Abstract
Individuals with autism spectrum disorder (ASD) may demonstrate atypical autonomic (ANS) responses; however, research remains inconsistent. This study examined parasympathetic response during social evaluation in 241 youth (10–13 years) with ASD (n = 138) or typical development (TD; n = 103). Diagnosis, age, pubertal development, and body mass index (BMI) were hypothesized to be associated with ANS function. Linear mixed effects models demonstrated lower RSA in ASD relative to TD in a base model with no covariates. However, when accounting for differences in BMI, there was no evidence of atypical parasympathetic regulation in youth with ASD. As lower parasympathetic regulation may increase susceptibility for a number of conditions, it will be important to elucidate the link between BMI and the ANS, especially in ASD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is a complex and common neurodevelopmental disorder. Recent estimates suggest that 1 in every 54 children is diagnosed with ASD by the age of 8 years old, increasing public awareness and concern to how society may best support these individuals and their families (Maenner et al., 2020). ASD is characterized by deficits in two primary domains, including impaired social communication and interaction, as well as restricted and repetitive behaviors and interests (APA, 2013). For individuals with ASD, difficulties in social communication often manifest as decreased social reciprocity and less engagement with peers (Humphrey & Symes, 2011). The complexities of the social world require continuous interpretation and response to situations appropriate to context, which may be especially difficult and stressful for older children and adolescents with ASD because of increased social demands and onset of puberty (Picci & Scherf, 2015). By examining physiological stress responses to social stressors, we may be able to elucidate how youth with ASD interact with their social world, as well as the extent to which this changes during adolescence.
The Autonomic Nervous System (ANS) plays an important role in physiological regulation and response to stress. The parasympathetic (PNS) and sympathetic (SNS) branches of the ANS function primarily in opposition—the PNS acts to conserve energy (‘rest and digest’), the SNS is responsible for threat mobilization (‘fight or flight’). The ANS has been proposed as a behavioral regulator, with the balance and pattern of responses between the PNS and the SNS critical to shaping response to changing environmental conditions (Berntson et al., 2008). The dynamic ability of the autonomic system to flexibly adapt to changing demands may be important for emotional processing and responding, while an inflexible, invariable system is often characteristic of pathological states, such as anxiety disorders (Friedman, 2007; Friedman & Thayer, 1998; Thayer et al., 1996, 2000).
The PNS and SNS both project to the sinoatrial (SA) node of the heart, influencing beat-to-beat variability in heart rhythm (heart rate variability; HRV). Parasympathetic regulation is frequently indexed using respiratory sinus arrhythmia (RSA), which represents the high frequency heartbeat intervals that fluctuate with respiration (Berntson et al., 1993). RSA increases with greater output of the parasympathetically-mediated vagal nerve, and is sensitive to cholinergic but not adrenergic blockade, supporting its use as a sensitive marker of PNS functioning (Berntson et al., 1993; Cacioppo et al., 1994).
According to the Polyvagal Theory (Porges, 1995, 2001, 2003a, 2007), the default physiological state is one in which the parasympathetically-mediated vagus nerve maintains a ‘brake’ on the heart, slowing heart rate. In the presence of a stressor, however, vagal influence on the heart is said to withdraw, reducing cholinergic input to the SA node (Benarroch, 2012) and allowing for an adaptive increase in cardiac output without engaging the more metabolically demanding SNS (Porges, 2001, 2007; Wolff et al., 2012). For example, in a meta-analysis of 44 studies in children examining response to a stressor, greater parasympathetic withdrawal was associated with fewer externalizing and internalizing symptoms; however, clinical/at-risk groups (e.g., attention deficit/hyperactivity, social phobia, suicidal, etc.) displayed less decline in parasympathetic regulation in response to a stressor (Graziano & Derefinko, 2013). Further, young children who demonstrate increased vagal flexibility and changes in response to stressors also show diminished sympathetic activation to a number of stressful conditions when provided with social support (Wolff et al., 2012). The regulatory capacity of the vagal ‘brake’ may therefore have substantial long-term impacts on physical and psychological health, as chronic SNS hyperactivity is associated with a number of illnesses and psychopathologies, such as inflammation (e.g. Marvar & Harrison, 2012), depression (e.g. Gold, 2015; Thayer et al., 1998), and anxiety (e.g. Brosschot et al., 2006; Friedman, 2007; Thayer et al., 1996).
This vagal flexibility (Berntson et al., 2008) is also said to have an important role in regulating social behavior. The parasympathetically-mediated ‘social engagement system’ (Porges, 2001, 2003b) includes interconnected brainstem nuclei that regulate the myelinated vagus as well as cranial nerves directly involved in controlling muscles of the face and head. Calm, restful visceral states are said to promote these pro-social behaviors such as eye contact and language production; however, dysregulation of the vagal system and/or mobilization of the fight or flight system will block this social engagement system and prevent activation of the facial muscles (Porges, 2003a, b, 2007). Empirical support for the social engagement system includes evidence that individuals who demonstrate more social cooperation and engagement tend to have higher PNS regulation (Beffara et al., 2016; Kogan et al., 2014; Lischke et al., 2018). Additionally, young adults with higher vagal tone are found to be more socially engaged than their peers with lower PNS regulation (Geisler et al., 2013). Dysfunction within the vagal and social engagement system would therefore presumably lead to deficits in a number of socially adaptive behaviors, which may have significant implications for a number of clinical populations, including ASD.
Increasing evidence supports atypical autonomic regulation and arousal in individuals with ASD. A number of studies cite lower baseline parasympathetic regulation in ASD relative to individuals with typical development (TD) (e.g., Bal et al., 2010; Edmiston et al., 2016; Guy et al., 2014; Ming et al., 2005; Neuhaus et al., 2016; Vaughan Van Hecke et al., 2009). Young, preschool-aged children with ASD and higher resting PNS arousal have been reported to gesture more and engage in more sharing behavior (Patriquin et al., 2013). In contrast, in youth with ASD, those with lower baseline parasympathetic values demonstrate increasingly severe social symptoms (Edmiston et al., 2016; Vaughan Van Hecke et al., 2009). There is further evidence for differences in PNS stress reactivity or responsivity to various tasks. During a sensory challenge protocol, young children with ASD demonstrated significantly less change in RSA from rest to individual sensory stressors, providing support for reduced vagal flexibility in the ASD group (Schaaf et al., 2015). In response to an attention-demanding processing task, there further appears an atypical lack of vagal withdrawal in children ASD, who instead showed an increase in RSA to the task (Porges et al., 2013). In social contexts, school-aged youth with ASD have evidenced blunted RSA relative to TD peers during social interactions (Neuhaus et al., 2016; Vaughan Van Hecke et al., 2009), when higher RSA would be expected to promote the parasympathetically-mediated social engagement system (Porges, 2003b).
Despite the findings above, evidence for atypical autonomic functioning in ASD remains inconsistent and likely dependent on numerous influential factors, such as context, developmental influences, symptom severity, and more. Some studies have not found differences in parasympathetic (e.g., Kushki et al., 2014; Levine et al., 2012; Muscatello et al., 2020; Watson et al., 2012) or sympathetic (Edmiston et al., 2017b; Neuhaus et al., 2016; Schaaf et al., 2015) functioning at rest in youth with ASD. While some studies have examined parasympathetic regulation during various stressors and reported lower RSA across contexts (e.g. Edmiston et al., 2016; Guy et al., 2014), they have not reported a significant difference in parasympathetic reactivity to social evaluative stress between youth with and without ASD (Corbett et al., 2019a; Edmiston et al., 2016; Hollocks et al., 2014; Kushki et al., 2014; Sheinkopf et al., 2013). This suggests an important distinction between overall parasympathetic tone versus acute vagal flexibility and highlights the need to not only distinguish between the two, but also to further understand the potential implications of maladaptive states of each, especially for youth with ASD.
These inconsistencies within the ASD literature emphasize the need to examine associated factors which may influence physiological states and responses to stress. It is plausible that the type of stressor may contribute to the reported inconsistencies. Previous research examining another stress system, the hypothalamic pituitary axis (HPA) indexed by increases in salivary cortisol, has shown that situations that rely on social communication and interaction with peers often result in enhanced stress response in children with ASD (Corbett et al., 2010, 2014; Lopata et al., 2008; Schupp et al., 2013). Similarly, within the sympathetic branch of the ANS, there is evidence for increased sympathetic influence during interactions with a familiar partner in boys with ASD compared to TD (Neuhaus et al., 2016). However, other contexts such as social evaluation that trigger an adaptive stress response in TD youth, do not consistently promote a cortisol or sympathetic response in youth with ASD (e.g., Corbett et al., 2019a, 2021; Edmiston et al., 2017b; Lanni et al., 2012; Levine et al., 2012). These findings suggest that not all social contexts are equally salient for youth with ASD. Moreover, developmental factors, such as age and puberty, have contributed to differences between youth with ASD and with TD in the HPA axis (Corbett et al., 2010, 2021; Muscatello & Corbett, 2018; Schupp et al., 2013). Similar developmental effects might be present for the autonomic system but have not been explored.
It is also necessary to acknowledge the autonomic system may be influenced by physical health variables, such as body weight, height, and body mass index (BMI) (Masi et al., 2007; Molfino et al., 2009; Shibao, 2012; Thayer & Sternberg, 2006). Specifically, BMI, which is an index of the amount of body fat, can alter cardiac functioning and therefore impact ANS output. Youth with ASD have been reported to show elevated BMI relative to youth with TD (e.g., Corbett et al., 2020; Healy et al., 2019; Hill et al., 2015; McCoy & Morgan, 2020; Must et al., 2017).
Despite the potential influence of these recognized developmental (age, puberty) and physical (BMI) factors, research of autonomic functioning in school-age and adolescent youth with ASD has been inconsistent in controlling for many of these factors, which may be influencing the significant variability in findings. Previous research in TD populations have noted steadily increasing HRV throughout development (e.g., Eyre et al., 2014) but decreased PNS activity in obese children and adolescents (e.g., Eyre et al., 2014; Rabbia et al., 2003). Despite such findings, few studies in ASD have examined age (Harder et al., 2016; Kushki et al., 2014; Ming et al., 2005; Muscatello et al., 2021) or pubertal effects (Edmiston et al., 2016). To our knowledge, no studies have directly examined the effects of physical health variables such as BMI on ANS functioning in autism, and only a limited number of studies explicitly controlled for or matched diagnostic groups on BMI (Bricout et al., 2018; Harder et al., 2016; Muscatello et al., 2020).
The primary goal of the study was to examine parasympathetic regulation and response to a social evaluative threat paradigm in a large, well-characterized sample of youth, ages 10–13 years, with and without ASD. We sought to identify expected differences between groups in response to the Trier Social Stress Test (TSST; Kirschbaum et al., 1993). Further, in an effort to address inconsistencies in previous research, we evaluated factors expected to contribute to ANS response. Based upon the extant literature (e.g. Neuhaus et al., 2016; Thayer & Sternberg, 2006; Vaughan Van Hecke et al., 2009), it was hypothesized that: (1) youth with TD would demonstrate more parasympathetic regulation and vagal flexibility, with higher RSA on average as well as a larger decrease in RSA in response to the social evaluation, compared to youth with ASD who would show a blunted parasympathetic profile, (2) higher pubertal status and older age would predict higher resting RSA and greater change in RSA from baseline, and (3) higher BMI would predict lower RSA and a blunted slope in response to social evaluation in ASD and TD youth.
Methods
Participants
Data were collected as part of a longitudinal study on pubertal development and stress (Corbett, 2017). The current study includes data from Year 1 enrollment when the children were between 10-years-0-months and 13-years-11-months of age. The total enrolled sample included 241 youth with ASD (n = 138) or TD (n = 103). The ASD group comprised 102 males and 36 females, while the TD group included 57 males and 46 females. Demographic information for the full sample is presented in Table 1.
All participants were required to have an intelligence quotient (IQ) score ≥ 70 as estimated by the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II, (Wechsler, 2011)). Participants in the ASD group had a diagnosis of ASD based on the Diagnostic and Statistical Manual-5 (APA, 2013) and confirmed by: (1) a previous diagnosis by a psychologist, psychiatrist, or behavioral pediatrician with autism expertise; (2) current clinical judgment, and (3) corroborated by the Autism Diagnostic Observation Schedule (ADOS-2; Lord et al., 2012), a semi-structured interview-based instrument administered by research-reliable personnel. Additionally, TD participants were determined to have no other developmental delay or neurodevelopmental diagnosis and were required to score < 10 on the Social Communication Questionnaire (SCQ; Rutter et al., 2003), which is a parent-report questionnaire used to screen for ASD symptoms.
As the larger longitudinal study included examination of hormonal responses, exclusion criteria for both groups included current use of medications known to alter the Hypothalamic–Pituitary–Adrenal (HPA) axis [e.g., corticosteroids; see (Granger et al., 2009)] or HPG axis (e.g., growth hormone), or medical conditions known to impact pubertal development (e.g., Cushing’s Disease). Also, participants taking oral contraceptives, growth hormones, or nicotine all known to influence the HPA axis, were excluded (Foley & Kirschbaum, 2010; Kirschbaum et al., 1995). An estimated 42% of children with ASD have been reported to take at least one psychotropic medication (Mire et al., 2014), thus the current study did not include a completely medication-naïve sample to be more representative of the overall ASD population. In the ASD group, 65.2% of youth were taking at least one medication, while 17.5% of TD participants reported taking a daily medication.
The Vanderbilt Institutional Review Board (IRB) approved the study, which was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All parents/guardians provided informed written consent and youth participants gave verbal assent prior to inclusion in the study.
Procedures
Participation included two research visits to the University. On Visit 1, the diagnostic (ADOS-2) and cognitive measures (WASI-II) were administered. Parents completed the Social Responsiveness Scale, 2nd Edition (Constantino & Gruber, 2012), which covers several areas of social functioning symptoms, including social motivation, communication, awareness, and cognition, as well as repetitive behaviors and interests. Additionally, participants completed a brief physical examination, as described below. On Visit 2, participants were exposed to the stress paradigm, the TSST-Child Version (Buske-Kirschbaum et al., 1997; Kirschbaum et al., 1993).
Physical Examination (PE)
Participants completed a physical examination, conducted by trained, licensed, study physicians to assess pubertal development, height, and weight. Recent research with the sample comparing physical exam to parent- and self-report demonstrated that physical exam is the optimal approach for more precise pubertal measurement (Corbett et al., 2019b). Therefore, physical exam scores were used in the current study.
The PE was completed to reliably identify pubertal development and assign Tanner stage (Marshall & Tanner, 1969, 1970). The exam ascertained two measures with five stages for Genitals (G1-G5 for boys) or Breasts (B1-B5 for girls; GB stage) and Pubic hair (P1-P5 for both genders; PH stage). The exam, as described previously by Corbett et al., 2019b, consisted of visual inspection and categorization of pubertal and genital maturation. To be consistent with the original Tanner staging and to maximize participation, palpation of breasts or measurement of testes was not conducted.
Height and weight were measured once using a calibrated stand-on Health-o-meter TM Professional 499KL Waist High Digital Scale with Height Rod (Hogentogler & Co., MD, USA). Height was measured to the nearest inch and weight was measured to the nearest 0.1 lb. Children were weighed and measured in light clothing. BMI was calculated using the standard formula (lb/in2) × 703 for use with the Center for Disease Control (CDC) growth charts for children and adolescents (2 through 19 years; https://www.cdc.gov/healthyweight/bmi/calculator.html) in order to convert to percentiles for statistical analyses.
Social Stress Paradigm
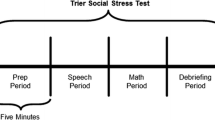
The TSST-Child Version (Buske-Kirschbaum et al., 1997; Kirschbaum et al., 1993) is a well-validated, experimentally induced psychosocial stressor known to reliably activate a physiological stress response in TD populations (Kudielka et al., 2004) including children and adolescents (Seddon et al., 2020). The TSST is a 20-min task divided into four subcomponents: (1) Intro/Preparation, (2) Present Speech, (3) Serial Subtraction, and (4) Debriefing/Recovery. The protocol involves a scenario in which the participant must deliver the ending to a short story in front of a panel of judges (unresponsive judges showing neutral facial expressions) who will, purportedly, be judging the child’s performance against that of other children. For the protocol, mixed-age (adult and peer), as well as mixed-sex judges were used since this contributes to a stronger effect than using female judges only (Seddon et al., 2020). The 5-min speech task is followed by a 5-min serial subtraction task. Of the 241 enrolled participants, 212 completed the full TSST protocol, with 29 participants failing to return for the second visit. There was no difference in number of youth with ASD (n = 17) or TD (n = 12) who did not return for Visit 2 [χ2(1) = 0.02, p = 0.87].
Respiratory Sinus Arrhythmia (RSA)
RSA was collected using MindWare Mobile Impedance Cardiograph units (MindWare Technologies LTD, Gahanna, OH) for synchronized electrocardiography (ECG) and respiration data collection using a seven-electrode configuration. Participants were told they would be wearing ‘stickers’ throughout the protocol. Following an explanation of the electrode locations on the torso, participants were given the opportunity to place an electrode on their hand prior to placement. After placement, there was a five-minute acclimation period before initiating the baseline collection protocol (explained below). Of the 212 participants that completed the stress paradigm, four participants with ASD did not have HRV data due to an inability to tolerate the sensory aspects of the protocol. An additional three participants were missing RSA data due to use of anticholinergic medication (1 ASD), atypically low RSA values (1 ASD), or atypical heart rhythms (1 TD).
Baseline RSA was acquired using a 5-min resting collection period in which participants were instructed to sit quietly without engaging in other tasks. Following the baseline period, participants were taken to a separate room for the TSST protocol, at which point they received instructions for the task from the two judges. The participants were escorted back to the first assessment room, where they were given five minutes to prepare their speech. RSA collection started immediately prior to the preparation period (Prep) and was collected continuously throughout the TSST paradigm, calculated on a minute-by-minute basis and averaged into five-minute epochs for each major period of the paradigm- Baseline, Prep, Speech, Math, and Recovery (see Fig. 1).
RSA was derived in accordance with guidelines set forth by the Society for Psychophysiological Research committee on heart rate variability (Electrophysiology, 1996). ECG signal was sampled at 500 Hz and analyzed using the Heart Rate Variability Software Suite provided by MindWare Technologies (MindWare Technologies LTD, Gahanna, OH). RSA was quantified as the integral power within the respiratory frequency band (0.12–0.40 Hz), and respiration was monitored by impedance cardiography. The respiration signal was displayed to ensure that values were within the designated frequency band. Respiratory frequency was confirmed to lie within the high frequency/RSA band (0.12–0.40 Hz) for all participants. Of the total processed data, 3.5% were excluded due to excessive motion artifact, equipment error, or cardiac arrhythmias. RSA was measured in ms2.
Statistical Analysis
All hypotheses were tested using linear mixed effects models with a random intercept for subject to account for correlation within subjects. To test the hypothesis that youth with TD would demonstrate higher RSA levels on average, we fit a baseline model to predict RSA levels adjusted for sex, diagnosis and a covariate indicating timepoint within the TSST protocol. To test whether there was a larger decrease in RSA in response to the social evaluation in TD youth, an interaction term between diagnosis and timepoint was included. We tested the second and third hypotheses by adding age, PH stage, BMI, and their interactions with TSST time point to the model. To test the second hypothesis that higher pubertal status and older age would be associated with higher resting RSA, age, PH stage and their interactions with time were tested separately. Finally, to test the third hypothesis that higher BMI would predict lower RSA, we tested BMI and the interaction between BMI and time. The model was repeated using GB stage as well. For all models, we performed Wald F-tests using Type 2 sum of squares in order to test the main effects prior to including the interaction effects. All analyses were performed using R version 4.0.3. R packages used are listed in Supplementary Materials.
Results
Table 1 presents demographic and clinical characteristics of participants including age, sex, race, medication use, IQ, ADOS, BMI, Pubic Hair (PH) stage, Genital/Breast (GB) stage, and RSA across the TSST. The median age was similar for both groups—11.67 for TD youth and 11.25 for ASD youth. Median BMI percentile, which accounts for age and sex, was much lower in TD youth (52.5) compared to ASD youth (71). A majority of participants were in Stage 1 or 2 of PH and GB stages. The distribution of the sample included 7.9% African American, 83.0% Caucasian, 0.4% Asian, and 8.7% Mixed. Results of RSA levels at each stage are presented in Table 1.
Results from Linear Effects Modeling
Baseline Model
In order to test the first hypothesis, we fit a model including sex, diagnosis, timepoint, and the interaction between diagnosis and timepoint to investigate the effect of diagnosis on RSA response to the TSST (Table 2). In this model, RSA varies significantly across time (p < 0.0001) and is different between groups. Specifically, lower RSA levels, consistent with decreased PNS regulation, are associated with ASD diagnosis (p = 0.022) (Fig. 2). However, there was no evidence that the pattern of change in RSA levels across time (PNS responsivity) are different between TD and ASD groups (diagnosis by timepoint interaction: p = 0.32). Both groups display similar patterns of increased RSA during the Prep and Recovery stages and decreased RSA in the Speech and Math stages. See Supplementary Table S1 for parameter estimates.
Models with Age, Puberty, and BMI
To test the second hypothesis that there are associations between development and RSA, we added age and PH stage and their interaction terms with time. Age and PH stage did not contribute substantially over the effects of the other covariates and the effect of diagnosis was reduced (p = 0.11). There were no interaction effects between age and time (p = 0.073) and PH stage and time (p = 0.52). Therefore, we did not find sufficient evidence for an association between age and PH stage with RSA values (Table 3).
To test the third hypothesis that higher BMI would be associated with lower RSA overall and would result in less change in RSA relative to baseline, we included an interaction between BMI and time in the model (Table 3; Supplementary Table S2 for parameter estimates). In contrast to the lack of associations for age and PH stage, results showed that higher BMI was strongly associated with lower RSA levels (p = 0.040), but there was no interaction effect between BMI and time (p = 0.68). Figure 3 shows the plot of RSA levels across stages for various BMI, demonstrating youth with elevated BMI tended to show lower overall RSA values.
To investigate whether results differed based on type of Tanner stage, we fit this same model with GB stage and found similar results (see Supplementary Tables S3 and S4). There were no interaction effects between age and time, GB stage and time, and BMI and time, though BMI was predictive of RSA levels (p = 0.029). Similar to the model fitting PH stage, diagnosis was not predictive of RSA.
Preliminary Post-Hoc Analysis of Social Communication Symptoms
The lack of diagnostic group differences suggests a need to consider individual traits and behaviors and their relation to autonomic functioning. Exploratory analyses were conducted to investigate the extent to which social communication symptoms, as measured by the SRS-2 (Constantino & Gruber, 2012), were associated with differences in RSA, regardless of diagnosis. A linear model including the main effects of PH stage, BMI, time, and SRS Total T score tested the association of individual physical and behavioral characteristics with RSA response to the TSST (Table 4). Significant effects were observed with SRS scores (p = 0.025) and with BMI (p = 0.049), where RSA levels decreased with increasing BMI and with more social symptoms (elevated SRS T score) (Fig. 4). Similar findings were observed in a model with GB Stage (Supplemental Table S5).
Estimated RSA during TSST across SRS Total T Scores. Plot depicts model results averaged across sex, PH stage, age, and BMI percentile. SRS scores have been chosen as the following: 10th percentile (43), 25th percentile (47), 50th percentile (64), 75th percentile (78) and 90th percentile (86). More severe social symptoms (high SRS score) are associated with lower RSA at every timepoint of the TSST
Discussion
The current study sought to examine parasympathetic response to the TSST in youth with and without ASD, predicting differences between youth with ASD and with TD at rest and in response to the TSST. Specifically, we hypothesized that youth with ASD would demonstrate an atypical parasympathetic response, illustrated by lower resting baseline RSA and blunted reactivity to social evaluation. Factors that may influence ANS response were considered, including age, puberty, and BMI, as we expected them to be associated with parasympathetic regulation and response.
The first model showed lower RSA in the ASD group compared to the TD group, supporting the hypothesis that youth with ASD would demonstrate reduced parasympathetic regulation. However, there was no diagnosis by timepoint interaction, suggesting groups did not differ in RSA reactivity and therefore, the first hypothesis was only partially supported. Both groups demonstrated significant changes in RSA in response to the TSST, including an initial increase in PNS regulation during the prep period, followed by the expected decrease in RSA in response to the TSST speech and math tasks. Importantly, both groups did not differ in the change in slopes over time, suggesting the groups display similar patterns of change and vagal flexibility during a social evaluative stress paradigm, though the youth with ASD experience slightly decreased parasympathetic regulation on average.
The reduced parasympathetic regulation in ASD youth suggests they may be in a state of heightened arousal with less influence from the PNS, which promotes calmer, relaxed states. The total extent to which elevated arousal may affect social or psychological functioning in ASD is unknown. Low vagal tone has been associated with increased incidence of anxiety (e.g., Beauchaine, 2015; Friedman & Thayer, 1998; Kushki et al., 2013; Thayer et al., 1996) as well as decreased social skills (e.g., Patriquin et al., 2019; Porges, 2007). In the current study, social symptoms often characteristic of ASD were correlated with lower RSA regulation, regardless of diagnosis. While we did not evaluate potential comorbid symptoms in this sample of youth, identifying physiological differences in a sample as young as 10–13 years of age suggest an important need to examine the relationship between autonomic and socioemotional functioning in ASD, especially as these youth transition through the adolescent period.
Interestingly, despite apparent differences in the overall parasympathetic regulation within this sample, no differences in reactivity were observed. This distinction may be an important one, whereas baseline vagal activity is suggested to be linked to temperamental states, but RSA reactivity may be linked to attention and engagement with the environment, as well as emotional regulation or mood stability (Beauchaine, 2001). As reviewed by Beauchaine (2001), depression and anxiety, among other emotional traits, are more commonly related to baseline parasympathetic tone, while emotional states, such as panic or anger, are related to atypical vagal flexibility or withdrawal. Therefore, in the context of our findings, youth with ASD may be more susceptible to symptoms of anxiety and depression even though attention and emotional processes involved in the TSST response do not significantly differ relative to youth with TD.
It is important to note that the current study stands in contrast to previous findings in other stress systems, such as the HPA axis and sympathetic nervous system. It has previously been shown that youth with ASD actually demonstrate less cortisol release or sympathetic activation (i.e., lower stress response) to the TSST relative to peers with TD, suggesting they do not perceive the paradigm to be particularly stressful (Corbett et al., 2019a, 2021; Lanni et al., 2012). Indeed, recent research has shown that children with ASD who demonstrate blunted cortisol response to the TSST also tend to more frequently mis-identify neutral faces (Corbett et al., 2019a). Further, more severe social symptoms characteristic of ASD are related to a blunted sympathetic response (Edmiston et al., 2017b). Impaired ability to recognize facial affect and other social cues may limit a child’s ability to identify the situation as threatening and evaluative. Given this, it was hypothesized that a similar pattern of responses would be observed in the current sample for parasympathetic regulation, with a blunted RSA withdrawal response to the stressor.
The lack of similar findings within the PNS could be due to several factors, most notable of which is the distinct roles these two systems play in regulating the body’s physiological stress response. The parasympathetic system and HPA axis have largely opposing functions within the body, where the HPA axis is tightly coupled to the sympathoexcitatory circuits of the ANS. The vagus nerve may also be involved in HPA axis regulation, exerting inhibitory control over cortisol release (Thayer & Sternberg, 2006; Thayer et al., 2006). Similarly, the sympathetic system, much like the HPA axis, tends to promote more metabolically-demanding stress responses and is inhibited by the vagal system (e.g., Thayer & Brosschot, 2005; Thayer & Lane, 2009; Ulrich-Lai & Herman, 2009). The sympathetic branch of the ANS can be described as mediating ‘fight or flight’ physiological responses, and thus may be more susceptible to real or perceived threats. Indeed, there is some evidence for atypical, possibly maladaptive sympathetic responses to stress in ASD (Edmiston et al., 2017b; Neuhaus et al., 2016; Schaaf et al., 2015). There exists a tight coupling between the branches of the ANS, though a simple, reciprocal relationship cannot be assumed (Berntson et al., 2008). Therefore, future research should investigate stress response across the PNS, SNS, and HPA axis to elucidate a more thorough and complete picture of the physiological profile in youth with ASD (Muscatello et al., 2020, 2021) and the extent to which responses of these systems relate to observed behaviors and emotional states.
It is further acknowledged that the chosen stress condition may not have been optimal to measure RSA reactivity. Differences in social context have revealed dissociations in stress response in youth with ASD between social evaluation (TSST) and social interaction (Corbett et al., 2012; Edmiston et al., 2017a; Lanni et al., 2012), such that social evaluative threat did not mount a significant stress response, whereas a friendly, social interaction resulted in elevated stress for youth with ASD. Similarly, the extent to which the TSST is an appropriate paradigm to look for possible underlying parasympathetic differences must be considered. In other words, the TSST may not activate the parasympathetically-mediated ‘social engagement system’ (Porges, 2001, 2003b) and therefore group differences would not be present. There is limited reciprocal communication in the TSST, as the raters are instructed not to provide feedback. Therefore, the task is more a ‘performance’ than a ‘reciprocal conversation.’ It may be the case that a task that promotes social cooperation and engagement would be more likely to lead to differences in PNS regulation and reactivity (Beffara et al., 2016; Kogan et al., 2014; Lischke et al., 2018; Muscatello et al., 2021). Additionally, findings of an association between SRS scores and RSA further suggest social functioning, communication, and engagement may play a critical role in regulating parasympathetic responses. The role of context may underlie the lack of difference in typical or atypical response depending upon the stressor, and future research should examine the extent to which different social scenarios activate the parasympathetic response.
Extending beyond diagnostic differences in parasympathetic response, we examined the hypotheses that age, puberty, and BMI would be related to RSA regulation and response. Concerning the hypothesized developmental effects, neither age nor pubertal stage (either GB or PH stage) were significant predictors of RSA. While previous evidence supports developmental effects on the HPA axis stress response in youth with ASD (Corbett et al., 2021; Muscatello & Corbett, 2018; Schupp et al., 2013), the extant research is more limited for autonomic response and development in ASD. Nevertheless, evidence within typically developing populations suggests age, and to a more limited extent, puberty, may influence parasympathetic response. For example, in a study of children ages 8–15 years, younger children demonstrated significantly larger decreases in RSA relative to baseline compared to older children during a cognitive tracing task (El-Sheikh, 2005). Notably, no group differences were observed during a social stressor task involving listening to an argument, which may be more closely aligned to the social components of the TSST paradigm. Trends in previous literature suggest resting parasympathetic regulation tends to increase through childhood (e.g. Gentzler et al., 2012; Hinnant et al., 2011; Michels et al., 2013), though there may be substantial variability in these trends by late childhood and early adolescence (Gentzler et al., 2012). Similarly, some limited relationships between pubertal status and RSA response have been observed, where higher stages of biological maturity are associated with lower levels of RSA suppression during a cognitive stressor (El-Sheikh, 2005). Though empirical evidence of pubertal effects on the parasympathetic system is minimal, biological maturity remains an important variable to consider in studies of RSA regulation and reactivity (Allen & Matthews, 1997; Hinnant et al., 2018). Notably, the majority of the sample was in Tanner stages 1 and 2 (73% TD and 72% ASD), and therefore it may be the case that as the youth mature over the course of the 4-year longitudinal study, pubertal and age effects may begin to emerge within the increased variability.
In contrast, the hypothesis that BMI would predict parasympathetic regulation was supported, as youth with elevated BMI also demonstrated significantly lower RSA on average. This finding is consistent with previous research showing a relationship between BMI and autonomic functioning (e.g., Eyre et al., 2014; Landsberg, 1986; Rabbia et al., 2003), including observations that these relationships are present not only in high-risk, obese patients, but also in non-obese, healthy individuals (Molfino et al., 2009), suggesting a close relationship between body mass and physiology. Further emphasizing this point, adult patients that lost at least 10% of their body weight demonstrated significant gains in parasympathetic control, along with decreases in the sympathetic system (Arone et al., 1995). Therefore, high parasympathetic regulation is generally considered to be a positive marker of health status and reduced risk for a variety of health conditions (Masi et al., 2007; Thayer & Sternberg, 2006). Similarly, decreasing BMI through weight loss reduces risk for a variety of health conditions such as type 2 diabetes or high blood pressure (Vidal, 2002).
Childhood obesity remains a significant public health concern in the United States, and rates of overweight and obesity are particularly high in ASD, with estimates ranging from 18.0 to 42.0% overweight and 10.0–30.4% obese (Corbett et al., 2020; Criado et al., 2018; Curtin et al., 2010, 2014; Whiteley et al., 2004; Zuckerman et al., 2014). Prevalence of elevated BMI ostensibly rise in youth with ASD as they enter the adolescent period, where recent studies have noted increases in BMI as youth with ASD advance through puberty (Corbett et al., 2020). There may be a variety of factors which contribute to this prevalence of overweight and obesity in ASD, with medication side effects (e.g., Curtin et al., 2014) or reduced physical activity (such as from peer isolation) (e.g., Must et al., 2014) being just a few contributing factors. No matter the reason, the physical and psychological consequences cannot be overstated, with an increased risk for several health conditions (e.g., type 2 diabetes, hypertension) (e.g., Bray, 2004), as well as elevated incidence of depression, low self-esteem, and peer victimization (see Rankin et al., 2016 for review) in those with overweight/obesity.
Finally, it should be noted that in the larger model including BMI, as well as puberty and age, there was no longer a significant main effect for diagnosis. In a recent report with the current sample, significant differences in BMI were observed such that adolescents with ASD showed significantly greater BMI on average compared to their peers with typical development (Corbett et al., 2020). It is likely these BMI differences also contributed to RSA regulation and response to the TSST, as demonstrated in Fig. 3, where youth with higher BMI had lower RSA regardless of diagnosis.
The use of physiological markers like RSA has become increasingly common, with studies investigating the feasibility of using RSA and heart rate variability to index emotional stress (Beauchaine, 2015), anxiety (Thayer et al., 1996), and depression (Rottenberg, 2007; Rottenberg et al., 2007). However, this technique is not without its limitations. There are a number of variables which may impact the quantification and interpretation of HRV, including, but not limited to, respiratory rates, physical activity or movement, and posture (see Berntson et al., 1997; Grossman & D’Augelli, 2007 for review). For example, ECG abnormalities and abnormal beats can alter the validity of RSA (Berntson et al., 1997), and obesity and extremes in BMI have been associated with these ECG abnormalities (Fraley et al., 2005). The findings from the current study reveal that BMI is a significant driver of RSA response and regulation. Within a sample that significantly differs in overweight/obesity status (Corbett et al., 2020), the contributions of BMI may be driving many of the observed diagnostic effects reported in initial models and therefore highlight the need to control for such covariates in future analyses given the possible effect on findings. Moreover, additional analyses considering social functioning, but not diagnosis, showed that those with more severe social symptoms, which are frequently associated with ASD, demonstrated significantly lower RSA on average. Given the notable heterogeneity of the autism diagnosis, future research should focus more on unique traits and characteristics to determine whether individuals with ASD and certain physical and behavioral profiles are more likely to experience atypical autonomic functioning.
Clinical implications
The nature of parasympathetic dysfunction and its relation to stress responses and social functioning in youth with ASD remains difficult to clearly elucidate. Although several of the hypotheses in the current study were not confirmed, an important finding is that BMI was a key predictor in the current results and potentially previous findings. While scientifically interesting, it is clinically concerning that BMI plays such a prominent role in physiological regulation and RSA. Regarding physical health, autonomic imbalance and reduced parasympathetic regulation increase risk for a number of cardiovascular diseases (see Thayer et al., 2010 for review). On the flip side, it has been shown that when individuals lose weight, there is a normalization of RSA response as well as many other encouraging health outcomes, providing support for the use of RSA as an index of poor health status. There is an emerging and alarming literature showing higher rates of overweight and obesity in youth with ASD (e.g., Corbett et al., 2020; Criado et al., 2018), yet there is little-to-no research investigating the extent to which BMI differences may be contributing to observed autonomic dysfunction in some with ASD. Due to the link between BMI and physiological regulation, it is plausible that normalization of body weight may also normalize RSA response, and possibly, further lead to improvements in other physical and mental health symptoms associated with parasympathetic dysregulation in ASD. Considerably more research is required in order to examine these hypotheses and to further understand the link between BMI, physiological regulation, and other diagnostic and health status markers in individuals with ASD.
Limitations and Future Directions
The study was strengthened by the relatively large, well-characterized sample of youth with and without ASD. Additionally, the use of physical exam by a medically-licensed study team member, as opposed to self- or parent-report, ensured accurate reporting of Tanner stage and body mass index. Nevertheless, there are study limitations that should be acknowledged. First, the sample included a relatively narrow age range of 10–13-year-olds, with most youth still in the earlier stages of puberty (Tanner stages 1 and 2). Thus, there may not have been enough variability in the sample to detect development effects. The sample is part of a four-year longitudinal study and follow-up analyses in future years may reveal the influence of age and puberty on physiological response. Second, the study did not enroll youth with ASD with accompanying intellectual impairment, and participants were predominantly White; thereby, results are not fully representative and generalizable to the larger population. Future research including a more racially and ethnically diverse sample of youth with and without intellectual impairment will be important in order to better understand the role of RSA in the broad spectrum of ASD. Lack of diagnostic group-based differences suggest a need to examine individual characteristics and differences in behaviors. While preliminary findings suggest social functioning is related to lower parasympathetic functioning, regardless of diagnosis, further research is needed to identify traits and behaviors which may leave one susceptible to atypical autonomic response. Finally, results of the current study emphasize the need to consider potential covariates when elucidating autonomic regulation and response. Consideration of other confounds such as posture, activity, and respiration, may more clearly elucidate the utility of RSA as a biomarker of stress or psychiatric comorbidities in youth with ASD while controlling for extraneous variables.
Conclusion
The current study demonstrated that when accounting for BMI differences among youth with and without ASD, there is no evidence of atypical parasympathetic reactivity to social evaluation in ASD youth. Physical health, such as BMI, influences resting autonomic regulation, while pubertal effects may become more apparent in later adolescence with more advanced physical maturation. Due to the impact of numerous covariates, diagnostic differences in autonomic functioning should be considered under multiple contexts (e.g., social evaluation vs. social interaction) while accounting for other contributing variables. Considering the potential implications with social functioning, cognition, or psychiatric comorbidities, a consistent and rigorous methodological approach is necessary to identifying the utility of RSA as a biomarker of these symptoms in ASD.
References
Allen, M. T., & Matthews, K. A. (1997). Hemodynamic responses to laboratory stressors in children and adolescents: The influences of age, race, and gender. Psychophysiology, 34(3), 329–339. https://doi.org/10.1111/j.1469-8986.1997.tb02403.x
APA. (2013). Diagnostic and statistical manual of mental disorders (DSM-5) (5th ed.). American Psychiatry Association.
Arone, L. J., Mackintosh, R., Rosenbaum, M., Leibel, R. L., & Hirsch, J. (1995). Autonomic nervous system activity in weight gain and weight loss. American Journal of Physiology, 269(1 Pt 2), R222-225. https://doi.org/10.1152/ajpregu.1995.269.1.R222
Bal, E., Harden, E., Lamb, D., Van Hecke, A. V., Denver, J. W., & Porges, S. W. (2010). Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders, 40(3), 358–370. https://doi.org/10.1007/s10803-009-0884-3
Beauchaine, T. P. (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13(2), 183–214. https://doi.org/10.1017/s0954579401002012
Beauchaine, T. P. (2015). Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology, 3, 43–47. https://doi.org/10.1016/j.copsyc.2015.01.017
Beffara, B., Bret, A. G., Vermeulen, N., & Mermillod, M. (2016). Resting high frequency heart rate variability selectively predicts cooperative behavior. Physiology & Behavior, 164(Pt A), 417–428. https://doi.org/10.1016/j.physbeh.2016.06.011
Benarroch, E. E. (2012). Central autonomic control. In D. Robertson, I. Biaggioni, G. Burnstock, P. A. Low, & J. F. R. Paton (Eds.), Primer on the autonomic nervous system (3rd ed., pp. 9–12). Academic Press.
Berntson, G. G., Cacioppo, J. T., & Quigley, K. S. (1993). Cardiac psychophysiology and autonomic space in humans: Empirical perspectives and conceptual implications. Psychological Bulletin, 114(2), 296–322. https://doi.org/10.1037/0033-2909.114.2.296
Berntson, G. G., Norman, G. J., Hawkley, L. C., & Cacioppo, J. T. (2008). Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology, 45(4), 643–652. https://doi.org/10.1111/j.1469-8986.2008.00652.x
Berntson, G. G., Thomas Bigger, J., Eckberg, D. L., Grossman, P., Kaufmann, P. G., Malik, M., Nagaraja, H. N., Porges, S. W., Philip Saul, J., Stone, P. H., & Van Der Molen, M. W. (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. https://doi.org/10.1111/j.1469-8986.1997.tb02140.x
Bray, G. A. (2004). Medical consequences of obesity. Journal of Clinical Endocrinology and Metabolism, 89(6), 2583–2589. https://doi.org/10.1210/jc.2004-0535
Bricout, V.-A., Pace, M., Dumortier, L., Favre-Juvin, A., & Guinot, M. (2018). Autonomic responses to head-up tilt test in children with autism spectrum disorders. Journal of Abnormal Child Psychology, 46(5), 1121–1128. https://doi.org/10.1007/s10802-017-0339-9
Brosschot, J. F., Gerin, W., & Thayer, J. F. (2006). The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research, 60(2), 113–124. https://doi.org/10.1016/j.jpsychores.2005.06.074
Buske-Kirschbaum, A., Jobst, S., Wustmans, A., Kirschbaum, C., Rauh, W., & Hellhammer, D. H. (1997). Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine, 59(4), 419–426. https://doi.org/10.1097/00006842-199707000-00012
Cacioppo, J. T., Uchino, B. N., & Berntson, G. G. (1994). Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology, 31(4), 412–419. https://doi.org/10.1111/j.1469-8986.1994.tb02449.x
Constantino, J. N., & Gruber, C. P. (2012). Social responsiveness scale (2nd ed.). Western Psychological Services.
Corbett, B. A. (2017). Examining stress and arousal across pubertal development in ASD. National Institute of Mental Health.
Corbett, B. A., Muscatello, R. A., & Baldinger, C. (2019a). Comparing stress and arousal systems in response to different social contexts in children with ASD. Biological Psychology, 140, 119–130. https://doi.org/10.1016/j.biopsycho.2018.12.010
Corbett, B. A., Muscatello, R. A., Tanguturi, Y., McGinn, E., & Ioannou, S. (2019b). Pubertal development measurement in children with and without autism spectrum disorder: A comparison between physical exam, parent- and self-report. Journal of Autism and Developmental Disorders, 49(12), 4807–4819. https://doi.org/10.1007/s10803-019-04192-w
Corbett, B. A., Muscatello, R. A., Horrocks, B. K., Klemencic, M. E., & Tanguturi, Y. (2020). Differences in body mass index (BMI) in early adolescents with autism spectrum disorder compared to youth with typical development. Journal of Autism and Developmental Disorders. https://doi.org/10.1007/s10803-020-04749-0
Corbett, B. A., Muscatello, R. A., Kim, A., Patel, K., & Vandekar, S. (2021). Developmental effects in physiological stress in early adolescents with and without autism spectrum disorder. Psychoneuroendocrinology, 125, 105115. https://doi.org/10.1016/j.psyneuen.2020.105115
Corbett, B. A., Schupp, C. W., & Lanni, K. E. (2012). Comparing biobehavioral profiles across two social stress paradigms in children with and without autism spectrum disorders. Molecular Autism, 3(1), 13. https://doi.org/10.1186/2040-2392-3-13
Corbett, B. A., Schupp, C. W., Simon, D., Ryan, N., & Mendoza, S. (2010). Elevated cortisol during play is associated with age and social engagement in children with autism. Molecular Autism, 1(1), 13. https://doi.org/10.1186/2040-2392-1-13
Corbett, B. A., Swain, D. M., Newsom, C., Wang, L., Song, Y., & Edgerton, D. (2014). Biobehavioral profiles of arousal and social motivation in autism spectrum disorders. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 55(8), 924–934. https://doi.org/10.1111/jcpp.12184
Criado, K. K., Sharp, W. G., McCracken, C. E., De Vinck-Baroody, O., Dong, L., Aman, M. G., McDougle, C. J., McCracken, J. T., Eugene Arnold, L., Weitzman, C., Leventhal, J. M., Vitiello, B., & Scahill, L. (2018). Overweight and obese status in children with autism spectrum disorder and disruptive behavior. Autism, 22(4), 450–459. https://doi.org/10.1177/1362361316683888
Curtin, C., Anderson, S. E., Must, A., & Bandini, L. (2010). The prevalence of obesity in children with autism: A secondary data analysis using nationally representative data from the national survey of children’s health. BMC Pediatrics, 10(1), 11–15. https://doi.org/10.1186/1471-2431-10-11
Curtin, C., Jojic, M., & Bandini, L. G. (2014). Obesity in children with autism spectrum disorder. Harvard Review of Psychiatry, 22(2), 93–103. https://doi.org/10.1097/HRP.0000000000000031
Edmiston, E. K., Blain, S. D., & Corbett, B. A. (2017a). Salivary cortisol and behavioral response to social evaluative threat in adolescents with autism spectrum disorder. Autism Research: Official Journal of the International Society for Autism Research, 10(2), 346–358. https://doi.org/10.1002/aur.1660
Edmiston, E. K., Jones, R. M., & Corbett, B. A. (2016). Physiological response to social evaluative threat in adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders, 46(9), 2992–3005. https://doi.org/10.1007/s10803-016-2842-1
Edmiston, E. K., Muscatello, R. A., & Corbett, B. A. (2017b). Altered pre-ejection period response to social evaluative threat in adolescents with autism spectrum disorder. Research in Autism Spectrum Disorders, 36, 57–65. https://doi.org/10.1016/j.rasd.2017.01.008
El-Sheikh, M. (2005). Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental Psychobiology, 46(1), 66–74. https://doi.org/10.1002/dev.20036
Eyre, E. L., Duncan, M. J., Birch, S. L., & Fisher, J. P. (2014). The influence of age and weight status on cardiac autonomic control in healthy children: A review. Autonomic Neuroscience, 186, 8–21. https://doi.org/10.1016/j.autneu.2014.09.019
Foley, P., & Kirschbaum, C. (2010). Human hypothalamus-pituitary-adrenal axis responses to acute psychosocial stress in laboratory settings. Neuroscience and Biobehavioral Reviews, 35(1), 91–96. https://doi.org/10.1016/j.neubiorev.2010.01.010
Fraley, M. A., Birchem, J. A., Senkottaiyan, N., & Alpert, M. A. (2005). Obesity and the electrocardiogram. Obesity Reviews, 6(4), 275–281. https://doi.org/10.1111/j.1467-789X.2005.00199.x
Friedman, B. H. (2007). An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology, 74(2), 185–199. https://doi.org/10.1016/j.biopsycho.2005.08.009
Friedman, B. H., & Thayer, J. F. (1998). Anxiety and autonomic flexibility: A cardiovascular approach. Biological Psychology, 49(3), 303–323. https://doi.org/10.1016/s0301-0511(98)00051-9
Geisler, F. C. M., Kubiak, T., Siewert, K., & Weber, H. (2013). Cardiac vagal tone is associated with social engagement and self-regulation. Biological Psychology, 93(2), 279–286. https://doi.org/10.1016/j.biopsycho.2013.02.013
Gentzler, A. L., Rottenberg, J., Kovacs, M., George, C. J., & Morey, J. N. (2012). Atypical development of resting respiratory sinus arrhythmia in children at high risk for depression. Developmental Psychobiology, 54(5), 556–567. https://doi.org/10.1002/dev.20614
Gold, P. W. (2015). The organization of the stress system and its dysregulation in depressive illness. Molecular Psychiatry, 20(1), 32–47. https://doi.org/10.1038/mp.2014.163
Granger, D. A., Hibel, L. C., Fortunato, C. K., & Kapelewski, C. H. (2009). Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology, 34(10), 1437–1448. https://doi.org/10.1016/j.psyneuen.2009.06.017
Graziano, P., & Derefinko, K. (2013). Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology, 94(1), 22–37. https://doi.org/10.1016/j.biopsycho.2013.04.011
Grossman, A. H., & D’Augelli, A. R. (2007). Transgender youth and life-threatening behaviors. Suicide & Life-Threatening Behavior, 37(5), 527–537. https://doi.org/10.1521/suli.2007.37.5.527
Guy, L., Souders, M., Bradstreet, L., DeLussey, C., & Herrington, J. D. (2014). Brief report: Emotion regulation and respiratory sinus arrhythmia in autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(10), 2614–2620. https://doi.org/10.1007/s10803-014-2124-8
Harder, R., Malow, B. A., Goodpaster, R. L., Iqbal, F., Halbower, A., Goldman, S. E., Fawkes, D. B., Wang, L., Goldman, S. E., & Goldman, S. E. (2016). Heart rate variability during sleep in children with autism spectrum disorder. Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society, 26(6), 423–432. https://doi.org/10.1007/s10286-016-0375-5
Healy, S., Aigner, C. J., & Haegele, J. A. (2019). Prevalence of overweight and obesity among US youth with autism spectrum disorder. Autism, 23(4), 1046–1050. https://doi.org/10.1177/1362361318791817
Hill, A. P., Zuckerman, K. E., & Fombonne, E. (2015). Obesity and autism. Pediatrics, 136(6), 1051–1061. https://doi.org/10.1542/peds.2015-1437
Hinnant, J. B., Elmore-Staton, L., & El-Sheikh, M. (2011). Developmental trajectories of respiratory sinus arrhythmia and preejection period in middle childhood. Developmental Psychobiology, 53(1), 59–68. https://doi.org/10.1002/dev.20487
Hinnant, J. B., Philbrook, L. E., Erath, S. A., & El-Sheikh, M. (2018). Approaches to modeling the development of physiological stress responsivity. Psychophysiology, 55(5), e13027. https://doi.org/10.1111/psyp.13027
Hollocks, M. J., Howlin, P., Papadopoulos, A. S., Khondoker, M., & Simonoff, E. (2014). Differences in HPA-axis and heart rate responsiveness to psychosocial stress in children with autism spectrum disorders with and without co-morbid anxiety. Psychoneuroendocrinology, 46, 32–45. https://doi.org/10.1016/j.psyneuen.2014.04.004
Humphrey, N., & Symes, W. (2011). Peer interaction patterns among adolescents with autistic spectrum disorders (ASDs) in mainstream school settings. Autism: The International Journal of Research and Practice, 15(4), 397–419. https://doi.org/10.1177/1362361310387804
Kirschbaum, C., Klauer, T., Filipp, S. H., & Hellhammer, D. H. (1995). Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine, 57(1), 23–31. https://doi.org/10.1097/00006842-199501000-00004
Kirschbaum, C., Pirke, K., & Hellhammer, D. H. (1993). The ‘trier social stress test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. https://doi.org/10.1159/000119004
Kogan, A., Oveis, C., Carr, E. W., Gruber, J., Mauss, I. B., Shallcross, A., et al. (2014). Vagal activity is quadratically related to prosocial traits, prosocial emotions, and observer perceptions of prosociality. Journal of Personality and Social Psychology, 107(6), 1051–1063. https://doi.org/10.1037/a0037509
Kudielka, B. M., Buske-Kirschbaum, A., Hellhammer, D. H., & Kirschbaum, C. (2004). HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology, 29(1), 83–98. https://doi.org/10.1016/s0306-4530(02)00146-4
Kushki, A., Brian, J., Dupuis, A., & Anagnostou, E. (2014). Functional autonomic nervous system profile in children with autism spectrum disorder. Molecular Autism, 5(1), 39. https://doi.org/10.1186/2040-2392-5-39
Kushki, A., Drumm, E., Pla Mobarak, M., Tanel, N., Dupuis, A., Chau, T., & Anagnostou, E. (2013). Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS ONE, 8(4), e59730. https://doi.org/10.1371/journal.pone.0059730
Landsberg, L. (1986). Diet, obesity and hypertension: An hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. The Quarterly Journal of Medicine, 61(236), 1081–1090.
Lanni, K. E., Schupp, C. W., Simon, D., & Corbett, B. A. (2012). Verbal ability, social stress, and anxiety in children with autistic disorder. Autism: The International Journal of Research and Practice, 16(2), 123–138. https://doi.org/10.1177/1362361311425916
Levine, T. P., Sheinkopf, S. J., Pescosolido, M., Rodino, A., Elia, G., & Lester, B. (2012). Physiologic arousal to social stress in children with autism spectrum disorders: A pilot study. Research in Autism Spectrum Disorders, 6(1), 177–183. https://doi.org/10.1016/j.rasd.2011.04.003
Lischke, A., Mau-Moeller, A., Jacksteit, R., Pahnke, R., Hamm, A. O., & Weippert, M. (2018). Heart rate variability is associated with social value orientation in males but not females. Scientific Reports, 8(1), 7336–7339. https://doi.org/10.1038/s41598-018-25739-4 Original Paper.
Lopata, C., Volker, M. A., Putnam, S. K., Thomeer, M. L., & Nida, R. E. (2008). Effect of social familiarity on salivary cortisol and self-reports of social anxiety and stress in children with high functioning autism spectrum disorders. Journal of Autism and Developmental Disorders, 38(10), 1866–1877. https://doi.org/10.1007/s10803-008-0575-5
Lord, C., Rutter, M., DiLavore, P. C., Risi, S., Gotham, K., & Bishop, S. L. (2012). Autism diagnostic observation schedule (ADOS-2) (2nd ed.). Western Psychological Services.
Maenner, M. J., Shaw, K. A., Baio, J., Washington, A., Patrick, M., DiRienzo, M., Christensen, D. L., Wiggins, L. D., Pettygrove, S., Andrews, J. G., Lopez, M., Hudson, A., Baroud, T., Schwenk, Y., White, T., Robinson Rosenberg, C., Lee, L.-C., Harrington, R. A., Huston, M., … Dietz, P. M. (2020). Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2016. Morbidity and Mortality Weekly Report. Surveillance Summaries, 69(SS–4), 1–12. https://doi.org/10.15585/mmwr.ss6904a1
Marshall, W. A., & Tanner, J. M. (1969). Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood, 44(235), 291–303. https://doi.org/10.1136/adc.44.235.291
Marshall, W. A., & Tanner, J. M. (1970). Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood, 45(239), 13–23. https://doi.org/10.1136/adc.45.239.13
Marvar, P. J., & Harrison, D. G. (2012). Stress-dependent hypertension and the role of T lymphocytes. Experimental Physiology, 97(11), 1161–1167. https://doi.org/10.1113/expphysiol.2011.061507
Masi, C. M., Hawkley, L. C., Rickett, E. M., & Cacioppo, J. T. (2007). Respiratory sinus arrhythmia and diseases of aging: Obesity, diabetes mellitus, and hypertension. Biological Psychology, 74(2), 212–223. https://doi.org/10.1016/j.biopsycho.2006.07.006
McCoy, S. M., & Morgan, K. (2020). Obesity, physical activity, and sedentary behaviors in adolescents with autism spectrum disorder compared with typically developing peers. Autism, 24(2), 387–399. https://doi.org/10.1177/1362361319861579
Michels, N., Clays, E., De Buyzere, M., Huybrechts, I., Marild, S., Vanaelst, B., De Henauw, S., & Sioen, I. (2013). Determinants and reference values of short-term heart rate variability in children. European Journal of Applied Physiology, 113(6), 1477–1488. https://doi.org/10.1007/s00421-012-2572-9
Ming, X., Julu, P. O. O., Brimacombe, M., Connor, S., & Daniels, M. L. (2005). Reduced cardiac parasympathetic activity in children with autism. Brain & Development, 27(7), 509–516. https://doi.org/10.1016/j.braindev.2005.01.003
Mire, S. S., Nowell, K. P., Kubiszyn, T., & Goin-Kochel, R. P. (2014). Psychotropic medication use among children with autism spectrum disorders within the Simons simplex collection: Are core features of autism spectrum disorder related? Autism: The International Journal of Research and Practice, 18(8), 933–942. https://doi.org/10.1177/1362361313498518
Molfino, A., Fiorentini, A., Tubani, L., Martuscelli, M., Rossi Fanelli, F., & Laviano, A. (2009). Body mass index is related to autonomic nervous system activity as measured by heart rate variability. European Journal of Clinical Nutrition, 63(10), 1263–1265. https://doi.org/10.1038/ejcn.2009.35
Muscatello, R. A., Andujar, J., Taylor, J. L., & Corbett, B. A. (2020). Exploring key physiological system profiles at rest and the association with depressive symptoms in autism spectrum disorder. Journal of Autism and Developmental Disorders. https://doi.org/10.1007/s10803-020-04516-1
Muscatello, R. A., & Corbett, B. A. (2018). Comparing the effects of age, pubertal development, and symptom profile on cortisol rhythm in children and adolescents with autism spectrum disorder. Autism Research: Official Journal of the International Society for Autism Research, 11(1), 110–120. https://doi.org/10.1002/aur.1879 Comparative Study.
Muscatello, R. A., Vandekar, S. N., & Corbett, B. A. (2021). Evidence for decreased parasympathetic response to a novel peer interaction in older children with autism spectrum disorder: A case–control study. Journal of Neurodevelopmental Disorders, 13(1), 6. https://doi.org/10.1186/s11689-020-09354-x
Must, A., Curtin, C., Hubbard, K., Sikich, L., Bedford, J., & Bandini, L. (2014). Obesity prevention for children with developmental disabilities. Current Obesity Reports, 3(2), 156–170. https://doi.org/10.1007/s13679-014-0098-7
Must, A., Eliasziw, M., Phillips, S. M., Curtin, C., Kral, T. V., Segal, M., Sherwood, N. E., Sikich, L., Stanish, H. I., & Bandini, L. G. (2017). The effect of age on the prevalence of obesity among US youth with autism spectrum disorder. Childhood Obesity, 13(1), 25–35. https://doi.org/10.1089/chi.2016.0079
Neuhaus, E., Bernier, R. A., & Beauchaine, T. P. (2016). Children with autism show altered autonomic adaptation to novel and familiar social partners. Autism Research: Official Journal of the International Society for Autism Research, 9(5), 579–591. https://doi.org/10.1002/aur.1543
Patriquin, M. A., Hartwig, E. M., Friedman, B. H., Porges, S. W., & Scarpa, A. (2019). Autonomic response in autism spectrum disorder: Relationship to social and cognitive functioning. Biological Psychology, 145, 185–197. https://doi.org/10.1016/j.biopsycho.2019.05.004
Patriquin, M. A., Scarpa, A., Friedman, B. H., & Porges, S. W. (2013). Respiratory sinus arrhythmia: A marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Developmental Psychobiology, 55(2), 101–112. https://doi.org/10.1002/dev.21002
Picci, G., & Scherf, K. S. (2015). A two-hit model of autism: Adolescence as the second hit. Clinical Psychological Science: A Journal of the Association for Psychological Science, 3(3), 349–371. https://doi.org/10.1177/2167702614540646
Porges, S. W. (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology, 32(4), 301–318. https://doi.org/10.1111/j.1469-8986.1995.tb01213.x Review.
Porges, S. W. (2001). The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 42(2), 123–146. https://doi.org/10.1016/s0167-8760(01)00162-3
Porges, S. W. (2003a). The polyvagal theory: Phylogenetic contributions to social behavior. Physiology & Behavior, 79(3), 503–513. https://doi.org/10.1016/s0031-9384(03)00156-2
Porges, S. W. (2003b). Social engagement and attachment: A phylogenetic perspective. Annals of the New York Academy of Sciences, 1008(1), 31–47. https://doi.org/10.1196/annals.1301.004
Porges, S. W. (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. https://doi.org/10.1016/j.biopsycho.2006.06.009 Review.
Porges, S. W., Macellaio, M., Stanfill, S. D., McCue, K., Lewis, G. F., Harden, E. R., Handelman, M., Denver, J., Bazhenova, O. V., & Heilman, K. J. (2013). Respiratory sinus arrhythmia and auditory processing in autism: Modifiable deficits of an integrated social engagement system? International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 88(3), 261–270. https://doi.org/10.1016/j.ijpsycho.2012.11.009
Rabbia, F., Silke, B., Conterno, A., Grosso, T., De Vito, B., Rabbone, I., Chiandussi, L., & Veglio, F. (2003). Assessment of cardiac autonomic modulation during adolescent obesity. Obesity Research, 11(4), 541–548. https://doi.org/10.1038/oby.2003.76
Rankin, J., Matthews, L., Cobley, S., Han, A., Sanders, R., Wiltshire, H. D., & Baker, J. S. (2016). Psychological consequences of childhood obesity: Psychiatric comorbidity and prevention. Adolescent Health, Medicine and Therapeutics, 7, 125–146. https://doi.org/10.2147/AHMT.S101631
Rottenberg, J. (2007). Cardiac vagal control in depression: A critical analysis. Biological Psychology, 74(2), 200–211. https://doi.org/10.1016/j.biopsycho.2005.08.010
Rottenberg, J., Clift, A., Bolden, S., & Salomon, K. (2007). RSA fluctuation in major depressive disorder. Psychophysiology, 44(3), 450–458. https://doi.org/10.1111/j.1469-8986.2007.00509.x
Rutter, M., Bailey, A., & Lord, C. (2003). Social communication questionnaire. Western Psychological Services.
Schaaf, R. C., Benevides, T. W., Leiby, B. E., & Sendecki, J. A. (2015). Autonomic dysregulation during sensory stimulation in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(2), 461–472. https://doi.org/10.1007/s10803-013-1924-6
Schupp, C. W., Simon, D., & Corbett, B. A. (2013). Cortisol responsivity differences in children with autism spectrum disorders during free and cooperative play. Journal of Autism and Developmental Disorders, 43(10), 2405–2417. https://doi.org/10.1007/s10803-013-1790-2
Seddon, J. A., Rodriguez, V. J., Provencher, Y., Raftery-Helmer, J., Hersh, J., Labelle, P. R., & Thomassin, K. (2020). Meta-analysis of the effectiveness of the trier social stress test in eliciting physiological stress responses in children and adolescents. Psychoneuroendocrinology, 116, 104582. https://doi.org/10.1016/j.psyneuen.2020.104582
Sheinkopf, S. J., Neal-Beevers, A. R., Levine, T. P., Miller-Loncar, C., & Lester, B. (2013). Parasympathetic response profiles related to social functioning in young children with autistic disorder. Autism Research and Treatment, 2013, 868396. https://doi.org/10.1155/2013/868396
Shibao, C. (2012). Obesity-associated hypertension. In D. Robertson, I. Biaggioni, G. Burnstock, P. A. Low, & J. F. R. Paton (Eds.), Primer on the autonomic nervous system (pp. 359–361). Academic Press.
Task Force of the European Society of Cardiology and The North American Society of Pacing and Electrophysiology. (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. European Heart Journal, 17, 354–381. https://doi.org/10.1161/01.cir.93.5.1043
Thayer, J. F., & Brosschot, J. F. (2005). Psychosomatics and psychopathology: Looking up and down from the brain. Psychoneuroendocrinology, 30(10), 1050–1058. https://doi.org/10.1016/j.psyneuen.2005.04.014
Thayer, J. F., Friedman, B. H., & Borkovec, T. D. (1996). Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry, 39(4), 255–266. https://doi.org/10.1016/0006-3223(95)00136-0
Thayer, J. F., Friedman, B. H., Borkovec, T. D., Johnsen, B. H., & Molina, S. (2000). Phasic heart period reactions to cued threat and nonthreat stimuli in generalized anxiety disorder. Psychophysiology, 37(3), 361–368.
Thayer, J. F., Hall, M., Sollers, J. J., 3rd., & Fischer, J. E. (2006). Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: Evidence for impaired inhibitory control of the HPA axis in heavy drinkers. International Journal of Psychophysiology, 59(3), 244–250. https://doi.org/10.1016/j.ijpsycho.2005.10.013
Thayer, J. F., & Lane, R. D. (2009). Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews, 33(2), 81–88. https://doi.org/10.1016/j.neubiorev.2008.08.004
Thayer, J. F., Smith, M., Rossy, L. A., Sollers, J. J., & Friedman, B. H. (1998). Heart period variability and depressive symptoms: Gender differences. Biological Psychiatry, 44(4), 304–306. https://doi.org/10.1016/s0006-3223(98)00008-0
Thayer, J. F., & Sternberg, E. (2006). Beyond heart rate variability: Vagal regulation of allostatic systems. Annals of the New York Academy of Sciences, 1088(1), 361–372. https://doi.org/10.1196/annals.1366.014
Thayer, J. F., Yamamoto, S. S., & Brosschot, J. F. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology, 141(2), 122–131. https://doi.org/10.1016/j.ijcard.2009.09.543
Ulrich-Lai, Y. M., & Herman, J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10(6), 397–409. https://doi.org/10.1038/nrn2647
Vaughan Van Hecke, A., Lebow, J., Bal, E., Lamb, D., Harden, E., Kramer, A., Denver, J., Bazhenova, O., & Porges, S. W. (2009). Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Development, 80(4), 1118–1133. https://doi.org/10.1111/j.1467-8624.2009.01320.x
Vidal, J. (2002). Updated review on the benefits of weight loss. International Journal of Obesity and Related Metabolic Disorders, 26(Suppl 4), S25-28. https://doi.org/10.1038/sj.ijo.0802215
Watson, L. R., Roberts, J. E., Baranek, G. T., Mandulak, K. C., & Dalton, J. C. (2012). Behavioral and physiological responses to child-directed speech of children with autism spectrum disorders or typical development. Journal of Autism and Developmental Disorders, 42(8), 1616–1629. https://doi.org/10.1007/s10803-011-1401-z
Wechsler, D. (2011). Wechsler abbreviated scale of intelligence (WASI-II) (2nd ed.). Pearson.
Whiteley, P., Dodou, K., Todd, L., & Shattock, P. (2004). Body mass index of children from the United Kingdom diagnosed with pervasive developmental disorders. Pediatrics International, 46(5), 531–533. https://doi.org/10.1111/j.1442-200x.2004.01946.x
Wolff, B. C., Wadsworth, M. E., Wilhelm, F. H., & Mauss, I. B. (2012). Children’s vagal regulatory capacity predicts attenuated sympathetic stress reactivity in a socially supportive context: Evidence for a protective effect of the vagal system. Development and Psychopathology, 24(2), 677–689. https://doi.org/10.1017/S0954579412000247
Zuckerman, K. E., Hill, A. P., Guion, K., Voltolina, L., & Fombonne, E. (2014). Overweight and obesity: Prevalence and correlates in a large clinical sample of children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(7), 1708–1719. https://doi.org/10.1007/s10803-014-2050-9
Acknowledgments
We are grateful to the children and families who participated and continue to support our research.
Funding
This study was funded by the National Institute of Mental Health (Grant No. MH111599 PI: Corbett) and an Autism Speaks Weatherstone Predoctoral Fellowship (Grant No. 10616 PI: Muscatello). Core support was provided by the National Institute of Child Health and Human Development (Grant No. U54 HD083211, PI: Neul) and the National Center for Advancing Translational Sciences (CTSA Grant No. UL1 TR000445). None of the funding sources were involved in the study design, collection, analysis and interpretation of the data, writing of the report, or the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
RAM contributed to the design of the study, led data acquisition, analyzed physiological data, and wrote the initial draft of the manuscript. AK and SV contributed to the statistical design, analysis, interpretations of the data, and to drafting and editing of the manuscript. BAC designed the study, oversaw data acquisition, analysis, and interpretation, and contributed to writing the initial draft of the manuscript. All authors participated in the preparation and editing of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical stands of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed written consent and assent was obtained from all parents and study participants, respectively, prior to inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muscatello, R.A., Kim, A., Vandekar, S. et al. Diagnostic and Physical Effects in Parasympathetic Response to Social Evaluation in Youth With and Without Autism Spectrum Disorder. J Autism Dev Disord 52, 3427–3442 (2022). https://doi.org/10.1007/s10803-021-05224-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-021-05224-0