Abstract
This study provides a life cycle assessment of the energy balance and the potential greenhouse gas impacts of heterotrophic microalgal-derived biodiesel estimated from the upstream biomass production to the downstream emissions from biodiesel combustion. Heterotrophic microalgae can be cultivated using a by-product from biodiesel production such as glycerol as a carbon source. The oils within the algal biomass can then be converted to biodiesel using transesterification or hydroprocessing techniques. This approach may provide a solution to the limited availability of biomass feedstock for production of biorefined transportation fuels. The life cycle assessment of a virtual production facility, modeled on experimental yield data, has demonstrated that cultivation of heterotrophic microalgae for the production of biodiesel is comparable, in terms of greenhouse gas emissions and energy usage (90 g CO2e MJ−1), to fossil diesel (85 g CO2e MJ−1). The life cycle assessment identified that improvement in cultivation conditions, in particular the bioreactor energy inputs and microalgae yield, will be critical in developing a sustainable production system. Our research shows the potential of heterotrophic microalgae to provide Australia’s transportation fleet with a secure, environmentally sustainable alternative fuel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of microalgae as alternative sources of energy has been explored since the 1950s, and the energy shocks in the 1970s prompted widespread interest in microalgal biofuels, such as hydrogen production by microalgae and methane production from wastewater treatment (Benemann 2000; Levin et al. 2004; Esper et al. 2006). From 1978 to 1996 the US Department of Energy funded a program known as the Aquatic Species Program to develop biodiesel from algae. The program focused on the production of biodiesel from high oil-content microalgae grown in ponds, utilizing waste CO2 from coal-fired power plants (Sheehan et al. 1998b). However, the program was discontinued due to federal budget cutbacks after a subsequent decrease in fossil oil prices. These studies demonstrated the promise of the technology, but the commercial success of large-scale production has been elusive. The renewed interest and demand in microalgal-derived biodiesel is now increasing globally due to growing concern with depletion of fossil fuels and anthropogenic climate change. These two factors are driving development of economical and environmentally sustainable, low greenhouse gas emission transportation fuels.
In addition to producing oils for biodiesel, microalgae are capable of producing high-value bioproducts, such as carotenoid pigments, industrial enzymes, omega-3 long-chain (≥C20) polyunsaturated fatty acids (LC-PUFA), and exopolysaccharides (EPS), and lower value by-products, such as algal meal, which may be of use in animal and fish feeds, adding greater value to the production process pipeline with improved process economics (Huntley and Redalje 2007; US DOE 2010; Li et al. 2008; Stephens et al. 2010; Wijffels and Barbosa 2010).
Numerous research and start-up companies have shown that heterotrophic cultivation could result in higher production of biomass and accumulation of high oil content in cells compared to that achieved using photoautotrophic cultivation (Brennan and Owende 2010; Liang et al. 2009). The majority of photoautotrophic systems for production of biofuels depend on photosynthesis of phototrophic microalgae in open raceway ponds or photobioreactors using CO2 and light as carbon and energy sources, respectively (De Boer et al. 2012; Passell et al. 2013). However, phototrophic cultivation provides low biomass dry weight yield per liter of cultivation medium, with microalgae concentration ranging from 0.5 g L−1 in an open raceway pond (Collet et al. 2011) to 8.3 g L−1 in a photobioreactor (Stephens et al. 2010). This means of cultivation, with low cell concentrations achieved, significantly increases processing costs (i.e., harvesting, dewatering, and oil extraction) and thus represents a significant economic barrier if the system is designed to produce only low-value commodity oils such as is used for biofuel production.
Alternatively, some microalgae can grow heterotrophically in stainless steel fermenters on organic substances (e.g., sugars, organic acids) as the only carbon and energy source. Yan et al. (2011) demonstrated that heterotrophic cultivation of the green alga Chlorella protothecoides has the potential to provide significantly higher biomass yields than photoautotrophic production. The cell density reached up to 70.9 g L−1 and the oil content 57.6 % of the cell dry weight in 178 h of cultivation, and established an equivalent bio-oil productivity of 8.3 mL L−1 day−1 (Yan et al. 2011). Solazyme, Inc. (South San Francisco, USA) has reported using heterotrophic microalgae to produce more than 37,854 L (10,000 gal) of oil at a quality that meets existing fuel standards (Peterka 2013). The company has successfully partnered with the US Navy to produce commercial quantities of algal fuel. Solazyme’s fuel feedstock has already been demonstrated and approved as a commercial aviation fuel blend. In addition, Solazyme is producing oil for a range of higher value applications such as chemicals, nutritional, cosmetic, and personal care products.
Heterotrophic cultivation of another microalgal group—the thraustochytrids—using by-product from biodiesel production such as glycerol as a carbon source can be used for biomass production (Pyle et al. 2008). The oils in this biomass feedstock can then be converted to biodiesel using transesterification to produce fatty acid methyl esters (FAME) or hydroprocessing to produce hydrocarbons from the oils and may provide a potential solution.
Thraustochytrids are heterotrophic protists found ubiquitously in the marine environment and play an important role in marine ecosystems; they can be bacterivores, detritivores, or parasites (Maas et al. 1999; Raghukumar 2002). Molecular phylogenetic studies have resulted in their classification into the class Labyrinthulomycota and phylum Heterokonta within the kingdom Chromista. This phylum also includes the chromophyte algae such as brown algae and diatoms (Cavalier-Smith et al. 1994; Leander et al. 2004; Porter 1990). They are known to produce high amounts (>30 % of total fatty acids) of omega-3 LC-PUFA, including docosahexaenoic acid (DHA, 22:6ω3) and eicosapentaenoic acid (EPA, 20:5ω3) (Jain et al. 2007; Lewis et al. 1999). Numerous studies have shown that dietary consumption of omega-3 LC-PUFA, in particular EPA and DHA, helps reduce the risk of cardiovascular diseases, neural disorders, arthritis, asthma, and skin diseases in humans (Danaei et al. 2009; Mozaffarian and Rimm 2006). DHA is also essential for neural and retinal development during foetal life and infancy (Forsyth and Carlson 2001; Ratledge 2004).
In a previous study, we demonstrated that recently isolated endemic Australian thraustochytrid strains were promising candidates for production of biodiesel, omega-3 LC-PUFA, and bioproducts such as carotenoid pigments, phytosterols, EPS, and odd-chain length fatty acids (Lee Chang et al. 2011, 2012, 2013). The fatty acid profile of the species examined contained high levels of saturated fatty acids (SFA), mainly palmitic acid (16:0, up to 52 % TFA), and LC-PUFA were mostly DHA (up to 39 % TFA) with low levels of docosapentaenoic acid (DPA-6, up to 4.2 %) (Lee Chang et al. 2013). The degree of unsaturation (number of double bonds) of the fatty acids is important with respect to its suitability for biodiesel production. Fatty acids with fewer double bonds are more stable to oxidation compared to the PUFA. Oxidation results in the formation of undesirable products, such as alcohols that reduce the flash point of biodiesel, aldehydes that cause rancidity, and shorter chain (≤C20) fatty acids that are corrosive to engine components(Knothe 2007; Monyem et al. 2000). Furthermore, SFA typically have higher melting points and cannot be used at lower temperatures. Therefore high levels of SFA and monounsaturated FA (MUFA) are more desirable for biodiesel production due to the increased oxidative stability as well as the thermal stability of biodiesel. Due to their high oil productivity, thraustochytrids have the potential for production of both omega-3 LC-PUFA rich oils as well as the shorter chain, less unsaturated fatty acids suitable for biodiesel (Johnson and Wen 2009).
The rise in biodiesel production from vegetable oils and animal fats has resulted in a surplus of low-cost glycerol (a by-product of biodiesel production by transesterification process) internationally, with crude glycerol prices dropping from US$0.11 kg−1 in 2004 to about US$0.02 kg−1 in 2006, although more recent trends show the price is stable at around US$0.04–0.11 kg−1 (Johnson and Taconi 2007; Pyle et al. 2008; Quispe et al. 2013). In Australia, there were previously 11 biodiesel plants with a combined total installed capacity of approximately 360 ML. In 2013, only four of these plants were still operating, and Australia was estimated to be producing about 115 ML of biodiesel from tallow and used cooking oil (BAA 2013). Biodiesel production will generate about 10 % (w/w of the bio-oil) glycerol as the main by-product (Dasari et al. 2005). These data indicate approximately 12.8 ML of crude glycerol by-product were produced in Australia.
For a large-sized biodiesel plant, crude glycerol can be refined into food or pharmaceutical grade glycerol. Pluske (2007) revealed that processing crude glycerol is not only costly and generally not economically feasible for small- to medium-sized plants, but also glycerol produced from small-sized plants will have little or no value due to difficulties in marketing small amounts of glycerol. Pluske (2007) suggested that crude glycerol can be used as a feed ingredient in the Australian pig industry for which the costs associated with processing are expected to be minimal. Many studies have proposed alternative uses for the crude glycerol including the following: animal feed (Pluske 2007; Lammers et al. 2008), combustion (Johnson and Taconi 2007), composting (Brown 2007), feedstock in fermentation processes to produce high-value LC-PUFA (Pyle et al. 2008; Lee Chang et al. 2013), or it can be used to increase the biogas production of anaerobic digesters (Athanasoulia et al. 2014). The use of a glycerol stream as a carbon source for a heterotrophic algal production system could not only reduce greenhouse gas emissions but also substantially reduce the cost of commercial production of algal-derived biodiesel. The key objective of this study was to investigate the greenhouse gas emissions from heterotrophic cultivation of thraustochytrids using glycerol to produce bio-oil through a preliminary life cycle assessment (LCA).
Materials and methods
Life cycle assessment
The goal of the current study was to conduct a LCA of heterotrophic microalgal-derived biodiesel by extrapolation of experimental data to estimate the greenhouse gas impacts of larger scale production. The LCA includes electricity sourced from coal, natural gas, and related upstream processes (i.e., gas extraction) when calculating the LCA impact. Capital equipment and infrastructure are rarely included when analyzing the footprint of the fossil fuel industry, and as such, they were excluded from this LCA (Grant et al. 2008; Stratton et al. 2011; Passell et al. 2013). It was assumed that the greenhouse gas impact of the construction of the algae bioreactors and processing plant would be negligible over the life of these capital items, in comparison to the operating impacts. In this study, the LCA impact of refining crude bio-oil to produce diesel was based on energy content allocation, as modeled in the Australasian Unit Process life cycle inventory (LCI) SimaPro7.
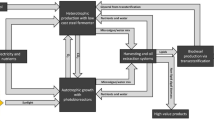
LCA involves taking into account both upstream and downstream emissions. In the context of biodiesel, this includes not only the combustion emissions from vehicles (referred to as downstream emissions) but also those associated with the overall production of the biofuel: extraction, transportation, processing, conversion, and distribution (referred to as upstream emissions) (Grant et al. 2008). The lack of algae-to-biofuel processes operating at scale, coupled with the commercial sensitivities of the sector, required the development of a hypothetical production scenario. This scenario was developed and later populated using information from the literature as well as results from engineering correlations of the impellor (Rushton turbine), sparging, centrifuge, pumps, cell disruptor, solvent stripper, hydroprocessing unit with energy use, and calculated greenhouse gas generation. The process diagram of heterotrophic thraustochytrid culture and subsequent processing and conversion into the biodiesel blend stock is shown in Fig. 1.
Heterotrophic microalgae production process diagram. The upgrade step could be production of biodiesel via either hydroprocessing to produce a hydrocarbon based fuel or transesterification to produce a fatty acid methyl ester based fuel. The residual biomass could be used elsewhere, for example, for stock or aquaculture feed or used on-site in an anaerobic digester to produce energy to be fed back into the system
The term “well-to-wheel emissions” is also used to describe the full-fuel LCA. The emissions related to vehicle manufacture, maintenance and disposal, and road building are relevant to total transport emissions, but they are not likely to vary significantly with the nature of the fuel used, and thus these factors were excluded from the LCA (Grant et al. 2008). Furthermore, the LCA excluded transport of upstream inputs like glycerol and downstream delivery of biodiesel due to the unknown distances and different modes of possible transport used. Apart from analyzing the well-to-wheel emission profile of a particular transport fuel, LCA is often applied to determine the energy returned on energy invested (ERoEI). The “invested energy,” in a transport fuel context, represents the energy inputs required to produce a unit of fuel. For example, in the case of microalgal-derived bio-oils, this represents the energy consumed during the growth, harvest, extraction, and refining stages (MJin). The “energy returned” represents the energy output from combusting the produced unit of fuel (MJout). Therefore, the amount of energy returned per energy input can be calculated (ERoEI = MJout / MJin). If the energy input is less than the available combustion energy in the refined fuel, the process is an energy source (ERoEI > 1). However, if the ERoEI < 1, the process is an energy sink. Importantly, energy cannot be created nor destroyed, and as such, the LCA software (SimaPro 7) includes the embedded energy content of limited fossil fuel resources (i.e., crude oil) as energy inputs.
LCA software
LCA was performed using SimaPro 7, which is an open structure program that can be used for different types of LCAs. The upstream production (heterotrophic cultivation) and use (processing) stages and the downstream end-of-life stage (combustion emissions) can be specified in as much detail as necessary by selecting processes from the database and by building process trees that can be drawn automatically by the program. The results are presented in graphs, varying from a list of substances (inputs and outputs), characterized scores, normalized scores, or evaluated scores. The program visual output provides for easy identification of processes that have a high impact to the overall life cycle.
Heterotrophic cultivation
This LCA was based on heterotrophic cultivation of thraustochytrids using a 0.2-ML industrial bioreactor run in a single batch. Cultivation data was based on experimental data from our 2-L lab-scale fed-batch bioreactors (Lee Chang et al. 2013). This work reported 71 g L−1 biomass with FAME content of 52 % (w/w) of dry weight by 69 h using glycerol as a carbon source. The data were extrapolated to the proposed full-scale bioreactor based on maintaining an equivalent aggregator tip speed and is one of the many scale-up techniques used in industry (Holland and Chapman 1966). Scale-up introduces obvious uncertainty, however, the selection of our experimental yield data (under yet to be optimized culture conditions) provides for the assessment of a baseline production target. Biomass of thraustochytrids may reach up to 200 g L−1 (oil content >50 %) in commercial-scale stirred fermentors (100 m3) (Barclay et al. 2010). The major carbon source in the medium was glycerol (sourced from biodiesel production) and was the only media carbon source included in this LCA. Minor media components include yeast extract, peptone, vitamins, and mineral supplements (Lee Chang et al. 2013). The embodied greenhouse gas impact of glycerol was economically allocated from the biodiesel production process based on the Ecoinvent LCI database. We also investigated substituting the main carbon (glycerol) with molasses (another renewable feed stock) for the heterotrophic cultivation stage to compare the total upstream emissions. The embodied greenhouse gas impact of molasses was evaluated as a by-product of the sugar extraction process by Beer et al. (2001) and that data was used in the current LCA. The bioreactor operating conditions—scaled up from Lee Chang et al. (2013) are shown in Table 1. Once the culture reached sufficient biomass (after 69 h), it was transferred for harvesting, oil extraction, and subsequent upgrading using transesterification or hydroprocessing techniques.
Processing
In this laboratory-based study, the dewatering step involved 0.2 ML of culture, containing the equivalent of 71 g L−1 of dry algae, being centrifuged (continuous flow), with the culture volume reduced to approximately 30 % of the original volume. Without mixing from impellors and sparging, thraustochytrid cells tend to clump and settle on the bottom of culture flask. The use of gravity sedimentation in a settling tank for pre-concentration of algae biomass before centrifugation could be incorporated, but as this (which is achieved by turning off the power) has very little impact on the carbon footprint, it was not included in the processing scheme.
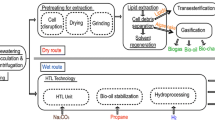
The small size of the algal cells and presence of a cell wall suggests that efficient oil extraction may benefit from the rupture of the microalgal cells prior to oil extraction. This was assumed to be achieved using a high-pressure industrial homogenizer as has been shown to be an effective technique to rupture Nannochloris oculata cell walls (Samarasinghe et al. 2012). Further research is needed to validate whether the cell rupture step may be omitted and which direct solvent extraction method could improve oil extraction from thraustochytrid cells at a commercial scale. In the LCA, the lysed cell solution was then mixed with solvent (hexane) in a mixer/settler to extract the oils. A stripper was used to recover the hexane solvent (recovery assumed to be 90 % v/v of the original hexane) from the oil-solvent solution, producing a crude algal bio-oil, which may subsequently be upgraded into a transportation fuel. The process requirements to produce crude algal bio-oil, as shown in Fig. 1, are summarized in Table 2. For this LCA, the crude algal bio-oil was upgraded into renewable biodiesel fuel using the transesterification or the petrochemical hydroprocessing techniques. The transesterification process reacts the bio-oil with alcohol to produce biodiesel consisting of FAME and the by-product glycerol (Fig. 2). However, due to the high levels of LC-PUFA, not all of the FAME are suitable for direct use as biodiesel. For the purpose of this LCA, the thraustochytrid was assumed to be a low LC-PUFA strain (Fisher et al. 2007). However, if a high LC-PUFA strain was used, the separation of the FAME suitable of biodiesel from the LC-PUFA fraction would require an additional processing step, such as the relatively mature methods of winterization and urea complexation, etc. (Mendes et al. 2007), that would reduce yields of biodiesel. In comparison, the hydroprocessing technique removes oxygen, nitrogen, and other heteroatoms, producing a hydrocarbon-based hydroprocessed biodiesel. This hydroprocessed biodiesel contains no aromatics, and thus, the product must be blended with conventional diesel to satisfy certification requirements. Input data for the hydroprocessing requirement in this LCA has been adapted from Stratton et al. (2011). After bio-oil extraction, the residual oil-free biomass stream may be processed further for energy or sugar recovery. In the examples presented, the residual oil-free biomass was fed into an anaerobic digester (Oswald and Golueke 1960), where the gases produced were combusted and the energy yielded was fed back into the process to reduce processing costs and help reduce total upstream emissions.
LCA network diagram at 1 MJ of biodiesel derived from heterotrophic microalgae production followed by extraction and transesterification and energy yields resulting from oil-free biomass back to anaerobic digestion. Only inputs greater than 3.5 % of the total CO2 are displayed to show the major inputs and to avoid an overly complicated network diagram
Combustion emissions
To evaluate the total life cycle footprint of biodiesel derived from thraustochytrids, the combustion emissions must also be considered. Combustion emission data are available in the literature and from engine certification testing (Dobes 1994; Penner et al. 1999; Wahlen et al. 2013). Importantly, although these data were based on conventional fossil fuel, alternative products must be compositionally similar to biodiesel to satisfy the certification requirements. Previous studies have shown variations of gaseous exhaust emissions from microalgal-derived biodiesels (Knothe et al. 2006; Wahlen et al. 2013). Thus, fossil diesel emission data were used in this LCA; however, a correction was applied to take into account biogenic CO2.
Under carbon accounting practices, CO2 emitted during the combustion of a biofuel—entitled biogenic CO2—was assumed to be equal to that which was absorbed during photosynthetic growth. Heterotrophic algae utilize organic carbon sources (in this case glycerol) and do not absorb atmospheric CO2. Nevertheless, the cultivation of plants, from which the organic carbon source was obtained, absorbed atmospheric CO2 as part of growth processes. Therefore, the associated combustion emissions were considered biogenic.
Combustion produces gaseous (i.e., carbon dioxide, methane, nitrous oxide, etc.) and particulate matter (volatile and non-volatile organics) emissions that must be considered when evaluating the biofuel life cycle. Total emissions were accounted through a CO2 equivalent (CO2e) value based on their global warming potential (GWP) as defined in the Kyoto Protocol, which was assessed with the characterization factors for a temporal horizon of 100 years. Selected exhaust data including methane and nitrous oxide has a GWP of 21 and 310, respectively; these data were adapted from the SimaPro 7 database.
Results and discussion
Total greenhouse gas impact and energy intensity
Combining the upstream and downstream use stages provides the total greenhouse gas impact and energy intensity of the heterotrophic microalgae production system (Table 3). Emission data are reported per megajoule of product produced (e.g., g CO2e MJ−1) from the batch cultivation system, with the energy intensity representing the ratio of energy output over input (e.g., ERoEI; MJ MJ−1). The life cycle impact of fossil diesel is included for comparison.
The results show that production of biodiesel using heterotrophic algae via hydroprocessing (71.5 g CO2e MJ−1) had a superior greenhouse gas footprint for the whole of life cycle compared with fossil diesel (85.1 g CO2e MJ−1) (Table 3). The greenhouse gas footprint of transesterified heterotrophic algal-derived FAME biodiesel (89.9 g CO2e MJ−1) was comparable with fossil diesel. The differences between the algal-derived hydroprocessed and FAME biodiesels were predominantly due to the different energy densities of the hydrocarbons and FAME of the biodiesels produced (energy out; Table 3). The elevated upstream emissions from heterotrophic algal-derived hydroprocessed and FAME biodiesels were due mostly to the upstream impact of the heterotrophic cultivation stage. Specifically, these were the glycerol carbon source (61.0 g CO2e MJ−1 for hydroprocessed biodiesel and 66.8 g CO2e MJ−1 for FAME biodiesel) and the electricity demand of the impeller (3.8 g CO2e MJ−1) and air sparging motor (3.6 g CO2e MJ−1) from a mostly fossil-based grid (coal). A major impact was also from the hexane used in the solvent extraction during the processing stage (21.8 g CO2e MJ−1 for hydroprocessed biodiesel and 24.2 g CO2e MJ−1 for FAME biodiesel). A breakdown of emissions contributing to 3.5 % or more of the total impact for FAME biodiesel production is shown in Fig. 2. The cumulative emission impact is represented by the thermometer bars shown on each process block. The CO2e input from the production of glycerol and hexane were quite high (3.79 and 0.41 kg CO2e kg−1 of input, respectively), and large amounts were used in the production process (33,817 and 1,338 kg, respectively) resulting in the high contributions from these sources (Fig. 2). Even though these upstream inputs for the algal-derived biodiesel (especially from glycerol) were high, the total CO2 emitted for the whole of life cycle was low because the downstream CO2 emitted was significantly reduced (Table 3) due to taking into account the biogenic CO2 from which the glycerol was obtained, namely, the by-product from transesterification in the production of biodiesel from terrestrial plants.
The limited availability of terrestrial biomass feedstock is expected to restrict industry uptake of biorefined transportation fuel in Australia (Rye et al. 2010). Other carbon sources derived from agroindustrial wastes (such as waste molasses, empty palm fruit bunches, etc.) have been explored recently for producing high oil content containing biomass through heterotrophic fermentation of thraustochytrids (Yan et al. 2011; Hong et al. 2013). However, biomass yields to date have been considerably lower than obtained using glycerol (Lee Chang et al. 2013). The biomass yields were lower for molasses; however, due to the high embodied carbon of the glycerol, substituting molasses as the carbon source for the heterotrophic cultivation stage and incorporating this process into the scenario reduced total upstream emissions to 16.5 g CO2e MJ−1 (Table 3). This was a 77 to 81 % reduction of total upstream emissions and highlights the importance of optimizing the culture conditions, including choice of processing technologies and major ingredients, at scale. Further studies are needed, including examining microalgae strain selection and developing new strategies to decrease the susceptibility of potential variation in the feedstock (e.g., use of molasses in full or part) on the cultivation process.
The ERoEI was 0.5 for the production of biodiesel from heterotrophic cultivation of thraustochytrids (Table 3). An ERoEI of less than one is an energy sink, as we need twice the energy to produce one unit of energy for use. This was mainly due to costs of running the impeller, centrifugation electricity consumption, and also the carbon source with the high-process footprint as discussed. The value of ERoEI reported for fossil diesel is 0.8 according to Sheehan et al. (1998a) and Hossain and Davies (2010). It is worth noting that when replacing the glycerol with molasses as the carbon source, the ERoEI improved to 1.4.
Identification of other low-cost carbon sources such as a lignocellulosic biomass, as well as use of an appropriate process to convert this feed into carbohydrates suitable for heterotrophic growth, could also be critical in both the economic and sustainable scale-up of this technology.
Use of residual oil-free biomass after extraction of the bio-oil may also improve the process economics through the generation of an additional income stream (e.g., stock feed sales) or reduce operating expenditure through energy or material recovery. In the current study, this oil-free biomass was used as an energy source, reducing the energy required by 11.9 MJ kg−1. Alternatively, it may be possible to extract the starch (carbohydrates) from the residual microalgae biomass and, using enzymes, convert the biomass into a carbon source for subsequent culture batches.
Conclusion
The LCA, modeled on baseline literature yield data, has demonstrated that cultivation of heterotrophic microalgae for the production of biodiesel stock is viable and comparable in terms of greenhouse gas emissions and energy usage to fossil diesel. The LCA identified that improvement in cultivation conditions, in particular the bioreactor energy inputs and algae yield, will be critical in developing a sustainable production system. The literature reports that the required yields are close to being demonstrated in the laboratory; however, even if the laboratory yields translate to an industrial scale, the identification of a suitably cheap and readily available sugar or other carbon source, such as glycerol, is critical.
To generate commercial interest in the heterotrophic cultivation of thraustochytrids for bio-oil for use in biodiesel production together with other co-products, there is a need for additional research for the optimization of growth conditions. This will need to include industrial scale research, to allow further assessment of the commercial feasibility and to remodel the LCA with a more current glycerol processing footprint. To date, the authors are not aware of any commercial operation producing biodiesel using heterotrophic thraustochytrids at a cost comparable to conventional diesel. Therefore, as our study has not focused on cultivation costs, further research is required to improve the understanding of both the upstream and downstream process economics.
References
Athanasoulia E, Melidis P, Aivasidis A (2014) Co-digestion of sewage sludge and crude glycerol from biodiesel production. Renew Energy 62:73–78
BAA (2013) Biodiesel production facilities in Australia, current as at 1 December 2013, Biofuels Association of Australia <http://www.biofuelsassociation.com.au/biodiesel-production-facilities-in-australia> Accessed 20 May 2014
Barclay W, Weaver C, Metz J, Hansen J (2010) Development of a docosahexaenoic acid production technology using Schizochytrium: historical perspective and update. In: Cohen Z, Ratledge C (eds) Single Cell Oils - Microbial and Algal Oils (2nd Edition). AOCS Press, Champaign, pp 75–96
Beer T, Grant T., Morgan G, Lapszewicz J, Anyon P, Edwards J, Williams D (2001). Comparison of transport fuels. Final report (EV45A/2/F3C) to the Australian greenhouse office on the stage 2.of life- cycle emissions analysis of alternative fuels for heavy vehicles. Aspendale, Vic (AU)
Benemann JR (2000) Hydrogen production by microalgae. J Appl Phycol 12:291–300
De Boer K, Moheimani NR, Borowitzka MA, Bahri PA (2012) Extraction and conversion pathways for microalgae to biodiesel: a review focused on energy consumption. J Appl Phycol 24:1681–1698
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577
Brown R (2007) Biodiesel co-product markets in Wyoming for Wyoming Department of Agriculture. Lakewood, CO: International Center for Appropriate & Sustainable Technology. http://www.ams.usda.gov/AMSv1.0/getfile?dDocName=STELPRDC5064918Accessed 20 May 2014
Cavalier-Smith T, Allsopp MTEP, Chao EE (1994) Thraustochytrids are chromists, not fungi: 18 s rRNA signatures of Heterokonta. Phil Trans R Soc London B 346:387–397
Collet P, Hélias A, Lardon L, Ras M, Goy RA, Steyer JP (2011) Life-cycle assessment of microalgae culture coupled to biogas production. Bioresour Technol 102:207–214
Danaei G, Ding EL, Mozaffarian D, Taylor B, Jr R, Murray CJL, Ezzati M (2009) The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 6(4):e1000058
Dasari MA, Kiatsimkul PP, Sutterlin WR, Suppes GJ (2005) Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl Catal A: Gen 281(1):225–231
Dobes L (1994) Alternative fuels in Australian transport. Bureau of Transport and Communications, Economics, Canberra
Esper B, Badura A, Rögner M (2006) Photosynthesis as a power supply for (bio-) hydrogen production. Trends Plant Sci 11:543–549
Fisher L, Nicholls D, Sanderson K (2007) Production of biodiesel. WIPO Patent No. 2008067605
Forsyth JS, Carlson SE (2001) Long-chain polyunsaturated fatty acids in infant nutrition: effects on infant development. Curr Opin Clin Nutr Metab Care 4:123–126
Grant T, Beer T, Campbell PK, Batten D (2008) Life cycle assessment of environmental outcomes and greenhouse gas emissions from biofuels production in Western Australia. Department of Agriculture and Food Government of Western Australia. CSIRO, Western Australia
Holland FA, Chapman FS (1966) Liquid mixing and processing in stirred tanks. Reinhold Pub. Corp, New York
Hong WK, Yu A, Heo SY, Oh BR, Kim CH, Sohn JH, Yang JW, Kondo A, Seo JW (2013) Production of lipids containing high levels of docosahexaenoic acid from empty palm fruit bunches by Aurantiochytrium sp. KRS101. Bioproc Biosyst Eng 36:959–963
Hossain AK, Davies PA (2010) Plant oils as fuels for compression ignition engines: A technical review and life-cycle analysis. Renew Energy 35:1–13
Huntley M, Redalje D (2007) CO2 mitigation and renewable oil from photosynthetic microbes: a new appraisal. Mitig Adapt Strateg Glob Chang 12:573–608
Jain R, Raghukumar S, Sambaiah K, Kumon Y, Nakahara T (2007) Docosahexaenoic acid accumulation in thraustochytrids: search for the rationale. Mar Biol 151:1657–1664
Johnson DT, Taconi KA (2007) The glycerin glut: options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ Prog 26:338–348
Johnson MB, Wen Z (2009) Production of biodiesel fuel from the microalga Schizochytrium limacinum by direct transesterification of algal biomass. Energy Fuel 23:5179–5183
Knothe G (2007) Some aspects of biodiesel oxidative stability. Fuel Process Technol 88:669–677
Knothe G, Sharp CA, Ryan TW III (2006) Exhaust emissions of biodiesel, petrodiesel, neat methyl esters, and alkanes in a new technology engine. Energy Fuel 20:403–408
Lammers PJ, Kerr BJ, Honeyman MS, Stalder K, Dozier WA, Weber TE, Kidd MT, Bregendahl K (2008) Nitrogen-corrected apparent metabolizable energy value of crude glycerol for laying hens. Poult Sci 87:104–107
Leander CA, Porter D, Leander BS (2004) Comparative morphology and molecular phylogeny of aplanochytrids (Labyrinthulomycota). Eur J Protistol 40:317–328
Lee Chang KJ, Mansour MP, Dunstan GA, Blackburn SI, Koutoulis A, Nichols PD (2011) Odd-chain polyunsaturated fatty acids in thraustochytrids. Phytochemistry 72:1460–1465
Lee Chang KJ, Dunstan GA, Abell G, Clementson L, Blackburn S, Nichols PD, Koutoulis A (2012) Biodiscovery of new Australian thraustochytrids for production of biodiesel and long-chain omega-3 oils. Appl Microbiol Biotechnol 93:2215–2231
Lee Chang KJ, Dumsday G, Nichols P, Dunstan G, Blackburn S, Koutoulis A (2013) High cell density cultivation of a novel Aurantiochytrium sp. strain TC 20 in a fed-batch system using glycerol to produce feedstock for biodiesel and omega-3 oils. Appl Microbiol Biotechnol:1-12
Levin DB, Pitt L, Love M (2004) Biohydrogen production: prospects and limitations to practical application. Int J Hydrog Energy 29:173–185
Lewis TE, Nichols PD, McMeekin TA (1999) The biological potential of thraustochytrids. Mar Biotechnol 1:580–587
Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N (2008) Biofuels from microalgae. Biotechnol Prog 24:815–820
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Maas PAY, Kleinschuster SJ, Dykstra MJ, Smolowitz R, Parent J (1999) Molecular characterization of QPX (Quahog Parasite Unknown), a pathogen of Mercenaria mercenaria. J Shellfish Res 18:561–567
Mendes A, da Silva TL, Reis A (2007) DHA concentration and purification from the marine heterotrophic microalga Crypthecodinium cohnii CCMP 316 by winterization and urea complexation. Food Technol Biotechnol 45:38
Monyem A, Canakci M, Van Gerpen JH (2000) Investigation of biodiesel thermal stability under simulated in-use conditions. Appl Eng Agric 16:373–378
Mozaffarian D, Rimm EB (2006) Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 296:1885–1899
Oswald WJ, Golueke CG (1960) Biological transformation of solar energy. Adv Appl Microbiol 11:223–242
Passell H, Dhaliwal H, Reno M, WU B, Ben Amotz A, Ivry E, Gay M, Czartoski T, Laurin L, Ayer N (2013) Algae biodiesel life cycle assessment using current commercial data. J Environ Manag 129:103–111
Penner JE, Lister D, Griggs DJ, Dokken DJ, McFarland M (1999) Aviation and the global atmosphere: a special report of the Intergovernmental Panel on Climate Change. Cambridge University Press
Peterka A (2013) Biofuels: Energy companies buckle up in hope of green aviation takeoff. Environmental and Energy Publishing database http://www.eenews.net/stories/1059974887 Accessed 20 May 2014
Pluske J (2007) Evaluation of glycerine as co-product of biodiesel production for the pig industry. Pork Co-operative Research Center, 1, 1-47 http://www.apri.com.au/1C-101_Glycerine_report.pdf Accessed 20 Apr 2014
Porter D (1990) Phylum Labyrinthulomycota. In: Margulis L, Corliss JO, Melkonian M, Chapman DJ (eds) Handbook of Protoctista. Jones and Bartlett, Boston, pp 388–398
Pyle DJ, Garcia RA, Wen Z (2008) Producing docosahexaenoic acid (DHA)-rich algae from biodiesel-derived crude glycerol: effects of impurities on DHA production and algal biomass composition. J Agric Food Chem 56:3933–3939
Quispe CAG, Coronado CJR, Carvalho JRJA (2013) Glycerol: production, consumption, prices, characterization and new trends in combustion. Renew Sustain Energy Rev 27:475–493
Raghukumar S (2002) Ecology of the marine portists, the Labyrinthulomycetes (thraustochytrids and labyrinthulids). Eur J Protistol 38:127–145
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 86:807–815
Rye L, Blakey S, Wilson CW (2010) Sustainability of supply or the planet: a review of potential drop-in alternative aviation fuels. Energy Environ Sci 3:17–27
Samarasinghe N, Fernando S, Lacey R, Faulkner WB (2012) Algal cell rupture using high pressure homogenization as a prelude to oil extraction. Renew Energy 48:300–308
Sheehan J, Camobreco V, Duffield J, Graboski M, Shapouri H (1998a) Life cycle inventory of biodiesel and petroleum diesel for use in an urban bus. Final report. National Renewable Energy Lab., Golden, Colorado (USA)
Sheehan J, Dunahay T, Benemann J, Roessler P (1998b) Look back at the U.S. Department of Energy’s Aquatic Species Program: biodiesel from algae; close-out report. National Renewable Energy Lab., US Department of Energy. Golden, CO. (USA)
Stephens E, Ross IL, King Z, Mussgnug JH, Kruse O, Posten C, Borowitzka MA, Hankamer B (2010) An economic and technical evaluation of microalgal biofuels. Nat Biotechnol 28:126–128
Stratton RW, Wong HM, Hileman JI (2011) Quantifying variability in life cycle greenhouse gas inventories of alternative middle distillate transportation fuels. Environ Sci Technol 45:4637–4644
US DOE (2010) National algal biofuels technology roadmap. U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Biomass Program/www1.eere.energy.gov/biomass/pdfs/algal_biofuels_roadmap.pdf. Accessed 18 Mar 2011
Wahlen BD, Morgan MR, McCurdy AT, Willis RM, Morgan MD, Dye DJ, Bugbee B, Wood BD, Seefeldt LC (2013) Biodiesel from microalgae, yeast, and bacteria: engine performance and exhaust emissions. Energy Fuel 27:220–228
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329:796–799
Yan D, Lu Y, Chen Y-F, Wu Q (2011) Waste molasses alone displaces glucose-based medium for microalgal fermentation towards cost-saving biodiesel production. Bioresour Technol 102:6487–6493
Acknowledgments
The authors thank the Commonwealth Scientific and Industrial Research Organisation (CSIRO) Energy Flagship and Food Futures Flagship for their support, Dr. David Batten for comments on an earlier version of this manuscript, and the helpful comments from two anonymous journal referees and Prof. Michael A. Borowitzka. Kim Jye Lee Chang was supported by a CSIRO Office of the Chief Executive (OCE) postdoctoral fellowship through the CSIRO Intelligent Processing Transformational Capability Platform (IP-TCP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee Chang, K.J., Rye, L., Dunstan, G.A. et al. Life cycle assessment: heterotrophic cultivation of thraustochytrids for biodiesel production. J Appl Phycol 27, 639–647 (2015). https://doi.org/10.1007/s10811-014-0364-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0364-9