Abstract
The nutritional profile of tropical mariculture wastewater in Hainan Island and the possibilities of artificial mariculture wastewater (AWW) bioremediation using microalgae and bacteria screened from the South China Sea were studied. A total of 34 sampling sites from field and water were selected to study the current status of mariaquaculture in Wenchang, an important aquaculture production base in Hainan. The mean value of pH, suspended solids (SS), NH4+-N, PO43−-P, and CODMn of field samples were 7.52, 229.44 mg/L, 1.44 mg/L, 0.88 mg/L, and 9.5 mg/L, respectively. Monocultures of microalgae (Chlorella vulgaris, Platymonas subcordiformis, and Chaetoceros müelleri) and microalgae-bacteria symbiosis (C. vulgaris and Bacillus spp.) were also studied in AWW indoor inoculation experiments. The results of monocultures indicated that C. vulgaris is the optimal strain for removing NH4+-N, NO2−-N, TN, and PO43−-P, with a removal rate of 97.15%, 99.45%, 95.90%, and 96.62% in 72 h, respectively. By comparison, symbiosis of C. vulgaris-Bacillus spp. with an initial concentration of 2.5 × 105 cells/mL microalgae + 5 mL Bacillus spp. (OD600 = 1.8) enhanced the removal of NH4+-N, PO43—P, and CODMn, with a removal rate of 100%, 100%, and 35.69% in 72 h, respectively. Combining microalgae and bacteria communities is probably effective in nutrients removal, enhancing microalgal biomass and biofuel production.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
According to Zheng and Shen (2021), the mariculture area of Hainan Province reached 5347 hm2 in 2018, more than twice that of 2009. Meanwhile, the mariculture output of Hainan Province has increased year by year for the last decade, reaching 124,554 tons in 2018, more than three times that of 2009. The growing food production industry increases high-density tropical mariculture, producing significant mariculture effluent. In general, surplus fishery feeder, excrement, and aquatic remains would build up in the cultivation system, resulting in a high concentration of particulate organic matter and a richness of dissolved nutrients in the wastewater, such as nitrogen and phosphorus, which could eventually cause eutrophication and disrupt the balance of aquaecosystem. Nevertheless, from the perspective of energy, these organic matters and nutrients can be a supply to bacteria and microalgae growth which can be utilized as an economical, efficient, and eco-friendly method to remove various pollutants in mariculture wastewater.
Typical technologies such as physical, chemical, and biological processes, which have been applied in the treatment of mariculture wastewater, include reverse osmosis, ion exchange, and constructed wetland (Santos & Pires, 2018; Sun et al., 2020). Physical methods such as sedimentation, sand filtration, or mechanical filtration are generally applied in the primary stage to clarify large-sized solids (Cai, Park, et al., 2013). Precipitation and ferrous chloride, in terms of chemical treatment processes, are commonly used to remove phosphorous compounds (Guldhe et al., 2017). The conventional biological treatment removes organic matter and nutrients through aerobic or anaerobic activities. Among the three conventional methods, some drawbacks also limit their application in practice. For instance, physical methods are of low efficiency and are not applicable to nutrients removal, chemical methods are costly and may produce some toxic compounds as byproducts, while the conventional biological method is expensive due to the large quantities of sludge production and high cost for drying and great amounts of nutrients would be wasted in the effluent (Guldhe et al., 2017). In contrast, microalgae-assisted bioremediation has the ability to assimilate inorganic nitrogen and phosphorus in a contaminated mariculture environment (Wang et al., 2016). Subsequently, it may offer more efficient and cost-effective alternatives to conventional treatment methods.
Furthermore, microalgae-based wastewater treatment technology can simultaneously remove other pollutants such as refractory organics and heavy metals from sewage. Likewise, it can be used to treat a variety of wastewater, including municipal sewage, aquaculture effluent, and industrial drainage. Especially, it is generally believed that the synergistic function between microalgae and bacteria is responsible for the purification of wastewater (Wang, Jiao, et al., 2020). Currently, higher removal efficiency has been observed based on the monoculture microalgae and microalgae-bacteria symbiosis technology (Chu et al., 2021; Koppel et al., 2019; Li et al., 2020; Liu et al., 2020). However, the differences between the two modes still need to be further investigated to optimize the mariculture wastewater.
This study investigated the nutritional profile of mariculture wastewater in Hainan and aimed to select proper microalgal strain and optimal symbiosis of microalgae and bacteria for the bioremediation of mariculture wastewater. Parameters such as ammonia nitrogen (NH4+-N), orthophosphate (PO43−-P), nitrite (NO2−-N), total phosphorus (TP), total nitrogen (TN), and chemical oxygen demand (CODMn) were tested.
2 Materials and Methods
2.1 Sampling of Tropical Mariculture Wastewater
A total of 34 sampling sites were selected surrounding the mariculture facility based on mariculture output, area, and mariculture wastewater receiving points at the sea site (as shown in Fig. 1).
Sampling was carried out on three consecutive sunny days (from October 30th to November 1st, 2017). All the mariculture wastewater samples were collected using 1-L polyethylene bottles at locations about 100 m away from drainage outlets and labeled as the sequence of sampling sites (see Supplementary Table S1). The samples were immediately delivered to the laboratory and stored at 4 ℃ in a refrigerator until further treatment and analysis; the parameters such as pH, suspended substance (SS), CODMn, PO43−-P, and NH4+-N content were measured.
2.2 Preparation of the Artificial Tropical Mariculture Wastewater
Artificial mariculture wastewater was prepared on the basis of the concentrations of pollutants in practical wastewater sampled from the 34 sites in Hainan. For details, the AWW was composed of glucose (200 mg/L), NH4Cl (5 mg/L), NaH2PO4 (1.5 mg/L), NaNO2 (3 mg/L), NaNO3 (3 mg/L), and stock of trace elements, as the same as that of f/2 medium in “Microalgal strain, medium, and cultivation condition.” As reported, a molar ratio of N:P lower than 5:1 would result in nitrogen limitation, whereas phosphorus limitation would occur at a ratio of higher than 30:1 (Larsdotter, 2006). In this study, the N:P molar ratio was 16.24, indicating that neither the nitrogen nor phosphorus limitation was reached. However, it was higher than that of the recommended medium of C. vulgaris (e.g., f/2 medium), in which N of 12.36 mg/L, P of 1.12 mg/L with an N:P ratio of 11.
2.3 Microalgal Strain, Medium, and Cultivation Condition
In this study, three microalgal species such as C. vulgaris, Platymonas subcordiformis, and Chaetoceros müelleri were selected to cultivate in mariculture wastewater. The strains were obtained from the microalgae culturing collection center at the College of Oceanology, Hainan University in China. The f/2 medium was based on Guillard(1975), which composed (per liter seawater, all analytical grade) NaNO3 (8.83 × 10−4 mol/L), NaH2PO4•2H2O (3.63 × 10−5 mol/L), Na2SiO3•9H2O (1.07 × 10−4 mol/L), CuSO4•5H2O (4 × 10−8 mol/L), ZnSO4•7H2O (8 × 10−8 mol/L), CoCl2•6H2O (5 × 10−8 mol/L), MnCl2•4H2O (9 × 10−7 mol/L), Na2MoO4•2H2O (3 × 10−8 mol/L), Na2EDTA (1 × 10−5 mol/L), FeCl3•6H2O (1 × 10−5 mol/L), vitamin B1 (3 × 10−7 mol/L), biotin (1 × 10−6 mol/L), and vitamin B12 (1 × 10−10 mol/L). The inoculum of microalgae was prepared in 250-mL flasks containing 120 mL fresh f/2 medium. The flasks containing fresh medium were sterilized at 121 ℃ and 0.22 MPa pressure conditions for 15 min. After cooling down to room temperature, the medium was inoculated to reach an initial cell density of 2.5 × 105 cells/mL. The microalgae were cultivated in an illumination incubator (MGC-100, Blue Pard) at 28 ± 1℃, with the illumination of 660 µmol photons/(m2•s) at 12:12 light: dark cycle under an array of fluorescence lights (DULUX L 36 W/840, OSRAM).
2.4 Bacteria strain, Medium, and Cultivation
Bacteria Bacillus spp. was the dominant strain in seawater around Hainan Island, screened by the Ecotoxicology Laboratory, Hainan University in China. Saline Lactose Broth (SLB) medium (per liter seawater, analytical grade) is composed of beef extract (5 g/L) and peptone (10 g/L) with a pH of 7.8 was used for the cultivation. After being sterilized and cooled down, the medium was inoculated with 2 mL Bacillus spp. with an initial optical density of 1.89 measured at a 600 nm (OD600) wavelength and kept at 30 ℃ and 160 rpm for 16 h in a shaker (ZQP-75D, Laboratory).
2.5 Nutrients Removal from Mariculture Wastewater Using Microalgae
Monoculture microalgae and microalgal-bacteria symbiosis were cultivated in AWW to evaluate their nutrient removal efficiencies from mariculture wastewater. For the monoculture experiment, three microalgal species of C. vulgaris, P. subcordiformis, and C. müelleri were separately inoculated into sterilized AWW, to reach an initial cell density of 2.5 × 105 cells/mL. The microalgal-bacteria symbiosis experiment was conducted by inoculating the aforementioned optimal microalgae and bacteria Bacillus spp. with different inoculation ratios to determine the optimal symbiosis for nutrients removal from AWW. An experimental sketch contained information on two monocultures (C and B) and three symbiosis (C-B5, C-B10, and C-B25) shown in Fig. 2.
In this study, each experiment was carried out with three replicates. Shake the flasks at least three times each day in the morning, noon, and afternoon of cultivation days, respectively. Besides, parameters such as optical density (OD), concentrations of NH4+-N, NO2−-N, PO43−-P, TN, and CODMn were determined daily. Other cultivation conditions were kept as the same as that in “Preparation of the artificial tropical mariculture wastewater.”
2.6 Analytical Methods
The biomass concentration of microalgae and bacteria was represented by the index of absorbance (OD), which was by the absorbance of microbial medium suspension in optical density at the wavelength of 680 nm (OD680) and 600 nm (OD600), respectively. The pH was determined by a Mettler Toledo Seven2Go pH meter, and SS was measured using the gravimetric method. Amount of PO43−-P was determined by the spectrophotometric determination of a blue phosphomolybdic complex that specifically absorbs at 882 nm compared with the deionized water as the blank solution (Strickland & Parsons, 1972). Nitrites were determined by a colorimetric chemical reaction forming an azo dye. While the concentration of NH4+-N and TN content follows the Phenate Method and Potassium Persulfate Oxidation Method compared with the ultrapure water as blank solution and NO2−-N content used deionized water as the blank solution. Additionally, the content of CODMn was determined by the method from Ansari et al. (2017).
2.7 Data Analysis
Data were presented as the average by recording in Microsoft Office Excel™. Correlational analysis was performed using the SPSS 23 software (IBM Corporation, Armonk, New York, USA). Graphical analyses were performed using Originlab Origin Pro 8.0™. The removal efficiencies and removal rates of nutrients were calculated according to Eqs. (1) and Eq. (2).
where \({\mathrm{RE}}_{i}\) (i = PO43−-P, NO2−-N, NH4+-N, TN, or CODMn) is the removal efficiencies of nutrients,\({\mathrm{R}}_{i}\) (i = PO43−-P, NO2−-N, NH4+-N, TN, or CODMn) is the removal rate of nutrients, \({\mathrm{S}}_{0,i}\) is the initial concentration value of substrate, and \({\mathrm{S}}_{t,i}\) shows corresponding substrate concentration at time t.
3 Results
3.1 Current Status of Tropical Mariculture Wastewater in Hainan
3.1.1 Mariculture Wastewater Discharge in Hainan
The field investigation showed that four main mariculture systems such as a high-level pond, low-level pond, common cage, and industrial factory are widely applied in Hainan, with an area of 692 hm2, 2489 hm2, 8 hm2, and 789,000 m3, respectively (2017). The main mariculture species include grouper (Epinephelus sp.), Penaeus sp., and high economic profits shellfish species including Babylonia lutosa and Scapharca inflate, with yields of 14,424 tons, 20,507 tons, and 11,930 tons, respectively. As investigated, the water exchange methods varied with the types of aquatic species, culturing period, and mariculture method (see Supplementary Table S2). It can be seen that large quantities of mariculture wastewater are directly discharged, resulting in adverse effects on the quality of the surrounding sea.
The current status of investigated sites is shown in Fig. 3. Dense aquaculture wastewater pipes are randomly distributed along the beachside (Fig. 3a,b). Moreover, some large-scale drainage ditches are also randomly distributed (Fig. 3c). Since many substances such as residual baits, excrement, and other culturing residues are produced in cultivation, most residues accumulate and decompose in the culturing system. Thus, the water quality would be affected significantly. As investigated, the seawater turns green, turbid, and stinky due to the accumulation of pollutants (Fig. 3d). Besides, the plastic sewage pipes are potential contributors of plastic debris and even micro-/nano-plastics to either beaches or seawater (Fig. 3e). In some places, the sands have become hardened and blacked due to the long time receiving the mariculture wastewater (Fig. 3f). The study also found many large-scaled culturing systems (e.g., industrial factories and high-level ponds) in Wenchang (Fig. 3g, h).
Representative present situation of mariculture sites in Wenchang City: (a) intake and drainage pipes on the beach, (b) coastal drainage pipes, (c) a drainage ditch, (d) water body color around the drainage outlet becomes green, (e) wasted pipe plastic garbage, (f) blackened and harden sands, (g) an industrial aquaculture pond, and (h) a high-level pond
3.1.2 Current Status of Mariculture Wastewater
For the 34 sampling sites, the temperature varied between 33 ℃ and 35 ℃ without drastic fluctuation (Table 1). The pH values of the samples ranged from 6.89 to 8.25, while the SS content ranged from 16.77 to 653.33 mg/L. The site with the highest SS content (653.33 mg/L) was site 21, which receives water samples from a high-level shrimp pond (Table 1).
The mean value of NH4+-N, PO43−-P, or SS was calculated on the basis of all 34 sampling sites (Table 1). The concentration of NH4+-N ranged from 0.33 mg/L (at site 10) to 3.15 mg/L (at site 21). Higher concentrations of NH4+-N were observed at sites 02, 09, 18, and 21, where Babylonia, fishes, or shrimps have been cultivated for a long period. Furthermore, the maximum NH4+-N value (i.e., 3.15 mg/L) was observed at site 21. For PO43−-P content of 34 sites ranged from 0.07 to 2.35 mg/L, in which three samplings showed a high level of PO43−-P from site 02 (1.94 mg/L), site 21 (1.89 mg/L), and site 27 (2.35 mg/L). Among them, site 27 was a fish culturing draining outlet, while site 02 and site 21 were close to the port. The range of CODMn was 0.43–18.44 mg/L for the 34 sampling locations. For CODMn with the maximum value of 18.44 mg/L was observed close to downtown and around under construction Shuxiang Park at site 25.
3.2 Effects of Free-celled Microalgae on Nutrients Removal from AWW
According to the above field investigation, the main pollutants included SS, NH4+-N, PO43−-P, and CODMn and the following experiments focused on the removal of these components.
3.2.1 Cell Growth of Microalgae in AWW
As shown in Fig. 4, an initial cell density of 1 × 106 cells/mL was inoculated with 25% (v/v) microalgal inoculum of C. vulgaris, P. subcordiformis, and C. müelleri. The growth profiles presented a slight increase in OD680 biomass(0.13–0.30) at the end of the cultivation period (P < 0.05). The maximum biomass concentration of C. vulgaris, P. subcordiformis, and C. müelleri with OD680 values of 0.18, 0.14, and 0.33 were obtained in AWW at 240 h of cultivation, respectively.
3.2.2 Phosphorus Removal by Microalgae from AWW
As a fundamental material of life module in DNA, RNA, ATP, proteins, and amino acid, phosphorus is regularly existing in wastewater as inorganic anionic types, including H2PO4−/HPO42− which is highly relative to microalgae metabolisms (Salama et al., 2017). In this experiment, three microalgal species exhibited high removal efficiency of PO43−-P in 24 h or 48 h of cultivation (Fig. 5). For example, near-zero residual PO43−-P level was achieved in monoculture C. vulgaris and P. subcordiformis in 24 h, while a remaining concentration of 0.86 mg/L PO43−-P in the treatment of C. müelleri, but it also reached a near-zero residual by 48 h of cultivation. The results indicated that PO43−-P could be removed entirely via cultivating such monoculture microalgae as C. vulgaris, P. subcordiformis, and C. müelleri.
3.2.3 Nitrogen Removal by Microalgae from AWW
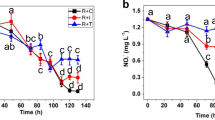
Ammonium could be absorbed by microalgae cells through active transport and directly utilized for amino acids synthesis (Han et al., 2019). Figure 6 shows nitrogen removal in terms of NH4+-N, NO2−-N, and TN in AWW by monoculture C. vulgaris, P. subcordiformis, and C. müelleri, respectively. The removal efficiencies of NH4+-N achieved by cultivating C. vulgaris, P. subcordiformis, and C. müelleri reached 88.79%, 61.42%, and 50.25% in 24 h, respectively (Fig. 6a). A near-zero residual NH4+-N level (0.01 ± 0.005 mg/L) was achieved by cultivating monoculture C. vulgaris, P. subcordiformis, and C. müelleri within 48 h. By comparison, C. vulgaris showed a relatively faster removal rate than P. subcordiformis and followed by C. müelleri.
NO2−-N can be eventually removed by monoculture either C. vulgaris, P. subcordiformis, or C. müelleri (Fig. 6b). For instance, NO2−-N was completely removed by C. vulgaris in a short cultivation time (i.e., 72 h), while by C. müelleri and P. subcordiformis in almost 96 h and 216 h, respectively. In the current study, C. vulgaris presented the optimal bioremediation in consideration of advantages such as shorter treatment time, higher removal efficiency, and vigorous cultures.
TN concentration in monoculture of C. vulgaris, P. subcordiformis, and C. müelleri declined in the first 48 h or 72 h. It then remained lower for the rest of the cultivation time (Fig. 6c). Microalgae C. vulgaris, P. subcordiformis, and C. müelleri can uptake almost all the TN from AWW, with the residue of 0.49 mg/L, 0.73 mg/L, and 0.55 mg/L in 48 h, respectively, corresponding to removal efficiencies of 90.24%, 85.36%, and 88.99%.
Overall, among the three monocultures of microalgal species, C. müelleri showed faster cell growth. While C. vulgaris exhibited a more rapid removal rate of NH4+-N, PO43−-P, TN, and NO2−-N than other microalgal species in AWW. Thus, the optimal microalgae C. vulgaris was selected for the combination of Bacillus spp. in the following tests of AWW biotreatment.
3.3 Effects of Microalgal-bacteria Symbiosis on Nutrients Removal in AWW
As aforementioned, the microalgal-bacteria symbiosis was based on C. vulgaris inoculation of 2.5 × 105 cells/mL with 5 mL, 10 mL, and 25 mL inoculum of Bacillus spp. (OD600 = 1.89), which are labeled as C-B5, C-B10, and C-B25, respectively. More specifically, we summarized and compared the microbial growth performance of both C. vulgaris and Bacillus spp. and the removal of NH4+-N, PO43—P, and CODMn from AWW in bioremediation between monoculture and symbiosis system in Table 2. As for the pollutants removal rates, in the same treatment period, all of them were higher in three consortium groups than that in the pure cultures of microalgae or bacteria groups. It should be noted that only NH4+-N was measured in this test in consideration of the main pollutants in AWW.
3.3.1 Cell Growth of Microalgal-bacteria Symbiosis
Cell growth of both C. vulgaris and Bacillus spp. increased during the period of treatment (P < 0.05), and the maximum DCW was achieved in 168 h. For symbiosis C-B5, C-B10, and C-B25, higher microalgal biomass concentrations of 344 mg/L, 457 mg/L, and 1,039 mg/L were obtained than monoculture C. vulgaris value of 318 mg/L. The supply of carbon dioxide and inorganic substances from bacteria benefitted the microalgal biomass accumulation. The DCW was significantly increased by C-B10 and C-B25 (P < 0.05), while no significant difference was observed in C-B5 (P > 0.05) when comparing the cell growth in microalgal-bacteria system with monoculture C. vulgaris. This indicated that Bacillus spp. contributed to the higher cell density of C. vulgaris, and C-B25 achieved the best cell growth. Additionally, the cell density of Bacillus spp. also exhibited the same trend as C. vulgaris.
3.3.2 NH 4 + -N Removal by Microalgal-bacteria Symbiosis
As shown in Table 2, NH4+-N contents decreased from 1.5 to 0.023 mg/L and 0.069 mg/L by cultivating monoculture of C. vulgaris and Bacillus spp. within 72 h, with the removal efficiency of 98.36% and 95.12%, respectively. By comparison, higher NH4+-N removal efficiency (up to approximate 98%) was achieved by microalgal-bacteria symbiosis in a shorter time (i.e., the first 48 h of cultivation time). It could be attributed to the cooperative interaction in microalgal-bacteria symbiosis.
Among the three symbioses of microalgal-bacteria, complete removal of NH4+-N (corresponds to 0 mg/L in the effluent) was achieved in the trial of C-B5 and C-B10 in 72 h, while 0.071 mg/L NH4+-N remained in the trial of C-B25 in 72 h, respectively. The results indicated that optimal bacteria was added and a relatively higher removal efficiency of NH4+-N was achieved (P < 0.05).
3.3.3 PO4 3−-P Removal by Microalgal-bacteria Symbiosis
High removal efficiency of PO43−-P was achieved by either monoculture or symbiosis of C. vulgaris and Bacillus spp. by 48 h of cultivation (P < 0.05). For instance, PO43−-P in almost all treatments was completely removed from 1.5 to 0–0.003 mg/L (corresponds to the rates of up to 1.490–1.497 mg/(L•d)). Zero residual of PO43−-P was achieved by C-B5 and C-B25 within 48 h. The fastest and highest PO43−-P removal was found in C-B5 (93.94% reduction within 12 h), followed by C-B10 (92.55% in 12 h) and C-B25 (90.24% in 12 h), while the lowest removal efficiency of PO43−-P (86.24%) was obtained in the monoculture of microalgae, which may be due to the lack of bacteria involvement and relatively poor PO43−-P removal capability.
3.3.4 COD Mn Reduction by Microalgal-bacteria Symbiosis
As shown in Table 2, the CODMn removal was similar in the treatments with the removal efficiencies of 27.56–44.93%, which indicated that there was no strong relationship between CODMn removal and initial inoculum ratios of monoculture or symbiosis microalgae or bacteria. Nevertheless, the CODMn removal efficiencies achieved by symbiotic bioremediation of microalgae and bacteria (35.69–44.93%) were slightly higher than monoculture C. vulgaris (27.56% in 168 h) and Bacillus spp (35.69% in 168 h). Moreover, it can be seen that the optimal efficiency of CODMn was achieved by C-B25, with the highest removal efficiency and reduction rate of 44.93% and 6.446 mg/(L•d), respectively.
4 Discussion
4.1 Current Status of Tropical Mariculture Wastewater in Hainan
The present study determined that the main pollutants are SS, NH4+-N, and PO43−-P. In general, SS values in 34 sites are high. High SS contents may be attributed to massive aquaculture feeds, antibiotics excretions of shrimp, and the algae baits in intensive shrimp mariculture mode.
A higher concentration of NH4+-N was observed in Babylonia, fishes, or shrimp cultivated sites for a long period. Under such circumstances, more bait residue, excrement, and plankton would be produced and accumulated, particularly at the late culturing period. Furthermore, when the drain outlet site is close to a port (i.e., Wenchang Port), the value of NH4+-N shows a high trend, which may result from the frequent shipping activities, including water cleaning ships bilges and fuel tanks, which produce large quantities of pollutants such as diesel fuels, oil, grease, food residues, solvents, cleaning agents, cigarette butts, paints, bacteria, and other solid particles (Bilgili et al., 2016).
Among 34 sites, three samplings showed a high level of PO43−-P. One of them was a fish culturing, draining accumulated feed residue and fish excreta. While the others were close to a port, the high nutrients might also be attributed to the geographical locations, feedings, and culturing methods, in the same as high NH4+-N content, which was mainly affected by various pollution sources around the port.
The highest concentration of CODMn may be contributed by that outlet receives not only mariculture wastewater but also municipal sewage. Particularly, municipal wastewater is generally with a high concentration of organic pollutants (e.g., COD containing 95.33–126.33 mg/L and SS containing 80–130 mg/L) and ammonia nitrogen (8.7–40 mg/L) (Cheah et al., 2016).
4.2 Effects of Free-celled Microalgae on Nutrients Removal from AWW
In this study, C. vulgaris, P. subcordiformis, and C. müelleri all grew well under the conditions of this experiment. However, the biomass accumulation of microalgae is relatively smaller as compared with other studies in Table 3, and that may be related to the low concentration of nitrogen and phosphorus in AWW, and they are insufficient to support the cell growth of microalgae. A 107.86 mg/(L•d) Chlorella sorokiniana biomass was obtained after phototrophic cultured in aquaculture wastewater with NO3−-N, NO2−-N, NH4+-N, and PO43−-P in concentrations of 40.67 mg/L, 5.52 mg/L, 5.32 mg/L, and 8.82 mg/L, respectively (Guldhe et al., 2017). Moreover, the biomass concentration of 3150 mg DCW/L was achieved when Neochloris oleoabundans was cultivated in artificial wastewater with sodium nitrate and phosphate concentrations of 140 mg/L and 47 mg/L, respectively (Wang & Lan, 2011). Interestingly, a previous study reported that C. vulgaris was cultivated in secondary treated domestic wastewater with a low NH4+-N concentration of 0.56 mg/L, but a higher biomass concentration of 1100 mg/L was reached, which may relate to the bubbling with sufficient air in a photobioreactor (Fernández-Linares et al., 2017).
In this study, PO43−-P can be completely removed via the cultivation of such monoculture microalgae as C. vulgaris, P. subcordiformis, and C. müelleri. Moreover, when C. vulgaris was continuously cultivated in aquaculture wastewater using a membrane photobioreactor, the PO43−-P removal efficiency of 82.7% (Gao et al., 2016). Under autotrophic, heterotrophic, and mixotrophic conditions, almost total nitrogen and phosphorus removals (> 99% for both total nitrogen and PO43−-P) were achieved by microalgae C. vulgaris in centrate wastewater (Ge et al., 2018). Peng et al. (2020) also proved the reduction in dissolved inorganic nitrogen dissolved inorganic phosphorus in marine aquaculture wastewater after being treated by microalgae (i.e., C. vulgaris) in a biofilm membrane photobioreactor.
By comparison, C. vulgaris showed a relatively faster removal rate of NH4+-N than that of P. subcordiformis, followed by C. müelleri, and this was also confirmed in other studies. For example, monoculture microalgae of Chlorella sp. greatly removed NH4+-N from aquaculture wastewater of L. calcarifer, with an initial concentration of 7 mg/L (Lananan et al., 2014).
Microalgal cells remove nitrogen through direct uptake of inorganic nitrogen such as NH4+-N, NO2−-N, NO3−-N, from which NH4+-N is the easiest to assimilate. However, priority assimilation may apply to some species. For example, when both nitrate and ammonium are provided, only NH4+-N is utilized by microalgal cells (Fernández-Linares et al., 2017).
The results obtained in this study showed that three microalgae completely removed NO2−-N at different removal rates. However, the removal efficiency is comparable to the previous research that Chlorella sorokiniana cultured in aquaculture wastewater removed NO2−-N from 5.52 to 0.2 mg/L after the 7-day cultivation period (Guldhe et al., 2017). Additionally, in the current study, C. vulgaris presented the optimal bioremediator despite advantages such as shorter treatment time, higher removal efficiency, and vigorous cultures. In comparison to other nitrogen species, NO2−-N must be converted into NH4+-N in two steps before being assimilated by microalgae. In the first step, NO3−-N is reduced to NO2−-N using nicotine-amide adenine dinucleotide phosphate (NADPH) as a reducing agent, and then NO2−-N is converted to NH4+-N using nitrite reductase in a six-electron transfer reaction catalyzed by ferredoxin.
Nutrients removal from mariculture wastewater in this study is comparable to that achieved in previous studies; for instance, all TN removal efficiencies of three species of Dunaliella sp., Nannochloropsis sp., and Tetraselmis sp. were reached higher than 90% in controlled simulated mariculture wastewater (Sacristán de Alva et al., 2018). The efficient TN removal is partly attributed to the uptaking of NH4+-N and organic nitrogen by microalgal cells under the investigated condition (Cai, Ge, et al., 2013). It may also be related to the fact that abiotic removal processes, such as chemical precipitation and stripping, can decrease TN (Salama et al., 2015).
4.3 Effects of Microalgal-bacteria Symbiosis on Nutrients Removal in AWW
In this study, the symbiosis of Bacillus spp. and C. vulgaris promoted both of their growth in mariculture wastewater. Both cooperative and competitive interactions contributed to the outstanding performance despite the ups and downs during the treatment process, which was similar to the findings of Sun et al. (2020). Likewise, in a wastewater treatment study, Qi et al. (2018) reported the dominance of three bacteria, Xiguobacterium aurantiacum, Stenotrophomonas acidaminiphila, and Chryseobacterium Scophthalmus species, in fermentation wastewater treated by microalgae. They found more than 77.8% of NH4+-N, 45.6% of total PO43−-P in all co-cultures. However, in TN contents, fluctuations occurred in the three pure cultures of bacteria, and the TN concentrations almost did not get reduced. In contrast, excellent removal efficiency was observed in three consortia groups, with TN nearly got completely removed by Chlorella sorokiniana L3 and C. scophthalmus. Muradov et al. (2015) compared the concentrations of nutrients in 25% diluted swine wastewater before and after treatment with C. protothecoides, T. suecica, and their pellets with A.fumigatus. After 48 h of Af/Cp incubation in 25% diluted wastewater, NH4+-N and PO43−-P concentrations reduced from 164.3 to 43.2 mg/L and from 38.7 to 17.2 mg/L with a corresponding elimination efficiency of 73.9% and 55.6%, respectively. This removal efficiency was higher than the efficiency of NH4+-N, and PO43−-P removal was achieved separately by C. protothecoides (36% and 25%, respectively) and by A. fumigatus (46% and 20%, respectively). The microalgae-bacteria consortium showed higher purification efficiency, which was consistent with the current study (Table 2).
Bacteria can enhance the sewage purification effect based on a microalgae monoculture and promote the deposition of sugars and fats in the microalgae cells, thereby significantly improving the wastewater purification function of microalgae (Ramanan et al., 2016). Furthermore, integrating microalgae into activated sludge treatment facilitates the decline of various organic pollutants and inorganic nitrogen species (NH3, NO3−, and NO2−) (Leong et al., 2020). Furthermore, up to a particular level, bacteria could be effective in the improvement of NH4+-N removal efficiency (Table 1). These findings are in agreement with the previous work, suggesting symbiosis of effective microorganisms (EM-1) and microalgae Chlorella sp. as an effective strategy to remove ammonia and phosphorus from aquaculture wastewater (Lananan et al., 2014). Likewise, the removal of PO43−-P in microalgal-bacteria symbiosis was consistent with the previous study, which focused on treating L. calcarifer aquaculture wastewater using symbiotic bioremediation Chlorella sp. and EM (Lananan et al., 2014). The better cell growth of both microalgae and bacteria and the higher removal of PO43−-P in the symbiosis could be explained as follows. First, microalgae can be a habitat for bacteria and protect them from adverse environmental conditions (Mandal et al., 2011). Second, microalgae (e.g., Amphidinium carterae) can release extracellular polymeric substances (EPS) to stimulate bacteria (Bacillus pumilus) growth (Mandal et al., 2011). Meanwhile, bacteria excreted growth-promoting factors to enhance microalgae generation (Subashchandrabose et al., 2011), and the EPS excreted by microalgae or bacteria surface adsorbed PO43−-P from mariculture wastewater via the formation of hydrogen bonds (Li et al., 2013). Then, PO43−-P was incorporated into organic compounds (e.g., DNA, RNA, lipids) through phosphorylation, involved in the generation of ATP from ADP (Cai, Park, et al., 2013) or were taken up and stored as intracellular polyphosphate (Schmidt et al., 2016). Moreover, higher pH resulting from microalgal growth would promote the precipitation of PO43−-P with calcium and magnesium ions via hydroxyapatite formation (Li et al., 2013; Liu et al., 2017).
The CODMn removal efficiencies achieved by symbiotic bioremediation of microalgae and bacteria were slightly higher than monoculture C. vulgaris. This enhancement could be explained by the fact that Bacillus spp. not only mineralizes organic carbon but also decomposes some organic matter into forms that are easier to be absorbed by microalgae (Zhang et al., 2012). Besides, CO2 produced in the metabolism of bacteria can be further utilized as a carbon source by microalgae (Muñoz and Guieysse, 2006). The low removal efficiencies achieved in the current study may be attributed to less nitrogen and phosphorus, which limited cell generation of C. vulgaris and Bacillus spp., and therefore inhibited the decomposition and absorption of organic matters since high phosphorus concentration (e.g., higher than 6 mg/L) could lead to the explosive growth of microalgae (Ahmad et al., 2011). In addition, previous studies also suggest that micro algae-microbe symbiosis also showed effective performance in the treatment of TN, TP, and OD, as summarized in Table 4.
4.4 Mechanisms of Microalgae-bacteria Symbiosis System for Nutrients Removal
Biodegradation is one of the major approaches in the microalgae-bacteria symbiosis system during wastewater remediation. Once microalgae and bacteria are co-cultivated in the wastewater treatment system, they would compensate each other with carbon dioxide or oxygen, and also some extracellular chemicals excreted by both of them may trigger or strict their growth which would further influence the efficiency of pollutants removal. Mutual interactions between bacteria and microalgae would occur via biodegradation, bioadsorption, and bioaccumulation during the pollutant removal process, as shown in Fig. 7. In the microalgae-bacteria symbiotic system, microalgae can capture light and nutrients from their surroundings and grow photoautotrophically, heterotrophically, and mixotrophically, facilitating biomass accumulation (polyphosphate, carboxylic acid, and polysaccharides) and decontamination efficiency (Hussain et al., 2021). On the other hand, Bacteria can assimilate organic matter and produce carbon dioxide and other inorganic substances as heterotrophic organisms. Bacterial respiration uses the oxygen produced by microalgal cells during photosynthesis. As a result, organic carbon such as carbohydrates, proteins, polysaccharides, and growth factors that produced to ensure the entire life process (Liu et al., 2017; Wang, Lei, et al., 2020). Simultaneously, inorganic substances produced by bacteria serve as raw materials such as NH4+-N, NO2−-N, and PO43−-P for microalgae photosynthesis with the help of enzymes and photosynthetic pigments and are eventually converted into amino acids, lipids, and proteins via nitrification, denitrification, substrate-level phosphorylation, oxidative phosphorylation, or photophosphorylation which can be regarded as a synergistic stage (Chu et al., 2021; Leng et al., 2021; Rahimi et al., 2020; Wang et al., 2014).
Unlike biodegradation, in bioabsorption and bioaccumulation, contaminants (e.g., heavy metals, antibiotics, organochlorines, and pesticides) are accumulated on the surface and or inside living organisms. Therefore, these two processes have become more popular because they are environmentally friendly, effective, and cheap. In addition, EPS secreted by microalgae and bacteria in the system can provide adsorption sites (like covalent bonding, electrostatic neutralization, and molecular interactions) (Kanamarlapudi et al., 2018; Wang et al., 2021). Composed of polysaccharides and proteins with carboxyl, phosphoric, amine, and hydroxyl groups, EPS is supposed to be regarded as a gap for mitigating the toxicity load and protecting the cell membrane (Wang et al., 2021).
5 Conclusions
The field investigation showed that the main culturing method is industrial factory and most of the wastewater is directly discharged into the surrounding sea in Hainan. The results of indoor experiments with monoculture microalgae indicated that nutrient removal efficiency of C. vulgaris was higher, followed by P. subcordiformis and C. müelleri, respectively. Furthermore, the symbiotic bioremediation of microalgae C. vulgaris and bacteria Bacillus spp. with C-B5 treatment was the optimal treatment strategy for treating tropical mariculture wastewater.
Data Availability
All the original data was integrated in the file named as Supplementary Information.
References
Ahmad, A. L., Mat Yasin, N. H., Derek, C. J. C., & Lim, J. K. (2011). Optimization of microalgae coagulation process using chitosan. Chemical Engineering Journal, 173(3), 879–882. https://doi.org/10.1016/j.cej.2011.07.070.
Ansari, F. A., Singh, P., Guldhe, A., Bux, F. (2017). Microalgal cultivation using aquaculture wastewater: integrated biomass generation and nutrient remediation. Algal research, 21, 169–177. https://doi.org/10.1016/j.algal.2016.11.015
Bilgili, M. S., Ince, M., Tari, G. T., Adar, E., Balahorli, V., & Yildiz, S. (2016). Batch and continuous treatability of oily wastewaters from port waste reception facilities: A pilot scale study. Journal of Electroanalytical Chemistry, 760, 119–126. https://doi.org/10.1016/j.jelechem.2015.11.024.
Cai, T., Ge, X., Park, S. Y., & Li, Y. (2013). Comparison of Synechocystis sp. PCC6803 and Nannochloropsis salina for lipid production using artificial seawater and nutrients from anaerobic digestion effluent. Bioresource Technology, 144(144C), 255–260. https://doi.org/10.1016/j.biortech.2013a.06.101.
Cai, T., Park, S. Y., & Li, Y. (2013). Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renewable and Sustainable Energy Reviews, 19(1), 360–369. https://doi.org/10.1016/j.rser.2012.11.030.
Cheah, W. Y., Ling, T. C., Show, P. L., Juan, J. C., Chang, J. S., & Lee, D. J. (2016). Cultivation in wastewaters for energy: A microalgae platform. Applied Energy, 179, 609–625. https://doi.org/10.1016/j.apenergy.2016.07.015.
Chu, R., Li, S., Zhu, L., Yin, Z., Hu, D., Liu, C., & Mo, F. (2021). A review on co-cultivation of microalgae with filamentous fungi: Efficient harvesting, wastewater treatment and biofuel production. Renewable and Sustainable Energy Reviews, 139, 110689. https://doi.org/10.1016/j.rser.2020.110689.
Fan S, Ji B, Abu Hasan H, Fan J, Guo S, Wang J, Yuan J (2021) Microalgal-bacterial granular sludge process for non-aerated aquaculture wastewater treatment. Bioprocess and Biosystems Engineering 44(8), 1733–1739. https://springerlink.bibliotecabuap.elogim.com/article/https://doi.org/10.1007/s00449-021-02556-0
Fernández-Linares, L. C., Barajas, C. G., Páramo, E. D., & Corona, J. A. B. (2017). Assessment of Chlorella vulgaris and indigenous microalgae biomass with treated wastewater as growth culture medium. Bioresource Technology, 244, 400–406. https://doi.org/10.1016/j.biortech.2017.07.141.
Fitzgerald, C. M., Camejo, P., Oshlag, J. Z., & Noguera, D. R. (2015). Ammonia-oxidizing microbial communities in reactors with efficient nitrification at low-dissolved oxygen. Water Research, 70, 38–51. https://doi.org/10.1016/j.watres.2014.11.041.
Gao, F., Li, C., Yang, Z. H., Zeng, G. M., Feng, L. J., Liu, J. Z., et al. (2016). Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecological Engineering, 92, 55–61. https://doi.org/10.1016/j.ecoleng.2016.03.046.
García, D., Posadas, E., Grajeda, C., Blanco, S., Martínez-Páramo, S., Acién, G., et al. (2017). Comparative evaluation of piggery wastewater treatment in algal-bacterial photobioreactors under indoor and outdoor conditions. Bioresource Technology, 245, 483–490. https://doi.org/10.1016/j.biortech.2017.08.135.
Ge, S., Qiu, S., Tremblay, D., Viner, K., Champagne, P., & Jessop, P. G. (2018). Centrate wastewater treatment with Chlorella vulgaris: Simultaneous enhancement of nutrient removal, biomass and lipid production. Chemical Engineering Journal, 342, 310–320. https://doi.org/10.1016/j.cej.2018.02.058.
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith, M.L. and Chanley, M.H., Eds., Culture of Marine Invertebrates Animals, Plenum Press, New York, pp. 29–60. https://doi.org/10.1007/978-1-4615-8714-9_3
Guldhe, A., Ansari, F. A., Singh, P., & Bux, F. (2017). Heterotrophic cultivation of microalgae using aquaculture wastewater: A biorefinery concept for biomass production and nutrient remediation. Ecological Engineering, 99, 47–53. https://doi.org/10.1016/j.ecoleng.2016.11.013.
Guo G, Cao W, Sun S, Zhao Y, Hu C (2017) Nutrient removal and biogas upgrading by integrating fungal-microalgal cultivation with anaerobically digested swine wastewater treatment. J. Appl.Phycol 29: 2857–2866. https://springerlink.bibliotecabuap.elogim.com/article/https://doi.org/10.1007/s10811-017-1207-2
Han, P., Lu, Q., Fan, L., Zhou, W. (2019). A review on the use of microalgae for sustainable aquaculture. Applied Sciences, 9(11), 2377. https://doi.org/10.3390/app9112377
Hussain, F., Shah, S. Z., Ahmad, H., Abubshait, S. A., Abubshait, H. A., Laref, A., et al. (2021). Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production. Renewable and Sustainable Energy Reviews, 137, 110603. https://doi.org/10.1016/j.rser.2020.110603.
Ji, M. K., Abou-Shanab, R. A., Hwang, J. H., Timmes, T. C., Kim, H. C., Oh, Y. K., & Jeon, B. H. (2013). Removal of nitrogen and phosphorus from piggery wastewater effluent using the green microalga Scenedesmus obliquus Journal of Environmental Engineering, 139(9), 1198–1205. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000726.
Kanamarlapudi, S., Chintalpudi, V. K., & Muddada, S. (2018). Application of biosorption for removal of heavy metals from wastewater. Biosorption, 18, 69. https://doi.org/10.5772/intechopen.77315.
Koppel, D. J., Adams, M. S., King, C. K., & Jolley, D. F. (2019). Preliminary study of cellular metal accumulation in two Antarctic marine microalgae-implications for mixture interactivity and dietary risk. Environmental Pollution, 252, 1582–1592. https://doi.org/10.1016/j.envpol.2019.06.003.
Lananan, F., Abdul Hamid, S. H., Din, W. N. S., Na, A., Khatoon, H., Jusoh, A., & Endut, A. (2014). Symbiotic bioremediation of aquaculture wastewater in reducing ammonia and phosphorus utilizing Effective Microorganism (EM-1) and microalgae (Chlorella sp.). International Biodeterioration and Biodegradation, 95, 127–134. https://doi.org/10.1016/j.ibiod.2014.06.013.
Larsdotter, K. (2006). Wastewater treatment with microalgae-a literature review. Solor Energy, 62(1), 31–38.
Leng, L., Li, W., Chen, J., Leng, S. Q., Chen, J. F., Wei, L., Peng, H. Y., Li, J., Zhou, W. G., Huang, H. J. (2021). Co-culture of fungi-microalgae consortium for wastewater treatment: A review. Bioresource Technology 125008. https://doi.org/10.1016/j.biortech.2021.125008
Leong, W. H., Kiatkittipong, K., Kiatkittipong, W., Cheng, Y. W., Lam, M. K., Shamsuddin, R., et al. (2020). Comparative performances of microalgal-bacterial co-cultivation to bioremediate synthetic and municipal wastewaters whilst producing biodiesel sustainably. Processes, 8(11), 1427. https://doi.org/10.3390/pr8111427.
Li, S. S., Li, J. H., Xia, M. S., Meng, Y. Y., & Zhang, H. (2013). Adsorption of nitrogen and phosphorus by intact cells and cell wall polysaccharides of Microcystis Journal of Applied Phycology, 25(5), 1539–1544. https://doi.org/10.1007/s10811-013-9992-8.
Li, M., Xiao, X., Wang, S., Zhang, X., Li, J., Pavlostathis, S. G., et al. (2020). Synergistic removal of cadmium and organic matter by a microalgae-endophyte symbiotic system (MESS): An approach to improve the application potential of plant-derived biosorbents. Environmental Pollution, 261, 114177. https://doi.org/10.1016/j.envpol.2020.114177.
Liu, J., Wu, Y., Wu, C., Muylaert, K., Vyverman, W., Yu, H. Q., et al. (2017). Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: A review. Bioresource Technology, 241, 1127–1137. https://doi.org/10.1016/j.biortech.2017.06.054.
Liu, B. L., Chai, W. S., Show, P. L., Chen, J. Y., & Chang, Y. K. (2020). Evaluation of dynamic binding performance of C-phycocyanin and allophycocyanin in Spirulina platensis algae by aminated polyacrylonitrile nanofiber membrane. Biochemical Engineering Journal, 161, 107686. https://doi.org/10.1016/j.bej.2020.107686.
Luo, Z. Z., Huang, W., Zheng, C. T., Li, J., Yun, L., Sun, H. M., et al. (2021). Identification of a microalgae-yeast coculture system for nutrient removal in shrimp culture wastewater. Journal of Applied Phycology, 33(2), 879–890. https://doi.org/10.1007/s10811-021-02379-2.
Ma, R. Y. (2019). Study on the purification technology of artificial mariculture wastewater by monoculture and symbiosis of microalgae-bacteria. Hainan University. https://doi.org/10.27073/d.cnki.ghadu.2019.000895.
Mandal, S. K., Singh, R. P., & Patel, V. (2011). Isolation and characterization of exopolysaccharide secreted by a toxic dinoflagellate, amphidinium carterae Hulburt 1957 and its probable role in harmful algal blooms (HABs). Microbial Ecology, 62(3), 518–527. https://doi.org/10.1007/s00248-011-9852-5.
Mendes, B., Brantes, L., Vermelho (2013). Allelopathy as a potential strategy to improve microalgae cultivation. Biotechnology Biofuels 6(1): 152. https://doi.org/10.1186/1754-6834-6-152
Ministry of Environmental Protection of the People’s Republic of China. China’s national environmental protection standard ‘‘Standards for Surface Water’’. GB11893–89 (1989)
Muñoz, R., & Guieysse, B. (2006). Algal-bacterial processes for the treatment of hazardous contaminants: A review. Water Research, 40(15), 2799–2815. https://doi.org/10.1016/j.watres.2006.06.011.
Muradov, N., Taha, M., Miranda, A. F., Wrede, D., Kadali, K., Gujar, A., et al. (2015). Fungal-assisted algal flocculation: application in wastewater treatment and biofuel production. Biotechnology Biofuels, 8(1), 1–23. https://doi.org/10.1186/s13068-015-0210-6.
Peng, Y. Y., Gao, F., Yang, H. L., Li, C., Lu, M. M., & Yang, Z. Y. (2020). Simultaneous removal of nutrient and sulfonamides from marine aquaculture wastewater by concentrated and attached cultivation of Chlorella vulgaris in an algal biofilm membrane photobioreactor (BF-MPBR). Science of the Total Environment, 725, 138524. https://doi.org/10.1016/j.scitotenv.2020.138524.
Qi, W., Mei, S., Yuan, Y., Li, X., Tang, T., Zhao, Q., et al. (2018). Enhancing fermentation wastewater treatment by coculture of microalgae with volatile fatty acid- and alcohol-degrading bacteria. Algal Research, 31, 31–39. https://doi.org/10.1016/j.algal.2018.01.012.
Rahimi, S., Modin, O., & Mijakovic, I. (2020). Technologies for biological removal and recovery of nitrogen from wastewater. Biotechnology Advances, 43, 107570. https://doi.org/10.1016/j.biotechadv.2020.107570.
Ramanan, R., Kim, B. H., Cho, D. H., Oh, H. M., & Kim, H. S. (2016). Algae-bacteria interactions: Evolution, ecology and emerging applications. Biotechnology Advances, 34(1), 14–29. https://doi.org/10.1016/j.biotechadv.2015.12.003.
Sacristán de Alva, M., Luna Pabello, V. M., Orta Ledesma, M. T., & Cruz Gómez, M. J. (2018). Carbon, nitrogen, and phosphorus removal, and lipid production by three saline microalgae grown in synthetic wastewater irradiated with different photon fluxes. Algal Research, 34, 97–103. https://doi.org/10.1016/j.algal.2018.07.006.
Salama, E. S., Kim, J. R., Ji, M. K., Cho, D. W., Abou-Shanab, R. A., Kabra, A. N., & Jeon, B. H. (2015). Application of acid mine drainage for coagulation/flocculation of microalgal biomass. Bioresource technology, 186, 232–237. https://doi.org/10.1016/j.biortech.2015.03.078
Salama, E. S., Kurade, M. B., Abou-Shanab, R. A., El-Dalatony, M. M., Yang, I. S., Min, B., & Jeon, B. H. (2017). Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renewable and Sustainable Energy Reviews, 79, 1189–1211. https://doi.org/10.1016/j.rser.2017.05.091.
Santos, F. M., & Pires, J. C. M. (2018). Nutrient recovery from wastewaters by microalgae and its potential application as bio-char. Bioresource Technology, 267, 725–731. https://doi.org/10.1016/j.biortech.2018.07.119.
Schmidt, J. J., Gagnon, G. A., & Jamieson, R. C. (2016). Microalgae growth and phosphorus uptake in wastewater under simulated cold region conditions. Ecological Engineering, 95, 588–593. https://doi.org/10.1016/j.ecoleng.2016.06.114.
Strickland, J. D. H., & Parsons, T. R. (1972). Practical handbook of seawater analysis, Fishery Research Board of Canada. Ottawa, 167, 49–52. https://doi.org/10.1002/iroh.19700550118.
Subashchandrabose, S. R., Ramakrishnan, B., Meghraj, M., Venkateswarlu, K., & Naidu, R. (2011). Consortia of cyanobacteria/microalgae and bacteria: Biotechnological potential. Biotechnology Advances, 29(6), 896–907. https://doi.org/10.1016/j.biotechadv.2011.07.009.
Sun, J., Li, N., Yang, P., Zhang, Y., Yuan, Y., Lu, X., & Zhang, H. (2020). Simultaneous antibiotic degradation, nitrogen removal and power generation in a microalgae-bacteria powered biofuel cell designed for aquaculture wastewater treatment and energy recovery. International Journal of Hydrogen Energy, 45(18), 10871–10881. https://doi.org/10.1016/j.ijhydene.2020.02.029.
Wang, B., & Lan, C. Q. (2011). Biomass production and nitrogen and phosphorus removal by the green alga Neochloris oleoabundans in simulated wastewater and secondary municipal wastewater effluent. Bioresource Technology, 102(10), 5639–5644. https://doi.org/10.1016/j.biortech.2011.02.054.
Wang, M., Kuo-Dahab, W. C., Dolan, S., & Park, C. (2014). Kinetics of nutrient removal and expression of extracellular polymeric substances of the microalgae, Chlorella sp. and Micractinium sp., in wastewater treatment. Bioresource Technology, 154, 131–137. https://doi.org/10.1016/j.biortech.2013.12.047.
Wang, L., Liu, J., Zhao, Q., Wei, W., & Sun, Y. (2016). Comparative study of wastewater treatment and nutrient recycle via activated sludge, microalgae and combination systems. Bioresource Technology, 211, 1–5. https://doi.org/10.1016/j.biortech.2016.03.048.
Wang, J., Lei, Z., Tian, C., Liu, S., Wang, Q., Shimizu, K., et al. (2020). Ionic response of algal-bacterial granular sludge system during biological phosphorus removal from wastewater. Chemosphere, 264, 128534. https://doi.org/10.1016/j.chemosphere.2020.128534.
Wang, Y., Wang, S., Sun, L., Sun, Z., & Li, D. (2020). Screening of a chlorella-bacteria consortium and research on piggery wastewater purification. Algal Research, 47, 101840. https://doi.org/10.1016/j.algal.2020.101840.
Wang, J., Chen, R., Fan, L., Cui, L. L., Zhang, Y. J., Cheng, J. J., et al. (2021). Construction of fungi-microalgae symbiotic system and adsorption study of heavy metal ions. Separation and Purification Technology, 268, 118689. https://doi.org/10.1016/j.seppur.2021.118689.
Wang, C. C., Jiao, C., Shen, Z. Y., Chen, L. (2020a). Effects of wastewater discharge of aquaculture and its prevention & treatment suggestion in China. People's Pearl River 41(1): 89–98. 10.3969/j.issn.1001-9235.2020.01.015
Ye, J., Song, Z., Wang, L., & Zhu, J. (2016). Metagenomic analysis of microbiota structure evolution in phytoremediation of a swine lagoon wastewater. Bioresource Technology, 219, 439–444. https://doi.org/10.1016/j.biortech.2016.08.013.
Zambrano, J., Krustok, I., Nehrenheim, E., & Carlsson, B. (2016). A simple model for algae-bacteria interaction in photo-bioreactors. Algal Research, 19, 155–161. https://doi.org/10.1016/j.algal.2016.07.022.
Zhang, Y., Su, H., Zhong, Y., Zhang, C., Shen, Z., Sang, W., et al. (2012). The effect of bacterial contamination on the heterotrophic cultivation of Chlorella pyrenoidosa in wastewater from the production of soybean products. Water Research, 46(17), 5509–5516. https://doi.org/10.1016/j.watres.2012.07.025.
Zheng, Y., & Shen, X. (2021). Research on current situation and countermeasures of marine fish culture industry in Hainan Province. Marine Development and Management, 1, 90–95. https://doi.org/10.3969/j.issn.1005-9857.2021.01.015.
Zheng, H., Wu, X., Zou, G., Zhou, T., Liu, Y., & Ruan, R. (2019). Cultivation of Chlorella vulgaris in manure-free piggery wastewater with high-strength ammonium for nutrients removal and biomass production: Effect of ammonium concentration, carbon/nitrogen ratio and pH. Bioresource Technology, 273, 203–211. https://doi.org/10.1016/j.biortech.2018.11.019.
Acknowledgements
This study was supported by the financial support from the Natural Science Foundation of Hainan Province, China (Grant No. 2019RC043), the National Natural Science Foundation of China (41766003), the start-up funding from Hainan University (kyqd(zr)1719), and the Natural Science Foundation of Hainan Province, China (518QN212). All authors gratefully acknowledge these financial supports.
Funding
This study was supported by the financial support from the Natural Science Foundation of Hainan Province, China (Grant No. 2019RC043), the National Natural Science Foundation of China (41766003), the start-up funding from Hainan University (kyqd(zr)1719), and the Natural Science Foundation of Hainan Province, China (518QN212).
Author information
Authors and Affiliations
Contributions
Ruiyang Ma contributed to the formal analysis, experiment investigation and original draft. Data curation was performed by Linhai Pu and Huiting Jia. Manuscript review and editing were prepared by Ruiyang Ma, Shiyu Xie, Linhai Pu, Huiting Jia, Licheng Peng and Tariq Mehmood. This study was supervised by Licheng Peng and Chengjun Ge. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This article does not contain any studies with animals performed by any of the authors.
Consent to Participate
All the people involved in this work gave their consent to participate.
Consent for publication
All the authors have given their consent for the publication of the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Mariculture wastewater pollution was detected in Wenchang, Hainan Province, China.
• High removal efficiency of N and P was achieved by Chlorella vulgaris.
• Complete removal of NH4+-N and PO43−-P was found in the symbiosis of C. vulgaris and Bacillus spp.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, R., Xie, S., Jia, H. et al. Nutritional Profile and Bioremediation of Tropical Marine Aquaculture with an Integrated Microalgae and Bacteria Symbiosis. Water Air Soil Pollut 233, 172 (2022). https://doi.org/10.1007/s11270-022-05615-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05615-8