Abstract
In manufacturing industries that use seaweeds containing high moisture as raw materials, the volume reduction of seaweeds is a basic requirement for their efficient storage, transport, and processing. In this study, fresh red algae, dulse Palmaria palmata was hot air–dried at 30 and 60 °C with or without pre-freezing for 1 week, and anti-inflammatory components, phycobiliprotein (P) and chlorophyll a-related compounds (C) were extracted together using water from the dried thalli. The water extractability of each was diminished by pre-freezing and markedly impaired by hot-air drying of the raw dulse. In contrast, the anti-inflammatory activity of thermolysin digested P and C mixtures, which was evaluated using lipopolysaccharide-stimulated murine macrophages, persisted despite exposure of the raw materials to the freezing and hot-air-drying processes. In addition, the deterioration in the extractability of P caused by hot-air drying was recovered by the enzymatic degradation of the cell wall hardened by drying, and the water extract showed strong anti-inflammatory activity. These results indicated that hot-air–dried dulse can be used as an anti-inflammatory material by utilizing the appropriate physicochemical procedure for restoring the extractability of the anti-inflammatory components, and enzymatic algal cell degradation is one such practical countermeasure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is a biological defensive reaction that protects the human body from infection by foreign matter such as fungi, bacteria, and viruses (Serhan et al. 2007). However, continuous excess inflammation in the living body due to unhealthy lifestyle has been recently shown to often induce various health problems, such as inflammatory disorders, and age-related diseases (Iwalewa et al. 2007). Therefore, maintaining a healthy lifestyle is expected to result in the development of a constitution in which continuous chronic inflammation in the body is unlikely to occur. In particular, the interest in food ingredients that suppress inflammation has been increasing in recent years, along with the important role of diet in reducing inflammation (Galland 2010; Wu and Schauss 2012); these findings have been the driving force behind screening studies of various anti-inflammatory food materials.
Seaweed is a resource-rich and highly reproducible marine biomass, which contains various types of unique bioactive compounds (Costa et al. 2010; Holdt and Kraan 2011; Gupta and Abu-Ghannam 2011). For instance, bioactive polysaccharides that are widely distributed in seaweeds have garnered much interest as a source of functional dietary fiber with immunomodulatory (Katayama et al. 2012), antitumor (Sheng et al. 2007), and antioxidant (Chandini et al. 2008) functions. In addition, red algae possess high anti-inflammatory activity derived from their characteristic photosynthetic tissue, phycobilisome; components of the phycobilisome and their degradation products exert inhibitory effects on the production of proinflammatory mediators in immune cells (Sakai et al. 2011; Islam et al. 2013; Lee et al. 2017).
For the industrial utilization of high-moisture seaweed as a functional material, the drying step is an essential procedure for improving transport efficiency and storage stability. For instance, dried brown seaweeds in Japan (called Konbu), used as a traditional raw material for seasoning Japanese food, are distributed as hot-air–dried seaweed, which helps in maintaining the extractability of monosodium glutamate (Kurihara 2009). Agar, which has multiple uses, such as in food and medical materials, is a polysaccharide mixture extracted from dried algae (Armisen and Galatas 1987).
However, the high-temperature treatment of seaweeds leads to the hardening of cells, thermal denaturation of proteins, and the decomposition of various components; this may impair the extraction of functional compounds and reduce the health benefits of seaweed. To solve this problem, various physicochemical methods have been developed such as ultrasonication (Sun et al. 2009), surfactant treatment (Mæhre et al. 2016), osmotic shock (Postma et al. 2018), and structural disruption by enzymes (Wijesinghe and Jeon 2012), while improving the extractability of industrial value-added components.
Dulse Palmaria palmata is a popular edible seaweed in Western countries (Mouritsen et al. 2013), but it is not such a popular resource in Asian countries, particularly Japan. Despite its abundant availability in northern Japan, it is consumed to an extremely small extent in comparison with traditionally used seaweeds. In a previous study (Lee et al. 2017), we reported that photosynthesis-related components (phycobiliprotein and chlorophyll a-related compounds), which were water-extracted from freeze-dried dulse, showed a strong suppression of local acute inflammation of mouse paw after being orally administered in vivo. This suggested the novel utilization of dulse as an edible anti-inflammatory material. To apply this knowledge to the industrial utilization of dulse as an anti-inflammatory food source, establishing a stable storage technology for phycobilisome components and maintaining extractability are essential. However, as described above, hot-air drying possibly affects the extractability of the anti-inflammatory components, such as phycobiliproteins and chlorophyll a-related compounds, in dulse. In addition, understanding the effect of heating on the anti-inflammatory function is extremely important for the future use of dulse as an anti-inflammatory material.
The purpose of this study was to investigate the effects of hot-air drying of dulse thallus on the extractability and anti-inflammatory functions of phycobiliprotein and chlorophyll a-related compounds, which are the major anti-inflammatory components of dulse (Lee et al. 2017). The effects of short-term cryopreservation before drying were also examined simultaneously because temporary frozen storage before drying is often required to adjust the processing capacity of the drying device and the harvest yield of fresh dulse. Furthermore, cellulase treatment was attempted to restore the extractability of the components impaired by hot-air drying in this study.
Materials and methods
Materials
Dulse was harvested in winter at the coast of Hakodate city, Hokkaido, on the northern island of Japan. Thermolysin (EC 3.4.24.27, from Bacillus thermoproteolyticus rokko) was supplied by Wako Pure Chemical Industries (Osaka, Japan). Cellulase (EC 3.2.1.4, from Aspergillus niger) was supplied by MP Biomedicals (Santa Ana, CA, USA). RAW264.7 cells (a cultivated cell line of murine macrophages) was supplied by the American Type Culture Collection via Dainippon Sumitomo Pharma Co., Ltd. (Osaka, Japan). The cell culture reagents were purchased from Life Technologies (Carlsbad, CA, USA). The antibodies used in enzyme-linked immunosorbent assay (ELISA), anti-murine tumor necrosis factor-α (TNF-α), biotinylated anti-murine TNF-α, horseradish peroxidase-conjugated streptavidin, and 3,3’,5,5’-tetramethylbenzidine were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Recombinant TNF-α and interferon-γ (IFN-γ) were purchased from Peprotech, Inc. (Rocky Hill, NJ, USA). Lipopolysaccharide (LPS: from Salmonella typhimurium) was obtained from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used in the study, unless otherwise described, were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan).
Preparation of dried thalli by freezing and drying processes
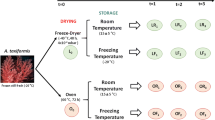
Fresh dulse brought to the laboratory on the day of harvest was gently washed with tap water, and the washed thalli were immediately subjected to a convective hot-air dryer equipped with the forced circulation system (Taiyo Seisakusho, Hokkaido, Japan) at 30 °C or 60 °C (relative humidity: 10%) at a flow rate of 1.5 m/s for 16–22 h. The change in the moisture content of the material was continuously monitored using an electronic balance equipped in the drying oven; the drying process was considered complete when the moisture evaporation terminated and the weight of the dried product reached a minimum. The obtained dried thalli were referred to as H30 and H60. A portion of the washed dulse was immediately frozen at − 25 °C for 7 days followed by hot-air drying in the same manner described above without thawing (referred to as FH30, and FH60). In addition, the washed dulse was freeze-dried (FD) as a control sample for the hot air–dried thalli. The moisture content of FD, H30, H60, FH30, and FH60 was 5.8, 6.1, 5.9, 9.9, and 9.2%, respectively. The five types of dried thalli thus obtained are summarized in Fig. 1.
Variation in dried dulse thallus used for water extractability assessment and sample preparation for the anti-inflammatory assay. Water extracts (WEx) containing phycobiliprotein and chlorophyll a-related compounds were prepared from five types of dried dulse thalli (FD, H30, H60, FH30, and FH60) for the water-extractability assessment. Furthermore, both of the anti-inflammatory components were purified from WEx and then thermolysin-digested for the anti-inflammatory assay
Preparation of water extract from dried thalli
The prepared thalli samples were pulverized by a dry matter crusher (Wonder blender WB-1, Osaka Chemicals, Osaka, Japan) and passed through a 250-µm metal mesh. The powder was suspended in 20-fold weight of distilled water, gently stirred at 4 °C for 12 h, and centrifuged at 15,000 × g for 10 min. The supernatant was collected as the water extract from each dried thallus (WEx). As described later, WEx contains phycobiliprotein and chlorophyll a-related compounds.
Enzymatic cell wall degradation of dried thalli and preparation of WEx
The thallus, which was previously washed with water, was frozen and hot air–dried at 50 °C (FH50) in the same way as shown in Fig. 1. In this experiment, dulse thalli were hot air–dried at 50 °C, which is commonly used in the drying process of the Japanese seaweed industry. The dried thallus was suspended in 40-fold weight of 0.1 M phosphate-buffered saline (pH 7.5) containing 0.1% cellulase and then incubated at 40 °C for 90 min. After the cellulase treatment, the suspension was centrifuged at 20,000 × g for 10 min, and the supernatant was collected as the WEx (referred as to CT (+) in Fig. 6). Non-cellulase-treated WEx from FH50 was also prepared as a negative control (CT (−)).
Quantitative analysis of extracted components from dried thalli
The amount of water-extracted protein in WEx was measured by the Bradford method (Bradford 1976) using the Bio-Rad protein assay kit (Bio-Rad) and bovine serum albumin fraction V (Sigma-Aldrich) as the standard protein. A 5- to 20-fold diluted sample was used for the measurement. Thus, protein concentration was quantified using the absorbance minus the measurement blank value with a reduced background. To measure chlorophyll a and its related compounds in the WEx, acetone was added to WEx at a final concentration of 80%. After sealing and stirring at 25 °C for 30 min, the acetone extract was subjected to the absorption spectrum assay; the concentration was calculated using the following equation: 12.25 × A663.6 − 2.55 × A646.6, where A663.6 and A646.6 were the absorbances at 663.6 nm and 646.6 nm obtained using a 1-cm cuvette and expressed as equivalents to chlorophyll a in µg/ml (Porra et al. 1989). The amount of the extracted components described above was expressed as a yield from 1.0 g of each dried thallus.
Composition analysis of water-extracted compounds from dried thalli
The WEx from each dried thallus was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) assay (Laemmli 1970), followed by protein staining using Bio-Safe CBB G-250 Stain (Bio-Rad, Richmond, CA, USA), and fluorescence imaging using a gel documentation system equipped with a light-emitting diode (LED) illuminator (the peak wavelength of LED for excitation: 525 nm, Light Capture II/VISRAYS-GL, ATTO, Tokyo, Japan). Each WEx was dissolved in an equal volume of 2% SDS–20 mM Tris–HCl (pH 8.0) containing 8 M urea and 2% 2-mercaptoethanol, and then heated in boiling water for 2 min. For the SDS-PAGE analysis, 12.5% acrylamide gel was used, and equal volumes of the SDS-treated samples were loaded in each gel lane. A protein marker kit (Cytiva, Tokyo, Japan) was used to estimate the molecular mass of the extracted protein subunits.
Preparation of phycobiliprotein and chlorophyll a-related compounds from dried thalli as anti-inflammatory components
Phycobiliprotein and chlorophyll a-related compounds, which show anti-inflammatory activity in vitro and in vivo, were prepared from the WEx of each dried thallus (Lee et al. 2017). In order to investigate their anti-inflammatory activity in the heat-treated thalli, both of the components were purified simultaneously from WEx followed by digestion. Briefly, the WEx was subjected to ammonium sulfate precipitation at 70% saturation three times to remove mycosporine-like amino acids and sugars. The anti-inflammatory components thus prepared were collected as the precipitate by centrifugation at 15,000 × g for 10 min, redissolved, and dialyzed in distilled water. After the protein concentration was adjusted to 10 mg/ml, the solution was digested with thermolysin (enzyme:protein = 1:100 [w/w]) at 70 °C for 3 h. The pH of the digested sample was adjusted to 8.0 with HCl during the reaction, and the enzymatic reaction was terminated by boiling for 15 min. The digested anti-inflammatory components thus obtained were lyophilized and stored at − 60 °C until the anti-inflammatory assay using RAW264.7 cells as described below.
Assessment of cell viability
The digested anti-inflammatory components from dried thalli were dissolved in sterilized phosphate-buffered saline (pH 6.8), passed through a 0.22-µm sterile filter, and used for functional assessments in the RAW264.7 cells. The cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% heat-inactivated fetal bovine serum (FBS-DMEM), 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.1 mM non-essential amino acids at 37 °C in 5% CO2. For the assessment of cell viability, the cells (200 µl, 2 × 105 cells/well) were seeded in 96-well culture plates, allowed to adhere for 2 h, cultured for 48 h with 500 μg/ml of each sample, and subjected to the WST-1 assay (Saigusa et al. 2015).
Assessment of anti-inflammatory activity
Each of the digested anti-inflammatory components prepared from WEx was added to LPS-stimulated RAW 264.7 cells and the suppressive effect on the secretion of inflammatory mediators was examined. For the analysis of nitric oxide production, RAW264.7 cells suspended in fresh 10% FBS-DMEM (without phenol red) were seeded in 96-well plates (200 μl, 2 × 105 cells/well) and allowed to adhere for 2 h. The plates were washed twice with phosphate-buffered saline and the cells were cultured in 200 μl of 10% FBS-DMEM containing the assay sample (0, 100, and 300 μg/ml), 0.5 ng/ml IFN-γ, and 2.5 ng/ml LPS for 24 h. The secretion of nitric oxide was directly measured from the culture supernatant by employing the Griess method (Baer et al. 1995) using sodium nitrite as a standard. To analyze TNF-α production, RAW264.7 cells seeded in a 96-well plate were stimulated by 2.5 ng/ml of LPS and cultured in FBS-DMEM contained in the assay sample. After cultivation for 24 h, the culture supernatants were analyzed using sandwich ELISA to measure TNF-α concentrations (Nishizawa et al. 2016).
Statistical analysis
The data were expressed as the mean ± standard deviation (n = 4–5). Statistical differences were determined using the Tukey–Kramer multiple comparison test with Statcel software ver. 1.0 (OMS-Publishing, Saitama, Japan). The letters in figures indicate significant differences (P < 0.05).
Results and discussion
Effect of freezing and hot-air drying on extractability of total protein and chlorophyll from dried thalli
It is apparent that the loss of extractability of anti-inflammatory components, phycobiliprotein and chlorophyll a-related compounds, affects the value of red algae as a biofunctional material to a large extent. To investigate the effect of freezing and heat-drying steps (Fig. 1) on the extractability of anti-inflammatory components, WEx was prepared from each dried thallus, followed by an examination of the yield of total protein containing phycobiliproteins and chlorophyll a-related compounds. In comparison with the freeze-dried thallus as a control (FD), the extractability losses of total protein (Fig. 2a) and chlorophyll a-related compounds (Fig. 2b) in the series of dried thalli occurred to larger extent due to the freezing and hot-air-drying processes. The yield of extracted total protein from H30 and H60 decreased considerably to 76 and 64% of FD, respectively. In addition, the freezing process in FH30 and FH60 further accelerated the loss of the extracted protein (44 and 34%, respectively). The yield of extracted chlorophyll a-related compounds also decreased by hot-air drying, but the freezing process had a weak effect on the loss of chlorophyll extraction. The loss of extractability of phycobiliproteins could have been caused by thermal denaturation and hardening of thallus due to hot-air drying. Furthermore, the fact that the freezing of raw thallus promoted the loss of extractability caused by hot-air drying suggests that the loss of stability of phycobiliprotein occurred during short-term frozen storage.
Amounts of extracted components in WEx from various dried thalli. a Total protein, b Chlorophyll a. Labeling of the dried thalli samples is shown in Fig. 1
The results of SDS-PAGE and spectrophotometric analyses to investigate the change in the extracted components from the thalli prepared are shown in Fig. 3. In protein staining (left side gel), the WEx of raw and FD thalli contained three major protein components of 55, 21, and 15 kDa; the components of 55 and 15 kDa were determined to be the large and small subunits of ribulose-1,5-bisphosphate carboxylase/oxygenase, respectively (Andersson and Taylor 2003), and the component of 21 kDa was determined as phycobiliprotein by the bright fluorescent band shown in right side gel (Oi et al. 1982). Each red-colored WEx contained the fluorescent phycobiliprotein, and the visible absorption peaks derived from phycoerythrin (PE) at 495 nm and 565 nm and phycocyanin (PC) at 610 nm were detected (Gantt 1981), as shown in Fig. 3b. The small amounts of protein components of approximately 15.9 and 35.0 kDa shown in some extracts appear to be linker proteins that maintain the structure of phycobilisome (Zhang et al. 2017). Furthermore, when all the WEx samples were diluted with acetone at a final concentration of 80%, they turned from red to green, and the visible absorption peak of chlorophyll a (Chl) at 665 nm (Bacon and Holden 1967) was detected in all the extracts (Fig. 3c). This result shows that water-insoluble chlorophyll a and water-soluble phycobiliproteins co-existed in WEx, suggesting that the photosystem II complex, containing chlorophyll and connecting with the phycobilisome (Cunningham et al. 1989), was simultaneously extracted from hot-air–dried thalli.
Comparison of water-extracted components from various dried dulse thalli using SDS-PAGE and spectrophotometric assays. a Electrophoresed samples were subjected to protein staining (left side gel) and fluorescent imaging assay (right side gel) in SDS-PAGE. PP phycobiliproteins, LSr large subunits of ribulose-1,5-bisphosphate carboxylase/oxygenase. Labeling of the dried thalli samples is shown in Fig. 1, and fresh raw thallus before drying was also examined. b Absorption spectra of water-extracted components from the series of dried thalli. PC phycocyanin, PE phycoerythrin. Numbers in the figure indicate the absorption peak (nm). c Absorption spectra of the water-extracted components in 80% acetone. Chl chlorophyll
Anti-inflammatory activity of phycobiliproteins and chlorophyll a-related compounds from the dried thalli
The digested anti-inflammatory components were prepared from five types of dried thalli, and they were subjected to cytotoxicity assessment. As shown in Fig. 4, the addition of 500 µg/ml of each sample had no effect on the cell viability of RAW264.7 cells. Each sample was continuously added to LPS-stimulated RAW264.7 cells at 100 and 300 µg/ml, and their anti-inflammatory activity was examined by monitoring the loss of nitric oxide and TNF-α production. The results are shown in Fig. 5a, b. In comparison with the positive control (not containing the sample), each sample considerably impaired the production of nitric oxide and TNF-α, indicating the presence of anti-inflammatory activity; in particular, TNF-α secretion was reduced to approximately 45% of the positive control. In addition, there was no difference in the strength of the anti-inflammatory effect among all the samples from the series of dried thalli. These results indicate that the anti-inflammatory activity of the functional components in dulse thalli was not affected by freezing and heat treatment.
Cytotoxicity assessment of anti-inflammatory components prepared from various dried thalli. The cell viability of Raw 264.7 cells was examined after the addition of 500 µg/ml of each of digested anti-inflammatory components. Labeling of the dried thalli samples is shown in Fig. 1; Negative control indicates the value for the negative control (no addition of sample). Each data point is expressed as a relative value, where the negative control is 100%
Suppressive effect of phycobiliprotein and chlorophyll a-related compounds from various dried thalli on the secretion of inflammatory mediators in macrophages. The digested anti-inflammatory components from five types of dried dulse shown in Fig. 1 were added to RAW264.7 cells at 100 or 300 µg/ml. The production of nitric oxide (a) and TNF-α (b) was examined using LPS-stimulated RAW 264.7 cells with or without IFN-γ, respectively. Positive control shows data for LPS-stimulated cells without addition of sample
Our previous study (Lee et al. 2017) has already shown that the anti-inflammatory components include phycobiliproteins and chlorophyll a-related compounds. Therefore, the results of Figs. 3 and 5 show that the anti-inflammatory function of both components is maintained even though the extraction of both components from the algae was inhibited by freezing and hot-air-drying processes of the raw thalli.
Improved extractability of anti-inflammatory components from dried thalli by cell wall degradation
The results shown in Fig. 5 suggest that frozen and hot-air–dried dulse has the potential to be used as a health functional food. Therefore, the enhancement of water extractability of the anti-inflammatory components from the thallus would lead to effective utilization of dulse. Although the extractability of phycobiliprotein impaired by heat denaturation cannot be restored, destruction of the heat-hardened cell wall may promote the extraction of the phycobiliprotein contained in it. Therefore, to enhance the extraction of anti-inflammatory components, the heat-hardened cell structures of the hot-air–dried thallus (FH50: obtained by hot-air drying at 50 °C with pre-freezing) were degraded by cellulase.
As shown in Fig. 6a, the cellulase treatment (CT (+) in the figure) of FH50 increased the extracted protein content in WEx up to 1.7-fold of untreated thalli (CT (−)), and the yield approached the FD thallus yield of 68 mg/g. Concurrently, the extractability of phycoerythrin and phycocyanin was markedly improved, as shown by the results of the spectrophotometric assay of the extracts (Fig. 6b). These results show that damage of the cell wall due to cellulase treatment improved the extraction of phycobiliproteins from heat-hardened thallus.
Recovery effect of enzymatic algal cell degradation on the extractability of phycobiliprotein and anti-inflammatory activity of WEx. a The amount of total protein in WEx from freeze-dried (FD) thallus and frozen and 50 °C hot air–dried thallus (FH50), where CT (+) and CT (−) indicate with and without cellulase treatment, respectively. b Absorption spectra of WEx of FH50: Solid line (CT (+)) and dotted line (CT (−)) indicate with and without cellulase treatment, respectively. PE and PC indicate the absorption peaks of phycoerythrin and phycocyanin, respectively. c TNF-α production of LPS-stimulated RAW 264.7 cells after the addition of 300 µg/ml of the anti-inflammatory components prepared from FH50/CT (+). Positive and negative controls show data for LPS-stimulated and unstimulated cells without addition of the sample, respectively
As described in Figs. 2, 5, the hot-air drying at 30 and 60 °C diminished the yield of anti-inflammatory components, which corresponds with CE (−), but it had no effect on their anti-inflammatory activity. Figure 6c showed the same result for the thallus dried at 50 °C. That is, the digested anti-inflammatory components prepared from the cellulase-treated thallus (FH50/CE (+)) also markedly suppressed TNF-α production in LPS-stimulated RAW264.7 cells. These findings clearly indicate that the disruption of the cell structure of dulse hardened by hot-air drying results in the effective extraction of anti-inflammatory components.
Phycobiliproteins of red algae are regularly arranged in the phycobilisome, which is bound to the thylakoid membrane, and are difficult to extract using water (Cunningham et al. 1989); however, enzymatic degradation of the cell membrane is reportedly an effective means to improve the extraction of phycoerythrin (Dumay et al. 2013). Furthermore, the results of Fig. 6 clearly showed that the enzymatic degradation of the heat-hardened cell wall was also effective for hot-air–dried dulse and that its utilization could make dried dulse an important marine anti-inflammatory material. In the future, the combination of enzymatic hydrolysis of the cell wall and various types of physicochemical degradation methods, such as ultrasonic treatment (Sun et al. 2009), fine mechanical grinding (Lin and Hong 2013), and osmotic shock (Postma et al. 2018), are expected to be efficient alternatives for harvesting anti-inflammatory components of dulse.
In conclusion, the extractability of the anti-inflammatory components of dulse was impaired by the hot-air-drying treatment aimed at reducing the volume and improving the storage stability of red algae dulse that has high water content. However, the freezing and hot-air-drying processes did not considerably affect the function of the anti-inflammatory components. Therefore, it is crucial that the recovery of anti-inflammatory components is efficiently performed using appropriate treatments, such as enzymatic algal cell degradation, when seaweed with high-moisture content is dried for use as an anti-inflammatory functional material.
References
Andersson I, Taylor TC (2003) Structural framework for catalysis and regulation in ribulose-1,5-bisphosphate carboxylase/oxygenase. Arch Biochem Biophys 414:130–140. https://doi.org/10.1016/S0003-9861(03)00164-4
Armisen R, Galatas F (1987) Production, properties and uses of agar. In: McHugh DJ (ed) Production and utilization of products from commercial seaweeds, FAO fisheries technical paper 288, 1st edn. FAO, Rome, pp 1–32
Bacon MF, Holden M (1967) Changes in chlorophylls resulting from various chemical and physical treatments of leaves and leaf extracts. Phytochemistry 6:193–210. https://doi.org/10.1016/S0031-9422(00)82763-6
Baer HP, Schmidt K, Mayer B, Kukovetz WR (1995) Pentamidine does not interfere with nitrite formation in activated RAW 264.7 macrophages but inhibits constitutive brain nitric oxide synthase. Life Sci 57:1973–1980. https://doi.org/10.1016/0024-3205(95)02183-J
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Chandini SK, Ganesan P, Bhaskar N (2008) In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem 107:707–713. https://doi.org/10.1016/j.foodchem.2007.08.081
Costa LS, Fidelis GP, Cordeiro SL et al (2010) Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed Pharmacother 64:21–28. https://doi.org/10.1016/j.biopha.2009.03.005
Cunningham FX, Dennenberg RJ, Mustardy L et al (1989) Stoichiometry of photosystem I, photosystem II, and phycobilisomes in the red alga Porphyridium cruentum as a function of growth irradiance. Plant Physiol 91:1179–1187. https://doi.org/10.1104/pp.91.3.1179
Dumay J, Clément N, Morançais M, Fleurence J (2013) Optimization of hydrolysis conditions of Palmaria palmata to enhance R-phycoerythrin extraction. Bioresour Technol 131:21–27. https://doi.org/10.1016/j.biortech.2012.12.146
Galland L (2010) Diet and Inflammation. Nutr Clin Pract 25:634–640. https://doi.org/10.1177/0884533610385703
Gantt E (1981) Phycobilisomes. Annu Rev Plant Physiol 32:327–347
Gupta S, Abu-Ghannam N (2011) Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci Technol 22:315–326. https://doi.org/10.1016/j.tifs.2011.03.011
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597. https://doi.org/10.1007/s10811-010-9632-5
Islam MN, Ishita IJ, Jin SE et al (2013) Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem Toxicol 55:541–548. https://doi.org/10.1016/j.fct.2013.01.054
Iwalewa E, McGaw L, Naidoo V, Eloff J (2007) Inflammation: the foundation of diseases and disorders a review of phytomedicines of South African origin used to treat pain and inflammatory conditions. African J Biotechnol 6:2868–2885
Katayama S, Nishio T, Kishimura H, Saeki H (2012) Immunomodulatory properties of highly viscous polysaccharide extract from the Gagome alga (Kjellmaniella crassifolia). Plant Foods Hum Nutr 67:76–81. https://doi.org/10.1007/s11130-011-0271-z
Kurihara K (2009) Glutamate: from discovery as a food flavor to role as a basic taste (umami). Am J Clin Nutr 90:719S-722S. https://doi.org/10.3945/ajcn.2009.27462D
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee D, Nishizawa M, Shimizu Y, Saeki H (2017) Anti-inflammatory effects of dulse (Palmaria palmata) resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Res Int. https://doi.org/10.1016/j.foodres.2017.06.040
Lin CC, Hong PKA (2013) A new processing scheme from algae suspension to collected lipid using sand filtration and ozonation. Algal Res 2:378–384. https://doi.org/10.1016/j.algal.2013.06.001
Mæhre HK, Jensen IJ, Eilertsen KE (2016) Enzymatic pre-treatment increases the protein bioaccessibility and extractability in dulse (Palmaria palmata). Mar Drugs. https://doi.org/10.3390/md14110196
Mouritsen OG, Dawczynski C, Duelund L et al (2013) On the human consumption of the red seaweed dulse (Palmaria palmata (L.) Weber and Mohr). J Appl Phycol 25:1777–1791. https://doi.org/10.1007/s10811-013-0014-7
Nishizawa M, Saigusa M, Saeki H (2016) Conjugation with alginate oligosaccharide via the controlled Maillard reaction in a dry state is an effective method for the preparation of salmon myofibrillar protein with excellent anti-inflammatory activity. Fish Sci 82:357–367. https://doi.org/10.1007/s12562-015-0959-3
Oi VT, Glazer AN, Stryer L (1982) Fluorescent phycobiliprotein conjugates for analyses of cells and molecules. J Cell Biol 93:981–986. https://doi.org/10.1083/jcb.93.3.981
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394. https://doi.org/10.1016/S0005-2728(89)80347-0
Postma PR, Cerezo-Chinarro O, Akkerman RJ et al (2018) Biorefinery of the macroalgae Ulva lactuca: extraction of proteins and carbohydrates by mild disintegration. J Appl Phycol 30:1281–1293. https://doi.org/10.1007/s10811-017-1319-8
Saigusa M, Nishizawa M, Shimizu Y, Saeki H (2015) In vitro and in vivo anti-inflammatory activity of digested peptides derived from salmon myofibrillar protein conjugated with a small quantity of alginate oligosaccharide. Biosci Biotechnol Biochem 79:1518–1527. https://doi.org/10.1080/09168451.2015.1031075
Sakai S, Komura Y, Nishimura Y et al (2011) Inhibition of mast cell degranulation by phycoerythrin and its pigment moiety phycoerythrobilin, prepared from Porphyra yezoensis. Food Sci Technol Res 17:171–177. https://doi.org/10.3136/fstr.17.171
Serhan CN, Brain SD, Buckley CD et al (2007) Resolution of inflammation: state of the art, definitions and terms. FASEB J 21:325–332. https://doi.org/10.1096/fj.06-7227rev
Sheng J, Yu F, Xin Z et al (2007) Preparation, identification and their antitumor activities in vitro of polysaccharides from Chlorella pyrenoidosa. Food Chem 105:533–539. https://doi.org/10.1016/j.foodchem.2007.04.018
Sun L, Wang S, Gong X et al (2009) Isolation, purification and characteristics of R-phycoerythrin from a marine macroalga Heterosiphonia japonica. Protein Expr Purificatio 64:146–154. https://doi.org/10.1016/j.pep.2008.09.013
Wijesinghe WAJP, Jeon YJ (2012) Enzyme-assistant extraction (EAE) of bioactive components: a useful approach for recovery of industrially important metabolites from seaweeds: a review. Fitoterapia 83:6–12. https://doi.org/10.1016/j.fitote.2011.10.016
Wu X, Schauss AG (2012) Mitigation of inflammation with foods. J Agric Food Chem 60:6703–6717
Zhang J, Ma J, Liu D et al (2017) Structure of phycobilisome from the red alga Griffithsia pacifica. Nature 551:57–63. https://doi.org/10.1038/nature24278
Funding
We are grateful to Dr. Hiroyuki Munehara and Mr. Atsuya Miyajima (Usujiri Fisheries Station, Field Science Center for Northern Biosphere, Hokkaido University) for conducting the sampling of dulse. This study was partially supported by “Knowledge Cluster Initiative” National Project (“Hakodate Marine Biocluster”), Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sugita, D., Joe, GH., Masuoka, M. et al. Effect of drying treatment on the extractability and anti-inflammatory function of photosynthesis-related components in dulse Palmaria palmata and their efficient recovery from dried thallus. Fish Sci 88, 645–652 (2022). https://doi.org/10.1007/s12562-022-01619-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-022-01619-9