Abstract
Haematococcus pluvialis is a microalga that can reduce the levels of inorganic and organic components in effluents, in addition to production of the high-value carotenoid astaxanthin. The present study proposed an integrated system for the simultaneous treatment of effluent from a Nile tilapia recirculation system and astaxanthin production using the microalga H. pluvialis. Cultures were developed in 2 L bottles, with freshwater previously treated and enriched with a modified Bold’s Basal Medium (BBM). The experiment was carried out on a laboratory scale using a completely randomized experimental design with five treatments: control (100% BBM), E25 (25% Nile tilapia effluent + 75% BBM), E50 (50% Nile tilapia effluent + 50% BBM), E75 (75% Nile tilapia effluent + 25% BBM), E100 (100% Nile tilapia effluent), with three replicates for each condition. H. pluvialis could grow in all proportions of effluent from Nile tilapia cultivation. The use of effluent as culture medium (E100), despite having lower cell density and biomass, resulted in biomass with high astaxanthin content (10 mg g−1) and antioxidant activity (89% inhibition of DPPH radicals). Meanwhile, this integrated system significantly contributed to the effluent remediation (98% N and 91% P removal). H. pluvialis can be used for cost-effective treatment of aquaculture effluent with simultaneous production of astaxanthin, with potential application as aquafeed, in a holistic bioeconomy approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a result of global population increase, several environmental problems such as contamination of soil and water bodies caused by improper disposal of effluents have intensified, affecting the ecosystem functioning of aquatic and terrestrial environments (Chai et al. 2021). In view of the pollution increase and scarcity of freshwater resources, the treatment and reuse of wastewater result in social, environmental, and economic benefits (Tortajada 2020). Among the generated effluents, industrial and domestic effluents stand out, in addition to those from aquaculture, which when released into rivers and lakes lead to eutrophication (Nogueira et al. 2022).
Fish rearing is a growing activity worldwide due to its economic, social, and nutritional impact, contributing significantly to achieve a few sustainable development goals of the 2030 Agenda (Ashour et al. 2021; Wang et al. 2021; Oliveira et al. 2022a). The increase in this activity resulted, almost in the same proportion, in the increase in the production of effluents, which can cause environmental impacts due to the release of inorganic compounds, mainly nitrogen and phosphorus, from leftover feed and fish excreta (Kurniawan et al. 2021). The recirculation aquaculture system (RAS) is a way to reduce the disposal of effluents containing high levels of inorganic compounds; however, due to the intensive mode of production, it is essential to treat waste both within the recirculation circuit and in the effluents of these systems (Rijn 2013). Nevertheless, the use of conventional chemical products can cause imbalance in the system’s microbiota; thus, the use of algae and plants has proven to be an efficient way to solve this problem.
Algae require macronutrients and micronutrients and can absorb these compounds from effluents (Mohsenpour et al. 2021). In recent years, studies have been carried out aimed at the use of microalgae for the bioremediation of different types of effluents (Daneshvar et al. 2019; Oliveira et al. 2020; Chai et al. 2021; Moraes et al. 2021). When cultivated in media rich in nitrogen, phosphorus, and organic carbon, microalgae can growth quickly, using heterotrophic or mixotrophic pathways—depending on the availability of light energy (Lv et al. 2018; Perez-Garcia et al. 2011). Furthermore, algae can be inserted in several industrial segments, including agriculture, human and animal nutrition, and energy plants (Yap et al. 2021; Oliveira et al. 2021). Haematococcus pluvialis is especially known for its high content of carotenoids, mainly astaxanthin (reaching a concentration of up to 5% of the cellular content) (Mota et al. 2022). Astaxanthin is a carotenoid with high antioxidant capacity, which acts to protect cells against free radical damage, combating oxidative stress and reducing the risk of diseases in humans and animals (Mota et al. 2022). In recent years, astaxanthin has gained attention as a dietary supplement for animals, particularly in aquaculture (Lim et al. 2018).
Studies have shown that astaxanthin feed supplementation in aquaculture can improve immune function, reduce inflammation, increase resistance to environmental stressors, and improve reproductive performance, growth, and coloration (Lim et al. 2018; Ashour et al. 2021). The use of H. pluvialis as a source of astaxanthin in animal feed offers several advantages: it is a natural and sustainable source, with greater antioxidant power than the synthetic form (Mota et al. 2022); it can be cultivated using wastewater as an environmentally friendly and low-cost culture medium (Pan et al. 2021); the inclusion of H. pluvialis in animal feed can lead to an improvement in product quality (Su et al. 2020).
Although several microalgae have been studied in terms of growth kinetics and nutrient uptake to treat aquaculture effluents, few studies have focused on evaluating the high-value commercial metabolites produced during the bioremediation process. In view of this, this study proposed an integrated system for the bioremediation of effluents from a tilapia recirculation system and astaxanthin production by H. pluvialis, with potential application as aquafeed.
Material and methods
Strain and culture conditions
H. pluvialis was obtained from the Live Food Production Laboratory, at the Fisheries and Aquaculture Department of the Federal Rural University of Pernambuco. Cultures were developed in 2 L bottles, with freshwater previously treated with chlorine (3 ppm), filtered (22 µm) and autoclaved (120 °C), and then enriched with a modified version of Bold’s Basal Medium (BBM) proposed by Moraes et al. (2023) that contains (in mg L−1) 118 NH4NO3; 25 CaCl2·2H2O; 25 NaCl; 31 KOH; 50 EDTA Na·2H2O; 75 K2HPO4; 175 KH2PO4; 4.98 FeSO4·7H2O; 75 MgSO4·7H2O; 11.42 H3BO3; 1.412 ZnSO4·7H2O; 0.232 MnCl2·4H2O; 0.252 CuSO4·5H2O; 0.192 Na2MoO4·2H2O; 0.08 Co(NO3)2·6H2O.
Experimental design
The experiment was carried out on a laboratory scale using a completely randomized experimental design with five treatments: control (100% BBM), E25 (25% Nile tilapia effluent + 75% BBM), E50 (50% Nile tilapia effluent + 50% BBM), E75 (75% Nile tilapia effluent + 25% BBM), and E100 (100% Nile tilapia effluent), with three replicates each.
H. pluvialis was inoculated in 2 L bottles with an initial concentration of 20,000 cells mL−1, using 60 mL of inoculum, at a temperature of 22 °C, photoperiod of 12 h, and 40 µmol photons m−2 s−1 irradiance, under continuous aeration. In the stationary phase, to stimulate the carotenogenesis (cystic phase), there was an increase in light intensity (40 to 100 µmol photons m−2 s−1), in addition to the natural nitrogen deprivation (Moraes et al. 2023).
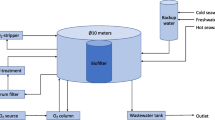
Effluent from Nile tilapia in Oasis® recirculation system
The effluent from a culture tank of Nile tilapia (Oreochromis niloticus) in Oasis® production system was used for H. pluvialis cultivation. The Oasis® is a RAS-based system that aims at high productivity, low water renewal, and full use of the waste generated. In this system, Nile tilapias of about 10.88 ± 0.77 cm and 31.47 ± 2.71 g were cultivated for 45 days using a feed of 45% of crude protein offered three times a day (ad libitum) and presenting a feed conversion factor of 0.98. The effluent was subjected to filtration (40 µm), chlorination with sodium hypochlorite at 3 ppm for 24 h (with aeration), and autoclaving at 120 °C for 15 min (Moraes et al. 2021). After these procedures, the effluent was characterized regarding the concentrations of nitrogenous compounds and phosphate (APHA 2012). This effluent contained 0.275 ± 0.04 mg L−1 of NH3-N, 0.846 ± 0.01 mg L−1 of NO2-N, 40.184 ± 0.06 mg L−1 of NO3-N, and 13.224 ± 0.03 mg L−1 of PO4-P.
Growth analysis and biomass harvesting
To evaluate growth, samples were taken daily and fixed in formaldehyde (2%) for quantification using a hemocytometer (Neubauer chamber). The growth curves with the average daily cell density were fitted by approximating the logistic curve (Sprouffske and Wagner 2016). The logistic equation describes the population size Nt at time t Eq. (1):
where N0 is the population size at the beginning of the growth curve, K is the maximum possible population size in a particular environment, and r is the specific growth rate. With this fit, the following parameters were calculated: specific growth rate (r, day−1) and doubling time (DT, division day−1). The maximum specific growth rate (µmax) was calculated according to the equations below.
The following asymmetric logistic Eq. (2) was used to fit the cell concentration (N(t)) versus time (t) data in order to accurately determine the specific growth rate:
where a, b, t0, and y0 are constants. The cell-specific growth rate (μ, day−1) was calculated using the best fit curve of Eq. (2); thus:
where µmax (day−1) is the maximum specific growth rate in the exponential phase according to Eq. (3).
For maximum cell density (Cmax, cells mL−1), it was considered the day of culture in which the population reached the maximum cell density.
The dry biomass (g L−1) and biomass productivity (g L−1 day−1) were determined by filtering 10 mL aliquots of suspended cells through a Whatman GF/C glass microfiber filter (1.2 µm) and drying at 75 °C for 24 h (Choi et al. 2018).
Water quality and nitrogen removal
The parameters of water quality, pH, temperature, ammonia-N (NH3-N), nitrite-N (NO2-N), nitrate–N (NO3-N), and phosphate-P (PO4-P) were analyzed in the culture medium on days 0 (first day), 12 (exponential phase), and 20 (last day). Samples of 10 mL were filtered through a Whatman GF/C glass microfiber filter (1.2 µm), and then, the filtered volume was collected for analysis of the nitrogen compounds and phosphate (APHA 2012). The pH and temperature were measured using a digital pHmeter (Kmoon pH/EC-983).
The determination of the percentages of nutrient removal from the culture media was carried out at the end of the experiment using Eq. 4:
Quantification of total carotenoids and astaxanthin
The concentrations of total carotenoids and astaxanthin were determined at the end of cultivation. Carotenoid and astaxanthin analyses were performed from a 10 mL and 1 mL aliquot of the algae suspension, respectively, and analyzed in a spectrophotometer, according to Moraes et al. (2023).
The concentration of total carotenoids was calculated by [4 × OD480], and astaxanthin concentration and content were calculated from the expressions [4.5 × OD490 × (Va / Vb)] and [P / W], respectively, where Va (mL) was the volume of DMSO, Vb (mL) was the volume of microalgae samples, P (mg L−1) was the concentration of astaxanthin, and W (g L−1) denoted the dry biomass of microalgae per unit volume of medium (Cheng et al. 2016).
Antioxidant activity
Extracts of H. pluvialis biomass were obtained using 0.1 g of dried biomass resuspended and homogenized in 2 mL of 99.9% dimethyl sulfoxide. Afterwards, the mixture was centrifugated at 1000 rpm for 2 min. The supernatant was collected subjected to serial dilution (50, 25, 12.5, 6.25, and 3.12 mg mL−1) for posterior antioxidant assays as proposed by Oliveira et al. (2022b). The antioxidant activity (inhibition %) of the extracts were evaluated by 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+•) (Guedes et al. 2013) and 2,2-diphenyl-1-picrylhydrazyl (DPPH•) (Dantas et al. 2019) methods. Trolox® was used as standards for calibration curve as well as positive control.
Statistical analysis
The response variables were submitted to the Shapiro–Wilk normality test and to the Bartlett’s test of homogeneity of variances. All data were submitted to ANOVA followed by Tukey’s test when a significant difference was observed. p values < 0.05 were considered statistically significant for all tests. Statistical analysis was performed using the R Core Team software (R Core Team 2023).
Results and discussion
Cell growth and biomass production of H. pluvialis
H. pluvialis could grow in all proportions of the Oasis® effluent from Nile tilapia rearing. The variables growth rate (r), doubling time (DT), and maximum specific growth rate (µmax) were higher for treatments E0 (control) and E75 followed by E100, while the lowest values were presented by E50 and E25. On the other hand, the maximum cell density (Cmax) was higher at E0 and E25; these treatments were significantly different from E50, E75, and E100 (Table 1).
Regarding the biomass, E0 and E25 obtained the highest values, with an average yield of 0.75 g L−1 and average productivity of 0.047 g L−1 day−1. While E50 and E75 showed an average yield and productivity of 0.61 g L−1 and 0.038 g L−1 day−1. E100 was significantly different for all treatments, except E75, and showed average yield and productivity of 0.53 g L−1 and 0.033 g L−1 day−1 (Table 1).
The growth curves of H. pluvialis which show the daily cell densities of each treatment are presented in Fig. 1. The control treatment (E0) presented a longer exponential phase than the others. This may have occurred due to the availability of mixed sources of nitrogen in the effluent. Once the higher affinity nitrogen source, ammonia, is depleted, there is a metabolic readjustment to absorb the following sources, nitrate and nitrite (Su 2021). This condition justifies the “pseudo-stationary phases” along the effluent treatment curves, which may influence the lower growth. Possibly, vegetative cells multiplied later compared to the control and treatment with lower percentage of effluent addition (i.e., E25), resulting in a lower cell density in treatments E75 and E100.
Besides the presence of nutrients, like nitrogen, other physicochemical aspects play a crucial role in cellular metabolism, such as temperature and pH. Maintaining minimal pH fluctuations ensures a more consistent chemical environment and, as a result, improved conditions for the division of H. pluvialis cells (Dos Santos and Lombardi 2017). In this study, the initial pH had no significant difference between treatments (p > 0.05), with an average of 7.2 ± 0.2; generally, at an initial pH of 7, greater growth of H. pluvialis is observed (Sarada et al. 2002). The pH variation until the end of cultivation was higher in treatments E50, E75, and E100 (8.4 ± 0.03, 8.26 ± 0.01, 8.37 ± 0.05, respectively), differing significantly of treatments E0 and E25 (7.8 ± 0.01, 7.9 ± 0.02). This may also have influenced the lower Cmax, biomass, and biomass productivity in treatments with a higher proportion of effluent (i.e., E50, E75, and E100). The temperature had no significant difference between treatments or over time, remaining at 22.2 ± 0.4 °C.
Removal of nitrogen and phosphate compounds by H. pluvialis
Regarding nitrogen sources, ammonia is the nitrogen compound most easily incorporated by microalgae (Su 2021). Thus, for microalgae in general, the order of preference for nitrogen utilization is NH4+ − > NO3 − > NO2, due to no redox reaction requirement and less energy cost to assimilate ammonia (Ramanna et al. 2014). Ammonia was more abundant (25 mg L−1) in the treatments with higher amounts of BBM, i.e., E0 and E25 (Fig. 2a), which showed higher Cmax, biomass, and biomass productivity (Table 1). Despite that, the total nitrogen concentration was similar for the treatments, with an average of 40 mg L−1. Hence, nitrogen in the form of ammonium contributed to greater biomass, like previous studies also conducted with H. pluvialis using different effluents (Mourya et al. 2023; Yap et al. 2022). For example, Pan et al. (2021) demonstrated a removal of 83.4% of NH4-N from potato juice wastewater after methanation and acidification of the effluent. Integrating processes with other microorganisms can optimize growth conditions, making other compounds bioavailable to algae, and increasing the uptake rates.
Concentrations of NH3-N (a), NO2-N (b), NO3-N (c), and PO4-P (d) over 20 days and uptake of nitrogen and phosphate compounds (e) by H. pluvialis under different proportions of BBM and effluent from the Nile tilapia Oasis.® system. Different letters indicate a significant difference among treatments by the Tukey’s post hoc test (p < 0.05)
H. pluvialis only uses other sources of nitrogen, such as nitrate and nitrite, when ammonia is no longer available in the environment (Markou and Georgakakis 2011). The average initial concentration of NO2-N in treatments E75 and E100 was 0.8 mg L−1, while for treatments E0 and E25, it was 0.04 and 0.2 mg L−1, respectively (Fig. 2b). Meanwhile, higher concentrations of NO3-N (27–40 mg L−1) were found in treatments with a high proportion of effluent (E100 and E75) (Fig. 2c). The nitrogen uptake by H. pluvialis in the effluent was more than 95% for NH3-N (except in E100 that reached 52%), 94% for NO3-N, and 100% for NO2-N (Fig. 2e). Regarding the total nitrogen concentration, there was an average absorption of 97.7% for all treatments, being the highest absorption in E75 (99.5%), whose growth velocity data r and µmax were higher.
In addition to nitrogen, phosphorus is an essential element as it assists in metabolic processes, photosynthesis, and energy conversion (Marinho et al. 2021). The initial concentration of PO4-P varied between 13 (E100) and 24 mg L−1 (E0) (Fig. 2d), being consumed by the microalgae throughout the cultivation, with a final removal efficiency of 84 to 98% (Fig. 2e). The highest phosphate absorption occurred in E75 and E100, which had a lower initial concentration of PO4-P.
In summary, the results of the study indicate that H. pluvialis has a significant ability to capture nutrients from effluent, improving, therefore, the levels of these compounds in the water, and this is in agreement with previous studies (Wu et al. 2013; Mourya et al. 2023; Pan et al. 2021; Yap et al. 2022).
Production of high-value metabolites from H. pluvialis
In addition to H. pluvialis acting in the bioremediation of effluent, it can produce high-value metabolites such as carotenoids, especially astaxanthin (80–99% of total carotenoids). As for the total carotenoids obtained in the cystic phase, higher concentrations (5.32–6.06 mg L−1) and content (7.6–10.1 mg g−1) were observed in the treatments E0, E25, and E100 (Fig. 3a, b). Following the same trend, higher concentrations (5.34–5.50 mg L−1) and content (7.26–10.25 mg g−1) of astaxanthin were obtained in treatments E0, E25, and E100 (Fig. 3c, d).
Concentrations (mg L−1) and contents (mg g.−1) of total carotenoids (a, b) and astaxanthin (c, d) produced by Haematococcus pluvialis cultivated in different proportions of BBM and effluent from the Nile tilapia Oasis® system (different letters indicate a significant difference by the Tukey’s post hoc test (p < 0.05)
Astaxanthin synthesis occurs under nutrient starvation (i.e., nitrogen and phosphate), which triggers the upregulation of genes associated with astaxanthin biosynthesis to act in defense metabolism to this stress condition (Liyanaarachchi et al. 2020). On day 20, total nitrogen depletion was observed in all treatments, accompanied by a predominance of cystic cells, with accumulation of astaxanthin. Furthermore, under nitrogen deprivation, the carbon flux is directed towards the synthesis of astaxanthin (Lu et al. 2019; Moraes et al. 2023). The use of effluent from the Nile tilapia recirculation system (E100) contributed to the high content of astaxanthin (10 mg g−1), similar to E0 and E25, although E100 had a low biomass compared to these treatments. The use of effluent replacing the culture medium was a stress factor for microalgae, primarily attributed to the significantly reduced initial concentration of PO4-P (1.8 times lower than E0), leading to low growth and greater astaxanthin production.
Astaxanthin is a carotenoid widely used in the pharmaceutical, nutraceutical, cosmetic, and food industries, in addition to aquaculture, both for pigmentation and to improve the immune response and zootechnical performance of aquatic organisms (Lim et al. 2018; Su et al. 2020). This carotenoid has anti-inflammatory, antitumor, antidiabetic, immunomodulatory, and antioxidant properties, highlighting its antioxidant activity, which is 10 times greater than that of β-carotene (Mota et al. 2022). The antioxidant activity of microalgal biomass extract cultured in effluent from Oasis® system was investigated using ABTS and DPPH assays (Table 2). As noted, all treatments exhibited antioxidant effects, even at varying degrees (47.4–88.7% inhibition). The highest percentages of inhibition of ABTS and DPPH radicals were presented by treatments E50 (73.9 ± 7.9%) and E100 (88.7 ± 1.5%), respectively. The percentage of inhibition observed in the present study was like that reported by Al-Tarifi et al. (2020), using the same solvent (DMSO) for astaxanthin powder, approximately 85% inhibition of DPPH radical. Therefore, the biomass produced in effluent showed greater antioxidant activity.
The findings indicate that the biomass of H. pluvialis, cultivated in effluent from Nile tilapia rearing—which is rich in astaxanthin and possesses high antioxidant properties—holds potential for utilization in aquaculture feed. This aquafeed could be used in the production of tilapia in the Oasis® system, as well as the treated water could be returned to replenish the cultivation system, in a circular economy approach. Furthermore, the astaxanthin from H. pluvialis biomass has promise as a bioactive compound in nutraceutical and pharmaceutical products for human applications. Thus, the use of effluent as a culture medium contributes to the sustainable production of H. pluvialis biomass and astaxanthin, considering the replacement of high-cost culture media, the valorization of effluents in high-value metabolites, and the treatment of effluents (potential source of contamination for the environment).
Conclusions
H. pluvialis was able to grow in all proportions of RAS effluent from the Nile tilapia rearing. The use of effluent as culture medium, despite having lower cell density and biomass, had a higher astaxanthin content. Therefore, the present study proposes an ecofriendly and low-cost medium formed only by effluent from the Nile tilapia recirculation system for astaxanthin production. Meanwhile, the integrated system contributed to the effluent remediation. H. pluvialis can be used for cost-effective treatment of aquaculture effluent with simultaneous production of astaxanthin with great potential for a holistic bioeconomy.
Data availability
The authors certify that all data support the published claims and comply with field standards.
Code availability
Not applicable.
References
Al-Tarifi BY, Mahmood A, Assaw S, Sheikh HI (2020) Comparison of different organic solvents on antioxidant activity of astaxanthin extracted from Hematococcus pluvialis using colorimetric and non-colorimetric methods. Orient J Chem 36(3):466–473. https://doi.org/10.13005/ojc/360316
APHA (2012). Standard methods for the examination of water and wastewater (22nd ed.). American Public Health Association, Washington
Ashour M, Alprol AE, Heneash AM, Saleh H, Abualnaja KM, Alhashmialameer D, Mansour AT (2021) Ammonia bioremediation from aquaculture wastewater effluents using Arthrospira platensis NIOF17/003: impact of biodiesel residue and potential of ammonia-loaded biomass as rotifer feed. Materials 14(18):5460. https://doi.org/10.3390/ma14185460
Chai WS, Tan WG, Munawaroh HSH, Gupta VK, Ho SH, Show PL (2021) Multifaceted roles of microalgae in the application of effluent biotreatment: a review. Environ Pollut 269:116236. https://doi.org/10.1016/j.envpol.2020.116236
Cheng J, Li K, Yang Z, Zhou J, Cen K (2016) Enhancing the growth rate and astaxanthin yield of Haematococcus pluvialis by nuclear irradiation and high concentration of carbon dioxide stress. Bioresour Technol 204:49–54. https://doi.org/10.1016/j.biortech.2015.12.076
Choi YY, Hong ME, Jin ES, Woo HM, Sim SJ (2018) Improvement in modular scalability of polymeric thin-film photobioreactor for autotrophic culturing of Haematococcus pluvialis using industrial flue gas. Bioresour Technol 249:519–526. https://doi.org/10.1016/j.biortech.2017.10.060
Daneshvar E, Zarrinmehr MJ, Koutra E, Kornaros M, Farhadian O, Bhatnagar A (2019) Sequential cultivation of microalgae in raw and recycled dairy effluent: microalgal growth, effluent treatment and biochemical composition. Bioresour Technol 273:556–564. https://doi.org/10.1016/j.biortech.2018.11.059
de Dantas DMM, Oliveira CYB, Costa RMPB, das Carneiro-da-Cunha MG, Gálvez AO, de Bezerra RS (2019) Evaluation of antioxidant and antibacterial capacity of green microalgae Scenedesmus subspicatus. Food Sci Technol Int 25:318–326. https://doi.org/10.1177/1082013218825024
Dos Santos AC, Lombardi AT (2017) Growth, photosynthesis and biochemical composition of Haematococcus pluvialis at various pH. J Algal Biomass Util 8:1–15
Guedes AC, Amaro HM, Gião MS, Malcata FX (2013) Optimization of ABTS radical cation assay specifically for determination of antioxidant capacity of intracellular extracts of microalgae and cyanobacteria. Food Chem 138:638–643. https://doi.org/10.1016/J.FOODCHEM.2012.09.106
Kurniawan SB, Abdullah SRS, Imron MF, Ahmad A, Mohd Said NS, Mohd Rahim NF et al (2021) Potential of valuable materials recovery from aquaculture effluent: an introduction to resource reclamation. Aquac Res 52(7):2954–2962. https://doi.org/10.1111/are.15180
Lim KC, Yusoff FM, Shariff M, Kamarudin MS (2018) Astaxanthin as feed supplement in aquatic animals. Rev Aquac 10(3):738–773. https://doi.org/10.1111/raq.12200
Liyanaarachchi V, Nishshanka G, Premaratne R, Ariyadasa T, Nimarshana P, Malik A (2020) Astaxanthin accumulation in the green microalga Haematococcus pluvialis: effect of initial phosphate concentration and stepwise/continuous light stress. Biotechnol Rep 28:e00538. https://doi.org/10.1016/j.btre.2020.e00538
Lu Z, Zheng L, Liu J, Dai J, Song L (2019) A novel fed-batch strategy to boost cyst cells production based on the understanding of intracellular carbon and nitrogen metabolism in Haematococcus pluvialis. Bioresour Technol 289:121744. https://doi.org/10.1016/j.biortech.2019.121744
Lv J, Liu Y, Feng J, Liu Q, Nan F, Xie S (2018) Nutrients removal from undiluted cattle farm wastewater by the two-stage process of microalgae-based wastewater treatment. Bioresour Technol 264:311–318. https://doi.org/10.1016/j.biortech.2018.05.085
Marinho YF, Malafaia CB, de Araújo KS, da Silva TD, dos Santos APF, de Moraes LB, Gálvez AO (2021) Evaluation of the influence of different culture media on growth, life cycle, biochemical composition, and astaxanthin production in Haematococcus pluvialis. Aquac Int 29:757–778. https://doi.org/10.1007/s10499-021-00655-z
Markou G, Georgakakis D (2011) Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and effluents: a review. Appl Energy 88(10):3389–3401. https://doi.org/10.1016/j.apenergy.2010.12.042
Mohsenpour SF, Hennige S, Willoughby N, Adeloye A, Gutierrez T (2021) Integrating micro-algae into effluent treatment: a review. Sci Total Environ 752:142168. https://doi.org/10.1016/j.scitotenv.2020.142168
Moraes LBS, Mota GCP, da Silva FR, Marinho YF, Silva LOB, Gálvez AO (2021) Growth and nitrogen uptake by Arthrospira platensis cultured in aquaculture effluent from Nile tilapia reared in biofloc system. Acta Sci Biol Sci 43:e57549. https://doi.org/10.4025/actascibiolsci.v43i1.57549
Moraes LBS, Mota GCP, dos Santos EP, Campos CVFDS, da Silva BAB, Olivera Gálvez A, de Souza Bezerra R (2023) Haematococcus pluvialis cultivation and astaxanthin production using different nitrogen sources with pulse feeding strategy. Biomass Convers Biorefin 13(2):1–13. https://doi.org/10.1007/s13399-023-03824-7
Mota GCP, Moraes LBS, Oliveira CYB, Oliveira DWS, Abreu JLD, Dantas DMM, Gálvez AO (2022) Astaxanthin from Haematococcus pluvialis: processes, applications, and market. Prep Biochem Biotechnol 52(5):598–609. https://doi.org/10.1080/10826068.2021.1966802
Mourya M, Khan MJ, Sirotiya V, Ahirwar A, Schoefs B, Marchand J et al (2023) Enhancing the biochemical growth of Haematococcus pluvialis by mitigation of broad-spectrum light stress in effluent cultures. RSC Adv 13(26):17611–17620. https://doi.org/10.1039/D3RA01530K
Nogueira GS, de SouzaBezerra G, Piana PA (2022) Treatment of aquaculture effluent with floating macrophytes: systemic review and meta-analysis. Res Soc Dev 11(4):e36811426533. https://doi.org/10.33448/rsd-v11i4.26533
Oliveira CYB, Abreu JL, de Oliveira CDL, Lima PC, Gálvez AO, Dantas DMM (2020) Growth of Chlorella vulgaris using effluent from Nile tilapia (Oreochromis niloticus) farming in a low-salinity biofloc system. Acta Sci Technol 42:1–11. https://doi.org/10.4025/actascitechnol.v42i1.46232
Oliveira CYB, Oliveira CDL, Prasad R, Ong HC, Araujo ES, Shabnam N, Gálvez AO (2021) A multidisciplinary review of Tetradesmus obliquus: a microalga suitable for large-scale biomass production and emerging environmental applications. Rev Aquac 13(3):1594–1618. https://doi.org/10.1111/raq.12536
Oliveira CYB, Abreu JL, Santos EP, Matos ÂP, Tribuzi G, Oliveira CDL, Veras BO, Bezerra RS, Müller MN, Gálvez AO (2022) Light induces peridinin and docosahexaenoic acid accumulation in the dinoflagellate Durusdinium glynnii. Appl Microbiol Biotechnol 106(18):6263–6276. https://doi.org/10.1007/s00253-022-12131-6
Oliveira CYB, Jacob A, Nader C, Oliveira CDL, Matos ÂP, Araújo ES, Shabnam N, Ashok B, Gálvez AO (2022) An overview on microalgae as renewable resources for meeting sustainable development goals. J Environ Manag 320:115897. https://doi.org/10.1016/j.jenvman.2022.115897
Pan M, Zhu X, Pan G, Angelidak I (2021) Integrated valorization system for simultaneous high strength organic effluent treatment and astaxanthin production from Haematococcus pluvialis. Bioresour Technol 326:124761. https://doi.org/10.1016/j.biortech.2021.124761
Perez-Garcia O, Escalante FM, De-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45(1):11–36. https://doi.org/10.1016/j.watres.2010.08.037
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: https://www.R-project.org/. Accessed 22 Mar 2023
Ramanna L, Guldhe A, Rawat I, Bux F (2014) The optimization of biomass and lipid yields of Chlorella sorokiniana when using effluent supplemented with different nitrogen sources. Bioresour Technol 168:127–135. https://doi.org/10.1016/j.biortech.2014.03.064
Rijn JV (2013) Waste treatment in recirculating aquaculture systems. Aquac Eng 53:49–56. https://doi.org/10.1016/j.aquaeng.2012.11.010
Sarada R, Tripathi U, Ravishankar GA (2002) Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem 37(6):623–627. https://doi.org/10.1016/S0032-9592(01)00246-1
Sprouffske K, Wagner A (2016) Growthcurver: an R package for obtaining interpretable metrics from microbial growth curves. BMC Bioinform 17:172. https://doi.org/10.1186/s12859-016-1016-7
Su Y (2021) Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci Total Environ 762:144590. https://doi.org/10.1016/j.scitotenv.2020.144590
Su F, Yu W, Liu J (2020) Comparison of effect of dietary supplementation with Haematococcus pluvialis powder and synthetic astaxanthin on carotenoid composition, concentration, esterification degree and astaxanthin isomers in ovaries, hepatopancreas, carapace, epithelium of adult female Chinese mitten crab (Eriocheir sinensis). Aquaculture 523:735146. https://doi.org/10.1016/j.aquaculture.2020.735146
Tortajada C (2020) Contributions of recycled wastewater to clean water and sanitation Sustainable Development Goals. NPJ Clean Water 3(1):22. https://doi.org/10.1038/s41545-020-0069-3
Wang C, Li Z, Wang T, Xu X, Zhang X, Li D (2021) Intelligent fish farm—the future of aquaculture. Aquac Int 29:2681–2711. https://doi.org/10.1007/s10499-021-00773-8
Wu YH, Yang J, Hu HY, Yu Y (2013) Lipid-rich microalgal biomass production and nutrient removal by Haematococcus pluvialis in domestic secondary effluent. Ecol Eng 60:155–159. https://doi.org/10.1016/j.ecoleng.2013.07.066
Yap JK, Sankaran R, Chew KW, Munawaroh HSH, Ho SH, Banu JR, Show PL (2021) Advancement of green technologies: a comprehensive review on the potential application of microalgae biomass. Chemosphere 281:130886. https://doi.org/10.1016/j.chemosphere.2021.130886
Yap SM, Lan JCW, Kee PE, Ng HS, Yim HS (2022) Enhancement of protein production using synthetic brewery effluent by Haematococcus pluvialis. J Biotechnol 350:1–10. https://doi.org/10.1016/j.jbiotec.2022.03.008
Acknowledgements
The authors would like to thank the Laboratory of Aquatic Animal Health, Laboratory of Shrimp Farming and Laboratory of Aquaculture Production at the Department of Fisheries and Aquaculture, and Laboratory of Molecular Biology Applied to Animal Production at the Department of Animal Science of the Federal Rural University of Pernambuco for providing the instruments used for the analysis.
Funding
The study was financially supported by the Federal Rural University of Pernambuco; the Coordination of Improvement of Higher Education Personnel (CAPES) grants to Laenne Moraes (88882.436230/2019–01); the Foundation for the Support of Science and Technology of Pernambuco (FACEPE) grants to Géssica Mota (IBPG-1574–5.04/22); and the National Council for Scientific and Technological Development (CNPq) for the scholarships granted to Alfredo Olivera Gálvez (PQ 308063/2019–8) and Ranilson de Souza Bezerra (PQ 307107/2019–1).
Author information
Authors and Affiliations
Contributions
LM: writing—original draft, conceptualization, investigation, methodology, formal analysis, and data curation. GM and BS: investigation, methodology, and formal analysis. CO: formal analysis, data curation, and writing—review and editing. JS: resources and data curation. AG: resources, supervision, and writing—review and editing. RB: study conception and design, resources, and project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: ronan sulpice
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Moraes, L.B.S., Mota, G.C.P., da Silva, B.A.B. et al. Integrated system for bioremediation of Nile tilapia rearing effluent and astaxanthin production by Haematococcus pluvialis. Aquacult Int 32, 4345–4358 (2024). https://doi.org/10.1007/s10499-023-01377-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01377-0