Abstract
The atmospheric CO2 concentration has been rising since the industrial revolution, and will continue to rise from the present 375 to about 1,000 ppmv by 2100 (Pearson and Palmer, 2000), increasing dissolution of CO2 from the air and altering the carbonate system of Surface Ocean (Stumm and Morgan, 1996; Takahashi et al., 1997; Riebesell et al., 2007). For example, an increase in atmospheric CO2 from 330 to 1,000 ppmv will lead to an increase in CO2 concentration from 12.69 to 38.46 μM in seawater (at 15°C and total alkalinity of 2.47 eq m−3) and an increase in the concentration of dissolved inorganic carbon (DIC, i.e., CO2(aq), HCO3 −, and CO3 2−) from 2.237 to 2.412 mM, with a concurrent decrease in the pH of the surface seawater from 8.168 to 7.735 (Raven, 1991; Stumm and Morgan, 1996). Increasing atmospheric CO2 and its associated changes in the carbonate system can influence the physiology and ecology of seaweeds.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Intertidal Seaweed
- Articulated Coralline Alga
- Corallina Pilulifera
- Gloiopeltis Furcata
- Sargassum Hemiphyllum
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The atmospheric CO2 concentration has been rising since the industrial revolution, and will continue to rise from the present 375 to about 1,000 ppmv by 2100 (Pearson and Palmer, 2000), increasing dissolution of CO2 from the air and altering the carbonate system of Surface Ocean (Stumm and Morgan, 1996; Takahashi et al., 1997; Riebesell et al., 2007). For example, an increase in atmospheric CO2 from 330 to 1,000 ppmv will lead to an increase in CO2 concentration from 12.69 to 38.46 μM in seawater (at 15°C and total alkalinity of 2.47 eq m−3) and an increase in the concentration of dissolved inorganic carbon (DIC, i.e., CO2(aq), HCO3 −, and CO3 2−) from 2.237 to 2.412 mM, with a concurrent decrease in the pH of the surface seawater from 8.168 to 7.735 (Raven, 1991; Stumm and Morgan, 1996). Increasing atmospheric CO2 and its associated changes in the carbonate system can influence the physiology and ecology of seaweeds.
Seaweeds (Chlorophyta, Rhodophyta, and Phaeophyta) are usually distributed in intertidal and subtidal zones of coastal waters. They play an important role in the coastal carbon cycle (Reiskind et al., 1989) and contribute remarkably to sea-farming activities. The rate of primary production of some species is comparable with those of the most productive land plants; therefore, seaweeds have a great potential for CO2 bioremediation (Gao and Mckinley, 1994). On the other hand, increasing pCO2 in seawater would affect physiology of seaweeds. Therefore, a number of studies have been performed to envisage the impacts of CO2 enrichment on photosynthesis, growth, nutrients metabolism, and cell components of seaweeds. Results showed that increased CO2 concentration may enhance, inhibit, or not affect the growth of the species investigated. This work is intended to examine how the macroalgal species respond and acclimate to elevated CO2 levels.
Inorganic Carbon Limitation
The effects of elevated CO2 concentrations on seaweeds largely depend on the degree of carbon limitation present in natural systems. Photosynthesis of seaweeds would be severely limited under current atmospheric conditions if it were dependent only on diffusional entry of CO2 from the medium to the site of fixation via the carbon-assimilating enzyme Rubisco. There are several aspects of CO2 limitation of carbon assimilation in seaweeds (Beardall et al., 1998): (1) rather low dissolved CO2 concentration; (2) low diffusion rate of CO2 in seawater, being four orders of magnitude slower than in air; (3) the slow spontaneous formation of CO2 from HCO −3 dehydration; and (4) the high K m values (40–70 μM) of Rubisco of algae. Nevertheless, photosynthesis in the investigated species can be fully or nearly saturated with the current ambient dissolved inorganic carbon (Ci) composition because of the presence of CO2-concentrating mechanisms (CCMs) that enable the algae to efficiently utilize the bulk HCO −3 pool in seawater (Beer, 1994; Beer and Koch, 1996; Raven, 1997; Larsson and Axelsson, 1999; Zou et al., 2004; Giordano et al., 2005), which is about 150 times more abundant than free CO2. Some species, however, exhibit Ci-limited photosynthesis in natural seawater (e.g., Johnston et al., 1992; Andría et al., 1999a; Zou et al., 2003).
HCO −3 is usually dehydrated extracellularly as mediated by periplasmic carbonic anhydrase (CA) to release CO2, which is then taken up into the cell. Another important approach for Ci acquisition of algae is the active uptake of HCO −3 through the plasma membrane facilitated by an anion exchange protein (Drechsler et al., 1993, 1994; Axelsson et al., 1995). Additionally, H+-ATPase-driven HCO −3 uptake has also been recognized in several marine seaweeds (Choo et al., 2002; Snoeijs et al., 2002). Seaweeds show different capacities to take advantage of the HCO −3 pool in seawater (Axelsson and Uusitalo, 1988; Maberly, 1990; Mercado et al., 1998). Therefore, they can exhibit heterogeneous, often species-specific responses to elevated CO2. Their physiological responses to elevated CO2 levels can also depend on their acclimation strategies and the environmental constraints under which CO2 enrichment is imposed.
Growth
When juveniles of Porphyra yezoensis germinated from the chonchospores were grown at enriched CO2 levels of 1,000 or 1,600 ppmv for 20 days, their growth was significantly enhanced (Gao et al., 1991; Fig. 1). Similar findings were reported in Gracilaria sp., Gracilaria chilensis, and Hizikia fusiforme (Gao et al., 1993a; Zou, 2005). Although these species are capable of using bicarbonate, they still showed carbon-limited photosynthetic rates in natural seawater. Growth of a nonbicarbonate-user, the red alga Lomentaria articulata, was stimulated by enriched CO2 (Kübler et al., 1999). The enhancement could be attributed to the accelerated photosynthetic carbon fixation by increasing Ci availability or the depression of photorespiration by elevating the ratio of CO2 to O2 in the culture medium. It was interesting that growth of a green alga, Ulva rigida, which showed efficient ability of HCO −3 utilization and saturated photosynthesis at the current Ci concentration of seawater (Björk et al., 1993; Mercado et al., 1998), was also enhanced at high CO2 concentrations (Björk et al., 1993; Gordillo et al., 2001). Such an enhancement of growth was suggested to be caused by the enhanced N-assimilation (Gordillo et al., 2001), but could also be attributed to downregulation of HCO −3 uptake and consequent energy saving for its operation. On the other hand, a decrease in growth rate caused by elevated CO2 has been reported in G. tenuistipitata (Garcìa-Sánchez et al., 1994), P. leucostica (Mercado et al., 1999), and P. linearis (Israel et al., 1999). Such an inhibition of growth was associated with lowered photosynthetic activity even measured at high CO2 concentrations (Garcìa-Sánchez et al., 1994). However, such a negative effect could also be caused by acidification of the medium (Israel et al., 1999). A more recent study by Israel and Hophy (2002) reported that the growth rates of 13 species (representing Chlorophyta, Rhodophyta, and Phaeophyta) cultivated in normal seawater were comparable with their growth in CO2-enriched seawater. The authors ascribed such nonresponsive behavior to the presence of CCMs that rely on the utilization of HCO −3 . Obviously, researches show that enrichment of CO2 in seawater may affect, positively, neutrally, or negatively, the growth of seaweeds in direct or indirect ways.
Enhanced growth of Porphyra yezoensis when 50 juveniles each (germinated from the same bunch of chonchospores released from the same chonchocelis, about 5 mm long at the beginning of the culture) were grown at different CO2 concentrations in aeration. The photo images were taken after 20 days culture (Gao et al., 1991).
Photosynthesis
Photosynthetic Ci Utilization
The response of macroalgal photosynthesis to elevated pCO2 in seawater is species-specific. When cultured in high CO2, the light-saturated photosynthetic rate was reduced in Fucus serratus (Johnston and Raven, 1990), G. tenuistipitata (Garcìa-Sánchez et al., 1994), and P. yezoensis (Gao, unpublished data) when measured at normal Ci of seawater. When the photosynthetic rate was measured at elevated DIC levels, it was significantly higher in the thalli grown at enriched CO2 levels in P. yezoensis (Gao et al., 1991) and Gracilaria sp. (Andría et al., 1999b). In P. leucostica, Mercado et al. (1999) found no significant difference between the maximal gross photosynthetic rates of the thalli grown at enriched and current inorganic carbon concentrations.
The photosynthetic affinity for Ci and the capacity of HCO −3 utilization are usually lowered in seaweeds following exposures to high CO2 (Johnston and Raven, 1990; Björk et al., 1993; Mercado et al., 1997; Andría et al., 1999a, b; Zou et al., 2003). Growing the cells at high CO2 levels decreased activity of the external (periplasmic) or total CA activity in Ulva sp. (Björk et al., 1993), G. tenuistipitata (Garcìa-Sánchez et al., 1994), P. leucosticta (Mercado et al., 1997), and H. fusimorme (Zou et al., 2003). Such a decrease reflects a decline in the capacity of HCO −3 utilization. Israel and Hophy (2002) showed that the enzymatic features of Rubisco did not differ in the seaweeds when compared between the CO2-enriched and control cultures, though enrichment of CO2 was reported to decrease the content of Rubisco in G. tenuistipitata (Garcìa-Sánchez et al., 1994), Gracilaria sp. (Andría et al., 1999a), and P. leucosticta (Mercado et al., 1997).
Photochemical Efficiency
Photosynthetic acclimation in seaweeds to high levels of Ci generally resembles their responses to high irradiances, resulting in a decrease in pigment contents. For example, the phycobiliprotein (phycoerythrin and phycocyanin) and Chl a contents were reduced in Gracilaria sp. (Andría et al., 1999b, 2001), G. tenuistipitata (Garcìa-Sánchez et al., 1994), and P. leucosticta (Mercado et al., 1999) grown at high levels of Ci than those at normal Ci level. On the other hand, both maximum quantum yield and effective quantum yield were downregulated in P. leucostica when grown under high Ci conditions (Mercado et al., 1999), suggesting that enriched CO2 lowered the demand of energy for the HCO −3 utilization mechanism.
Emersed Photosynthesis of Intertidal Seaweeds
Intertidal seaweeds experience continual alternation of living in air and in water as the tidal level changes. Their photosynthesis undergoes dramatic environmental changes between the aquatic and terrestrial exposures. When the tide is high, they are submerged in seawater, where HCO −3 pool is available for their photosynthesis (Beer and Koch, 1996; Beardall et al., 1998). When the tide is low, intertidal seaweeds are exposed to air, large buffering reservoir of HCO −3 in seawater is no longer present, and atmospheric CO2 becomes the only exogenous carbon resource for their photosynthesis. The acquisition of CO2 is less constrained in air than in seawater, through which CO2 diffuses about 10,000 times slower (Raven, 1999). However, this constraint can be offset by the abundance of HCO −3 , as many intertidal algae can use HCO −3 as the exogenous inorganic carbon source for photosynthesis (Maberly, 1990; Gao and McKinley, 1994). Thus, carbon limitation during photosynthesis in intertidal species may be potentially more important in air than in water.
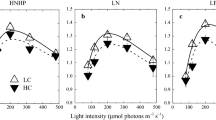
It is known that intertidal seaweeds can tolerate the emersed conditions, and the photosynthesis during emersion contributes significantly to their total carbon fixation budget (e.g., Gao and Aruga, 1987; Maberly and Madsen, 1990). Our previous works (Gao et al., 1999; Zou and Gao, 2002; Zou and Gao, 2004a, b, 2005; Zou et al., 2007) showed that elevated atmospheric CO2 might have a fertilizing effect increasing photosynthesis while exposed to air at low tide in most of the tested species, i.e. the red seaweeds P. haitanensis, Gloiopeltis furcata, and Gigartina intermedia, the brown seaweeds Ishige okamura, H. fusiformis, and Sargassum hemiphyllum, and the green seaweeds Enteromopha linza and Ulva lactuca. The relative photosynthetic enhancement by the elevated CO2 levels increased with desiccation, although the absolute photosynthetic rate decreased with desiccation. The enhancement of daily photosynthetic production by elevated CO2 concentration during emersion differs among species owing to their zonational depths and exposure durations and the daily timing of emersion (Gao et al., 1999; Zou and Gao, 2005; Zou et al., 2007). Additionally, the CO2 compensation points increased with enhanced desiccation, with higher CO2 concentrations required to maintain positive photosynthesis (Gao et al., 1999; Zou and Gao, 2002, 2005).
Calcification
It is estimated from more than two million surveys that the oceans have absorbed more than one third of the anthropogenic CO2 released to the atmosphere (Sabine et al., 2004). With increasing atmospheric CO2 concentration, CO2 dissolves in seawater to reach new equilibrium in the carbonate system. This leads to an increase in the concentrations of HCO −3 and H+ and a decrease in the concentration of CO 2−3 and of saturation state of calcium carbonate (Gattuso et al., 1999; Gattuso and Buddemeier, 2000; Caldeira and Wickett, 2003; Orr et al., 2005). The surface water of the ocean is known to have been acidified by 0.1 pH unit (corresponding to a 30% increase of H+) since 1800 (Orr et al., 2005), and will be further acidified by another 0.3–0.4 unit (about 100–150% increase of H+) by 2100 (Brewer, 1997; Caldeira and Wickett, 2003). Such an ocean-acidifying process has been suggested to harm marine-calcifying organisms by reducing the rate of calcification of their skeletons or shells (e.g., Gao et al., 1993b; Gattuso et al., 1999; Riebesell et al., 2000; Orr et al., 2005).
In the coastal waters where seaweeds are distributed, pH of seawater fluctuates within a larger range than pelagic waters because of inputs from terrestrial systems and fisheries. Nevertheless, additional CO2 input can still affect the biological activities in coastal waters, because ocean acidification will lower the pH regimes, shifting the pH range to a lower one. Therefore, increased pCO2 and decreased pH and CO 2−3 will affect calcifying seaweeds. Gao et al. (1993b) showed that enrichment of CO2 to 1,000 and 1,600 ppmv in aeration inhibited the calcification in the articulated coralline alga Corallina pilulifera. It has also been shown that the increase in CO2 concentrations significantly slowed down calcification of temperate and tropical corals and coralline macroalgae (Gattuso et al., 1998; Langdon et al., 2000). For the marine-calcifying phytoplankton Emiliania huxleyi, calcification was reported to be reduced by the enriched CO2 (Riebesell et al., 2000), while a recent study showed that its calcification increased with elevated CO2 (Iglesias-Rodriguez et al., 2008). On the other hand, when pH was controlled at a constant level, elevated concentrations of DIC enhanced the calcification of Bossiela orbigniana (Smith and Roth, 1979) and C. pilulifera (Gao et al., 1993b).
Nitrogen Metabolism
Zou (2005) reported that both the nitrate uptake rate and the activity of nitrate reductase (NR) in the brown algae H. fusiforme were increased following cultures at high CO2 levels. It was also shown that elevated CO2 concentrations in culture stimulated the uptake of NO −3 in Gracilaria sp. and G. chilensis (Gao et al., 1993a), Ulva lactuca (Zou et al., 2001), and U. rigida (Gordillo et al., 2001), and enhanced the activity of NR in P. leucosticta (Mercado et al., 1999) and U. rigida (Gordillo et al., 2001, 2003). This indicates that elevated CO2 concentrations can enhance nitrogen assimilation, as more nitrogen is required to support higher growth rate. The regulation of NR activity in seaweed by CO2 might be through a direct action on de novo synthesis of the enzyme, rather than through physiological consequences in carbon metabolism as occurring in terrestrial higher plants (Gordillo et al., 2001, 2003). Contrarily, decreased uptake rate of NO −3 by high CO2 in G. tenuistipitata (Garcìa-Sánchez et al., 1994) and G. gaditana (Andría et al., 1999b) was also reported. Mercado et al. (1999) stated that NO −3 uptake and reduction might be uncoupled when algae are grown at high CO2. Responses of macroalgal nitrogen assimilation to elevated CO2 could be species-specific; however, the results from different studies might be also generated from different culture systems or methods.
C/N Ratio
Growth under enrichment of CO2 would alter the cellular components of seaweeds. Contents of soluble proteins and phycobiliprotein were decreased in Graciaria tenuisitipitata (Garcìa-Sánchez et al., 1994), Gracilaria sp. (Andría et al., 1999b), and P. leucosticta (Mercado et al., 1999) when they were grown at high DIC levels. In contrast, the content of soluble carbohydrate was increased in Gracilaria sp. (Andría et al., 1999b). As a result of these changes, C/N ratios were increased in the seaweeds grown at elevated CO2 levels (Garcìa-Sánchez et al., 1994; Kübler et al., 1999; Mercado et al., 1999). Although phycobiliprotein, soluble proteins, and Rubisco contents were found to decrease under DIC-enriched conditions, internal N content was not significantly affected by the DIC levels. Andría et al. (1999b) thereby suggested that the exposure and acclimation to high CO2 would involve the reallocation of resources, such as N, away from Rubisco and other limiting components (electron transport) towards carbohydrate synthesis and nonphotosynthetic processes.
Summary
Atmospheric CO2 rise leads to a proportional increase in pCO2 of seawater and alters the carbonate chemistry, reducing the carbonate ions and pH while increasing that of bicarbonate. Physiological responses of seaweeds to elevated CO2 concentrations are highly variable, depending on the species, growing conditions, and duration of CO2 enrichment. In the species investigated, growth was enhanced, inhibited, or not affected by enrichment of CO2, while photosynthetic performance varied according to Ci acquisition mechanisms or the acclimation strategies. Usually, net photosynthesis was enhanced in elevated DIC levels for the species with less efficiency in bicarbonate utilization or CCMs. Growing the seaweeds in high CO2 downregulated their CCMs and possibly the electron transport demanded for its operation. On the other hand, calcification of calcifying seaweeds is negatively affected; nitrogen metabolism and the cellular C/N ratio would be increased in high-CO2-grown cells. For the intertidal species, large buffering reservoir of HCO −3 in seawater is no longer present and atmospheric CO2 becomes the only exogenous carbon resource for their photosynthesis at low tide, elevation of atmospheric CO2 might have a fertilizing effect, increasing their photosynthesis during emersion. More research efforts on biochemical and molecular aspects for a wider range of species grown at high CO2/low pH conditions are needed to further evaluate the impacts of increasing atmospheric CO2 concentrations on seaweeds. At the same time, physiological approaches are required to distinguish the effects of high CO2 from that of lowered pH.
References
Andría, J.R., Pérez-Lloréns, J. and Vergara, J.J. (1999a) Mechanisms of inorganic carbon acquisition in Gracilaria gaditana nom. prov. (Rhodophyta). Planta 208: 561–573.
Andría, J.R., Vergara, J.J. and Perez-Llorens, J.L. (1999b) Biochemical responses and photosynthetic performance of Gracilaria sp. (Rhodophyta) from Cadiz, Spain, cultured under different inorganic carbon and nitrogen levels. Eur. J. Phycol. 34: 497–504.
Andría, J.R., Brun, F.G., Pérez-Lloréns, J.L. and Vergara, J.J. (2001) Acclimation responses of Gracilaria sp. (Rhodophyta) and Enteromorpha intestinalis (Chlorophyta) to changes in the external inorganic carbon concentration. Bot. Mar. 44: 361–370.
Axelsson, L. and Uusitalo, J. (1988) Carbon acquisition strategies for marine macroalgae. I. Utilization of proton exchanges visualized during photosynthesis in a closed system. Mar. Biol. 97: 295–300.
Axelsson, L., Ryberg, H. and Beer, S. (1995) Two modes of bicarbonate utilization in the marine green macroalga Ulva lactuca. Plant Cell Environ. 18: 439–445.
Beardall, J., Beer, S. and Raven, J.A. (1998) Biodiversity of marine plants in an arc of climate change: some predictions based on physiological performance. Bot. Mar. 4: 113–123.
Beer, S. (1994) Mechanisms of inorganic carbon acquisition in marine maroalgae (with reference to the Chlorophyta). Prog. Phycol. Res. 10: 179–207.
Beer, S. and Koch, E. (1996) Photosynthesis of seagrasses and marine macroalgae in globally changing CO2 environments. Mar. Ecol. Prog. Ser. 141: 199–204.
Björk, M., Haglund, K., Ramazanov, Z. and Pedersen, M. (1993) Inducible mechanism for HCO –3 utilization and repression of photorespiration in protoplasts and thallus of three species of Ulva (Chlorophyta). J. Phycol. 29: 166–173.
Brewer, P.G. (1997) Ocean chemistry of the fossil fuel CO2 signal: the haline signal of “business as usual”. Geophys. Res. Lett. 24: 1367–1369.
Caldeira, K. and Wickett, M.E. (2003) Anthropogenic carbon and ocean pH. Nature 425: 365.
Choo, K.S., Snoeijs, P. and Pedersen, M. (2002) Uptake of inorganic carbon by Cladophora glomerata (Chlorophyta) from the Baltic Sea. J. Phycol. 38: 493–502.
Drechsler, Z., Sharkia, R., Cabantchik, Z.I. and Beer, S. (1993) Bicarbonate uptake in the marine maxroalga Ulva sp. is inhibited by classical probes of anion exchange by red blood cells. Planta 191: 34–40.
Drechsler, Z., Sharkia, R., Cabantchik, Z.I. and Beer, S. (1994) The relationship of arginine groups to photosynthetic HCO -3 uptake in Ulva sp. mediated by a putative anion exchanger. Planta 194: 250–255.
Gao, K. and Aruga, Y. (1987) Preliminary studies on the photosynthesis and respiration of Porphyra yezoensis under emersed condition. J. Tokyo Univ. Fish. 47: 51–65.
Gao, K. and McKinley, K.R. (1994) Use of macroalgae for marine biomass production and CO2 remediation: a review. J. Appl. Phycol. 6: 45–60.
Gao, K., Aruga, Y., Asada, K., Ishihara, T., Akano, T. and Kiyohara, M. (1991) Enhanced growth of the red alga Porphyra yezoensis Ueda in high CO2 concentrations. J. Appl. Phycol. 3: 356–362.
Gao, K., Aruga, Y., Asada, K. and Kiyohara, M. (1993a) Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. and G. chilensis. J. Appl. Phycol. 5: 563–71.
Gao, K., Aruga, Y., Asada, K., Ishihara, T., Akano, T. and Kiyohara, M. (1993b) Calcification in the articulated coralli alga Corallina pilulifera, with special reference to the effect of elevated atmospheric CO2. Mar. Biol. 117: 129–132.
Gao, K., Ji, Y. and Aruga, Y. (1999) Relationship of CO2 concentrations to photosynthesis of intertidal macrioalgae during emersion. Hydrobiologia 398/399: 355–359.
Garcìa-Sánchez, M.J., Fernández, J.A. and Niell, F.X. (1994) Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata. Planta 194: 55–61.
Gattuso, J.-P. and Buddemeier, R.W. (2000) Calcification and CO2. Nature 407: 311–312.
Gattuso, J.-P., Frankignoulle, M. and Bourge, I. (1998) Effect of calcium carbonate saturation of seawater on coral calcification. Glob. Planet Change 18: 37–46.
Gattuso, J.-P., Allemand, D. and Frankignoulle, M. (1999) Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. Am. Zool. 39: 160–183.
Giordano, M., Beardall, J. and Raven, J.A. (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 56: 99–131.
Gordillo, F.J.L., Niell, F.X. and Figueroa, F.L. (2001) Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta 213: 64–70.
Gordillo, F.J.L., Figueroa, F.L. and Niell, F.X. (2003) Photon- and carbon-use efficiency in Ulva rigida at different CO2 and N levels. Planta 218: 315–322.
Iglesias-Rodriguez, M.D., Halloran, P.R., Rickaby, R.E.M. et al. (2008) Phytoplankton calcification in a high-CO2 world. Science 320: 336–340.
Israel, A. and Hophy, M. (2002) Growth, photosynthetic properties and Rubisco activies and amounts of marine macroalgae grown under current and elevated seawater CO2 concentrations. Glob. Change Biol. 8: 831–840.
Israel, A., Katz, S., Dubinsky, Z., Merrill, J.E. and Friedlander, M. (1999) Photosynthetic inorganic carbon utilization and growth of Porphyra linearis (Rhorophyta). J. Appl. Phycol. 11: 447–453.
Johnston, A.M. and Raven, J.A. (1990) Effects of culture in high CO2 on the photosynthetic physiology of Fucus serratus. Br. Phycol. J. 25: 75–82.
Johnston, A.M., Maberly, S.C. and Raven, J.A. (1992) The acquisition of inorganic carbon for four red macroalgae. Oecologia 92: 317–326.
Kübler, J.E., Johnston, A.M. and Raven, J.A. (1999) The effects reduced and elevated CO2 and O2 on the seaweed Lomentaria articulata. Plant Cell Environ. 22: 1303–1310.
Langdon, C., Takahashi, T., Sweeney, C. et al. (2000) Effect of carbonate saturation state on the calcification rate of an experimental coral reef. Glob. Biogeochem. Cycles. 14: 639–654.
Larsson, C. and Axelsson, L. (1999) Bicarbonate uptake and utilization in marine macroalgae. Eur. J. Phycol. 34: 79–86.
Maberly, S.C. (1990) Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae. J. Phycol. 26: 439–449.
Maberly, S.C. and Madsen, T.V. (1990) Contribution of air and water to the carbon balance of Fucus spiralis. Mar. Ecol. Prog. Ser. 62: 175–183.
Mercado, J.M., Niell, F.X. and Figueroa, F.L. (1997) Regulation of the mechanism for HCO –3 use by the inorganic carbon level in Porphyra leucosticta thus in Le Jolis (Rhotophyta). Planta 201: 319–325.
Mercado, J.M., Gordillo, F.J.L., Figueroa, F.L. and Niell, F.X. (1998) External carbonic anhydrase and affinity for inorganic carbon in intertidal macroalgae. J. Exp. Mar. Biol. Ecol. 221: 209–220.
Mercado, J.M., Javier, F., Gordillo, L., Niell, F.X. and Figueroa, F.L. (1999) Effects of different leverls of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta. J. Appl. Phycol. 11: 455–461.
Orr, J.C., Fabry, V.J., Aumont, O. et al. (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437: 681–686.
Pearson, P.N. and Palmer, M.R. (2000) Atmospheric carbon dioxide concentrations over the past 60 million years. Nature 406: 695–699.
Raven, J.A. (1991) Physiology of inorganic C acquisition and implications for resource use efficiency by marine phytoplankton: relation to increased CO2 and temperature. Plant Cell Environ. 14: 779–794.
Raven, J.A. (1997) Inorganic carbon acquisition by marine autotrophs. Adv. Bot. Res. 27: 85–209.
Raven J.A. (1999) Photosynthesis in the intertidal zone: algae get an airing. J. Phycol. 35: 1102–1105.
Reiskind, J.B., Beer, S. and Bowes, G. (1989) Photosynthesis, photorespiration and ecophysiological interactions in marine macroalgae. Aquat. Bot. 34: 131–152.
Riebesell, U.L.F., Zondervan, I., Rost, B., Tortell P.D., Zeebe R.E. and Morel F.M.M. (2000) Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407: 3633–3667.
Riebesell, U., Schulz, K.G., Bellerby, R.G.J., Botros, M., Fritsche, P., MeyerhÃfer, M., Neill C., Nondal, G., Oschlies, A., Wohlers, J. and ZÃllner, E. (2007) Enhanced biological carbon consumption in a high CO2 ocean. Nature 450: 545–548.
Sabine, L.C., Feely, R.A., Gruber, N. et al. (2004) The oceanic sink for anthropogenic CO2. Nature 305: 367–371.
Smith, A.D. and Roth, A.A. (1979) Effect of carbon dioxide concentration on calculation in the red coralline alga Bossiella orbigniana. Mar Biol. 52: 217–225.
Snoeijs, P., Klenell, M., Choo, K.S., Comhaire, I. Ray, S. and Pedersen, M. (2002) Strategies for carbon acquisition in the red marine macroalgae Coccotylus truncatus from the Baltic Sea. Mar. Biol. 140: 435–444.
Stumm, W. and Morgan, J.J. (1996) Aquatic Chemistry, 3rd edn. Wiley, New York.
Takahashi, T., Feely, R.A., Weiss, R.F., Wanninkhof, R.H., Chipman, D.W., Sutherland, S.C. and Timothy, T.T. (1997) Global air-sea flux of CO2 difference. PNAS 94: 8292–8299.
Zou, D.H. (2005) Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture 250: 726–735.
Zou, D.H. and Gao, K.S. (2002) Effects of desiccation and CO2 concentrations on emersed photosynthesis in Porphyra haitanensis (Bangiales, Rhodophyta), a species farmed in China. Eur. J. Phycol. 37: 587–592.
Zou, D.H. and Gao, K.S. (2004a) Comparative mechanisms of photosynthetic carbon acquisition in Hizikia fusiforme under submersed and emersed conditions. Acta Bot. Sinica 46: 1178–1185.
Zou, D.H. and Gao, K.S. (2004b) Exogenous carbon acquisition of photosynthesis in Porphyra haitanensis (Bangiales, Rhodophyta) under emersed state. Prog. Nat. Sci. 14(2): 34–40.
Zou, D.H. and Gao, K.S. (2005) Ecophysiological characteristics of four intertidal marine macroalgae during emersion along Shantou Coast of China, with a special reference to the relationship of photosynthesis and CO2. Acta Oceanol. Sinica. 24(3): 105–113.
Zou, D.H., Gao, K.S. and Ruan, Z.X. (2001) Effects of elevated CO2 concentration on photosynthesis and nutrients uptake of Ulva lactuca. J. Ocean Univ. Qingdao 31: 877–882 (in Chinese with English abstract).
Zou, D.H., Gao, K.S. and Xia, J.R. (2003) Photosynthetic utilization of inorganic carbon in the economic brown alga, Hizikia fusiforme (Sargassaceae) from the South China Sea. J. Phycol. 36: 1095–1100.
Zou, D.H., Xia, J.R. and Yang, Y.F. (2004) Photosynthetic use of exogenous inorganic carbon in the agarphyte Gracilaria lemaneiformis (Rhodophyta). Aquaculture 237: 421–431.
Zou, D.H., Gao, K.S. and Run, Z.X. (2007) Daily timing of emersion and elevated atmospheric CO2 concentration affect photosynthetic performance of the intertidal macroalga Ulva lactuca (Chorophyta) in sunlight. Bot. Mar. 50: 275–279.
Acknowledgments
This work was supported by the Chinese 973 Project (No. 2009CB421207), the Key Project of Chinese Ministry of Education (No. 207080), and the National Natural Science Foundation (No. 40930846).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Zou, D., Gao, K. (2010). Physiological Responses of Seaweeds to Elevated Atmospheric CO2 Concentrations. In: Seckbach, J., Einav, R., Israel, A. (eds) Seaweeds and their Role in Globally Changing Environments. Cellular Origin, Life in Extreme Habitats and Astrobiology, vol 15. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-8569-6_7
Download citation
DOI: https://doi.org/10.1007/978-90-481-8569-6_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-8568-9
Online ISBN: 978-90-481-8569-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)