J Appl Phycol 11:455–461
Millero FJ, Graham TB, Huang F, Bustos-Serrano H, Pierrot D (2006) Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Mar Chem 100:80–94
Morand P, Merceron M (2005) Macroalgal population and sustainability. J Coast Res 21:1009–1020
Murru M, Sandgren CD (2004) Habitat matters for inorganic carbon acquisition in 38 species of red macroalgae (Rhodophyta) from Puget Sound, Washington, USA. J Phycol 40:837–845
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, NewYork
Pérez-Mayorga DM, Ladah LB, Zertuche-González JA, Leichter JJ, Filonov AE, Lavín MF (2011) Nitrogen uptake and growth by the opportunistic macroalga Ulva lactuca (Linnaeus) during the internal tide. J Exp Mar Biol Ecol 406:108–115
Raven JA (1997) Inorganic carbon acquisition by marine autotrophs. Adv Bot Res 27:85–209
Raven JA, Giordano M, Beardall J, Maberly SC (2011) Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth Res 109:281–296
Reymond CE, Lloyd A, Kline DI, Dove SG, Pandolfi JM (2013) Decline in growth of foraminifer Marginopora rossi under eutrophication and ocean acidification scenarios. Glob Change Biol 19:291–302
Runcie JW, Ritchie RJ, Larkum AW (2003) Uptake kinetics and assimilation of inorganic nitrogen by Catenella nipae and Ulva lactuca. Aquat Bot 76:155–174
Sarker MY, Bartsch I, Olischläger M, Gutow L, Wiencke C (2013) Combined effects of CO2, temperature, irradiance and time on the physiological performance of Chondrus crispus (Rhodophyta). Bot Mar 56:63–74
Stengel DB, Conde-Álvarez R, Connan S, Nitschke U, Arenas F, Abreu H, Barufi JB, Chow F, Robledo D, Malta EJ, Mata M, Konotchick T, Nassar C, Pérez-Ruzafa Á, López D, Marquardt R, Vaz-Pinto F, Celis-Plá PSM, Hermoso M, Ruiz E, Ordoñez G, Flores P, Zanolla M, Bañares-España E, Altamirano M, Korbee N, Bischof K, Figueroa FL (2014) Short-term effects of CO2, nutrients and temperature on three marine macroalgae under solar radiation. Aquat Biol 22:159–176
Suárez-Álvarez S, Gómez-Pinchetti JL, García-Reina G (2012) Effects of increased CO2 levels on growth, photosynthesis, ammonium uptake and cell composition in the macroalga Hypnea spinella (Gigartinales, Rhodophyta). J Appl Phycol 24:815–823
Sunda WG, Cai WJ (2012) Eutrophication induced CO2-acidification of subsurface coastal waters: interactive effects of temperature, salinity, and atmospheric pCO2. Environ Sci Technol 46:10651–10659
Valiela I, McClelland J, Hauxwell J, Behr PJ, Hersh D, Foreman K (1997) Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnol Oceanogr 42:1105–1118
Wang Y, Zhou B, Tang X (2009) Effects of two species of macroalgae—Ulva pertusa and Gracilaria lemaneiformis—on growth of Heterosigma akashiwo (Raphidophyceae). J Appl Phycol 21:375–385
Wu H, Zou D, Gao K (2008) Impacts of increased atmospheric CO2 concentration on photosynthesis and growth of micro- and macro-algae. Sci China C Life Sci 51:1144–1150
Xu J, Gao K (2012) Future CO2-induced ocean acidification mediates the physiological performance of a green tide alga. Plant Physiol 160:1762–1769
Xu Z, Zou D, Gao K (2010) Effects of elevated CO2 and phosphorus supply on growth, photosynthesis and nutrient uptake in the marine macroalga Gracilaria lemaneiformis (Rhodophyta). Bot Mar 53:123–129
Ye NH, Zhang XW, Mao YZ, Liang CW, Xu D, Zou J, Zhuang ZM, Wang QY (2011) ‘Green tides’ are overwhelming the coastline of our blue planet: taking the world’s largest example. Ecol Res 26:477–485
Yong YS, Yong WTL, Anton A (2013) Analysis of formulae for determination of seaweed growth rate. J Appl Phycol 25:1831–1834
Young CS, Gobler CJ (2016) Ocean acidification accelerates the growth of two bloom-forming macroalgae. PLoS One 11:e0155152
Zhang N, Song J, Cao C, Ren R, Wu F, Zhang S, Sun X (2012) The influence of macronitrogen (NO3 − and NH4 +) addition with Ulva pertusa on dissolved inorganic carbon system. Acta Oceanol Sinica 31:73–82
Zou D (2005) Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture 250:726–735
Zou D (2014) The effects of severe carbon limitation on the green seaweed, Ulva conglobata (Chlorophyta). J Appl Phycol 26:2417–2424
Zou D, Gao K (2014) The photosynthetic and respiratory responses to temperature and nitrogen supply in the marine green macroalga Ulva conglobata (Chlorophyta). Phycologia 53:86–94
Acknowledgement
This work was supported by a 2-Year Research Grant of Pusan National University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, J.W., Chung, I.K. The effects of eutrophication and acidification on the ecophysiology of Ulva pertusa Kjellman. J Appl Phycol 29, 2675–2683 (2017). https://doi.org/10.1007/s10811-017-1087-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1087-5