Abstract
This paper provides age- and sex-specific reference values for short-term heart rate variability (HRV) data in children by time domain and frequency domain methods. Furthermore, HRV determinants will be determined. In 460 children (5–10 years), 5-minute HRV measurements in supine position were undertaken with Polar chest belts. The data were manually edited and processed with time and frequency domain methods. Age, time point, physical activity (accelerometry), physical fitness (cardiopulmonary fitness, upper and lower limb muscular fitness) and body composition (body mass index, fat%, fat and fat-free mass) were analysed as determinants using multiple regression analysis stratified by sex. Sex- and age-specific reference values were produced. Overall, girls had lower HRV. Age-related parasympathetic increases and sympathetic decreases were seen with sometimes age-related year-to-year wave-like changes in boys. The time point of recording had limited influence on HRV. Of the lifestyle related factors, fatness (only 7 % overweight) was not associated with HRV but fat-free mass, physical activity and in particular physical fitness (over and above activity) had a favourable association by increased parasympathetic activity. Future HRV studies in children should consider age, sex and physical fitness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart rate variability (HRV) is defined as the variability of the distance between consecutive R peaks of the electrical heart beat signal (caused by polarization and depolarization of the heart muscles) as measured with an electrocardiogram. The R-wave is the first positive deflection of the signal after the P-wave. HRV is increasingly used as a quantitative expression of the autonomic nervous system activity and its two branches because of the sympathetic and vagal parasympathetic innervations of the heart (SA and PA, respectively) (Task Force of ESC/NASPE 1996). These changes reflect the heart’s ability to respond to physiological and environmental stimuli. Subsequently, a reduction of HRV (i.e. reduced PA with or without increased SA) is a pathway of increased morbidity and mortality (Thayer et al. 2010). Apart from its original clinical use as risk marker for cardiovascular mortality, a reduction of HRV has also been observed in non-cardiac pathologies such as stress-induced conditions (Chandola et al. 2010). HRV analysis has increasingly been used in child populations in which HRV analyses showed moderate-to-high reproducibility (Dietrich et al. 2010). In contrast to long-term recordings (24 h), short-term recordings have the advantage of being rapidly obtainable under standardized conditions.
Several physiological factors are known to influence HRV. Age and sex are well-described in adults (Umetani et al. 1998) with decline over age and lower values in women. However, there are conflicting data in children: (i) no sex difference (Fukuba et al. 2009; Goto et al. 1997), an overall sex difference (Faulkner et al. 2003) or an age- and measure-dependent sex difference (Galeev et al. 2002; Silvetti et al. 2001) have been observed as well as (ii) no age difference (Fukuba et al. 2009; Faulkner et al. 2003), an increase until 6 or 9 year with a decrease or stagnation afterwards (Goto et al. 1997; Finley and Nugent 1995; Silvetti et al. 2001; Massin and vonBernuth 1997) or more wave-like age-related year-to-year changes (Galeev et al. 2002). Therefore, establishment of age- and sex-specific reference values is definitely needed.
Associations of HRV have been described with physical activity (Gutin et al. 2005; Nagai and Moritani 2004; Krishnan et al. 2009; Buchheit et al. 2007) and body composition (Rabbia et al. 2003; Kaufman et al. 2007; Gutin et al. 2005; Nagai and Moritani 2004) in children. Association with physical fitness has rarely been studied (Gutin et al. 2005; Brunetto et al. 2005) with conflicting results (only one study showed enhanced HRV), notwithstanding a strong association with cardiovascular risk factors (Hurtig-Wennlof et al. 2007). Furthermore, large-scale studies often spread their fieldwork over a long period of the day because of logistic restrictions, but without correction for the time point of registration. Nevertheless, diurnal rhythms of HRV have also been shown in children over a 24 h period (Massin et al. 2000).
In a large healthy child population of 460 subjects, we aimed to provide age- and sex-specific reference values for an extensive battery of short-term HRV parameters. Moreover, the contribution of age, sex, time point, body composition, physical activity and particularly fitness was explored.
Methods
Participants and general procedures
Participating children were from the Belgian control region (i.e. Aalter, a city in Flanders, the northern, Dutch-speaking part of Belgium) of the IDEFICS study funded within the European Sixth Framework Programme. Children were selected by random cluster sampling. The 761 IDEFICS children in the Belgian control region were also allowed to participate in the national sub-study ChiBS (Children’s body composition and stress) that aimed at examining the association between stress and body composition evolution (Michels et al. 2012). Data were collected from February 2010 to June 2010, when children were between 5 and 10 years old. For the ChiBS measurements, parents had to make an appointment at the local sports park. The study was conducted according to the Declaration of Helsinki and the project protocol was approved by the Ethics Committee of the Ghent University Hospital. Written informed consent was obtained from the parents.
The HRV measurements were done in 475 of the 761 invited Belgian children (62.4 % participation rate). No difference in sex, age, body mass index and socio-economic status was observed between participants and non-participants. Exclusion criteria were cardiovascular diseases (1 case), diabetes (0 cases) and HRV measurements of too low quality (14 cases). Finally, 460 children were enrolled in this study.
Heart rate variability
Inter-beat RR intervals (RRI) were recorded at a sampling rate of 1,000 Hz with the elastic electrode belt Polar Wearlink 31 using a Windlink infrared computer transmitter. This low-cost device has been validated against an electrocardiogram device in children (Gamelin et al. 2008). Each child was individually examined in a quiet room in the supine position for 10 min. Children were asked to refrain from strenuous physical activity on the measurement day (9 a.m.–6 p.m.). Each child was encouraged to breath normally and not to speak or move. In the occasion of sudden irregular respiration, the registration was cancelled, as such minimizing breathing influences. The heart rate belt was fixed around the chest and measurements were started when the signal was stabilized. Further data processing was done with the free, professional HRV Analysis Software of the University of Kuopio, Finland (Niskanen et al. 2004). Very low frequency (VLF), low frequency (LF) and high frequency (HF) bands were analysed between 0.0033–0.04, 0.04–0.15 and 0.15–0.4 Hz, following the suggested default based on data from adults (Task Force of ESC/NASPE 1996). The RR series were detrended using the Smoothness priors method with alpha = 300 and a cubic interpolation at the default rate of 4 Hz was done. The middle 5 min were manually checked on their quality/stationarity and if necessary, another appropriate 5-min interval was selected. Quality was defined as no large RRI outliers, an equidistance between consecutive RRI points, minimal variation, stable mean and unimodal, Gaussians RRI and HR distribution graphics. By doing this, disturbing phenomena such as the Valsalva manoeuvre were excluded.
For time domain methods, the mean RRI (mRR), the standard deviation of the normal RRI (SDNN), the root mean square of successive differences (RMSSD) and the percentage of consecutive normal RRI differing more than 50 ms (pNN50) were determined.
For the frequency domain method, the nonparametric fast Fourier transform (FFT) model was used. Spectrum parameters were calculated with the Welch’s periodogram method using a standard 50 % overlap Hanning window as preprocessing technique and finally an integration (area under the curve). The power of LF and HF bands in absolute and normalized (nu, LF or HF divided by ‘LF + HF’) units and the LF/HF ratio were determined. Descriptive data but no reference values for the very low frequency (VLF) power will be given as the 5-min measurements are too short for estimating this frequency domain (Task Force of ESC/NASPE 1996). While HF, pNN50 and RMSSD can represent the vagal activity (=PA), LF and SDNN might reflect the activity of both branches of the nervous system (=PA and SA) and the LF/HF ratio is assumed to represent the sympathovagal balance. The normalized units have the advantage of minimizing the effect of total power differences (Task Force of ESC/NASPE 1996).
Other study variables
Sex, age, time point, physical activity, physical fitness and body composition were examined as possible determinants.
Time point was expressed as number of hours elapsed since 9 a.m. (the earliest measurement).
To monitor physical activity, children wore an uniaxial accelerometer (ActiGraph or ActiTrainer, Pensacola, FL, USA) on a hip belt over 3 consecutive days. Activity was counted in 15 s/min intervals. The average counts per minute (cpm) and the total time in moderate-to-vigorous activity (MVPA) following the cut-offs of Evenson (Trost et al. 2011) were used.
Physical fitness was measured with the Eurofit fitness test battery (Council of Europe 1988). Maximal oxygen uptake (VO2max) was estimated through the 20 m shuttle test of Léger and Lambert as objective criterion of cardiopulmonary fitness. Handgrip strength was measured by a handgrip dynamometer with adjustable grip (Takei TKK 5401, precision 0.1 kg). The sum of right and left arm strength was used. Lower limb muscular fitness was determined by the standing broad jump and the 40 m sprint. As both tests were repeated twice, the maximal jump distance (precision 1 cm) and the minimal sprint time (precision 0.1 s) were used.
For body composition, weight was recorded with an electronic scale (TANITA BC 420 SMA, 0.1 kg) and height was measured with a telescopic height measuring instrument (SECA 225, 0.1 cm). The children wore only underwear and T-shirts. Age- and sex- specific BMI z-scores were calculated. Body fat percentage (BF%) was measured by air-displacement plethysmography (BOPOD®, Life Measurement Inc, UK) in tight-fitting swimsuit using standardized procedures. Thoracic gas volume was predicted by the software with a validated child-specific equation, and fat mass (FM), fat-free mass (FFM) and BF% were calculated using the equation of Wells (Wells et al. 2010).
Statistical analyses
All statistical analyses were performed using SPSS/PASW version 19 (IBM Corp, NY, USA). Significance was set at p < 0.05. The positively skewed measurements (HF power, LF power and LF/HF) were log-transformed. Median and interquartile range were given.
Sex differences in HRV were examined by an independent samples t test. The LMS method (Cole and Green 1992) was used to generate smoothed percentile reference values across age for all HRV parameters stratified by sex. The LMS Chartmaker Pro software (version 2.3) uses cubic splines to fit smoothed L (skewness), M, (median) and S (coefficient of variation) curves across each age category by maximized penalized likelihood. Q tests and detrended Q–Q plot were used to assess goodness-of-fit.
To identify determinants, sex and age effects and their interaction were first analysed using two-way ANOVA. The age category ‘10 years’ was merged with the 9-year-olds because of the smaller sample size.
Relationships between HRV parameters and possible determinants were quantified using multiple linear regression stratified by sex. In the basic model, age and time point were entered simultaneously. Then, all other possible determinants (i.e. physical activity, physical fitness and body composition) were entered separately to test their significant contribution after correction for age and time point. Also, their independent contributions were tested after mutual correction (corrected for FFM or for sprint time). Standardized coefficients were recorded. Finally, the multiple linear regression analyses were repeated with correction for mean HR.
Results
Descriptive statistics and reference values
In total, 460 children (240 boys, 220 girls; 7 % overweight) between 5 and 10 years old were included. Participation numbers for the age categories 5, 6, 7, 8, 9 and 10 years old were 72, 62, 89, 113, 84, 40, respectively, evenly distributed over the sexes. Table 1 gives the descriptive statistics and sex differences for all variables. Mean HR was higher in girls, while mRR, SDNN, RMSSD, pNN50, VLF, LF and HF were higher in boys. Sex differences were also seen in the examined determinants with an overall higher fatness in girls and higher physical fitness and activity in boys. Girls and boys were equally distributed over age and time point (p > 0.05). Age- and sex-specific reference values are given in Table 2 and the percentile curves are shown in Fig. 1. As no sex differences were seen for LFnu, HFnu and LF/HF, only one set of reference values was given for both sexes together.
Age- and sex specific percentile curves for HRV measurements in 460 children: 2.5, 10, 25, 50, 75, 90 and 97.5th percentile. FFT Fast Fourier transform, HF high frequency spectral power, LF low frequency spectral power, LF/HF ratio of low frequency power to high frequency power, mRR mean RR interval, nu normalized units, pNN50 percentage of consecutive normal RRI differing more than 50 ms, RMSSD root mean square of successive differences, SDNN standard deviation of normal-to-normal intervals
Determinants of HRV parameters
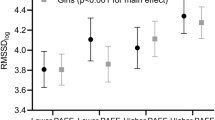
The sex and age effects on HRV parameters were examined using two-way ANOVA. A sex × age interaction was found for SDNN, RMSSD, pNN50 and HF, giving higher values for boys at the ages 5 and 6 and no sex differences at the older age. An age effect was seen for all variables except for LF. Detected sex differences were the same as for Table 1. When analysing the age effect with polynomial ANOVA, a cubic trend was seen for boys in all variables for which the sex × age interaction was significant. In girls, only linear trends were seen. Figure 2 shows these age and sex effects on mRR (example of the linear age effect) and RMSSD (example of the interaction effect).
Multiple regression analyses for age, time point and lifestyle factors (physical activity, physical fitness and body composition) were executed. Multiple regression results of physical fitness are shown separately in Table 3, as these were most significant and novel. Age was indeed correlated with both time and frequency domain parameters in both boys and girls, although more explicitly in girls (highest beta = 0.474). The time point had only very minimal influence and this was restricted to boys. The model with age and time point could explain 17.6 and 13.9 % of the variance in mRR for boys and girls, respectively. R 2 change values ranged between 3.3 and 3.9 % for physical activity, between 2.9 and 9.2 % for physical fitness and between 1.6 and 4.9 % for body composition. In boys, most physical fitness parameters and also physical activity were positive determinants, while body composition could barely serve as determinant (only one association with FFM). In girls, only FFM and arm strength showed a positive HRV association. Among physical fitness parameters, the sprint time had the largest effect. For body composition, only positive associations for FFM were seen, but no effect of FM, BF% and BMI was found. After correction for body composition (FFM), all physical fitness and activity associations remained significant. Physical fitness even had a relevant impact over and above physical activity (after correction for MVPA).
After correction for mean HR, age remained significant (except for mRR). All significances for time point and body composition disappeared and only sprint time and physical activity remained significant in boys (data not shown).
Discussion
To our knowledge, this is the first study giving age- and sex-specific reference values for an extensive battery of HRV parameters (both time and frequency domain parameters) in a large sample of children and using the sophisticated LMS software. It is crucial as most of the children’s HRV parameters were sex and age dependent. Moreover, physical fitness was a major positive determinant, especially in boys.
Descriptive statistics and reference values
Our data indicate sex and age differences in HRV parameters in young children. Generally time and frequency domain parameters were higher in boys and increased with age. There were no sex differences for LFnu, HFnu and LF/HF and for the age-related decreases in LF/HF and LFnu. Because of the age and sex differences and an equal distribution of our population over age and sex, we reproduced age- and sex-specific reference values. The LMS software had the advantage of allowing non-linear changes with age (Cole and Green 1992). It was indeed visible in the wave-like age-related year-to-year changes in boys, particularly in parameters with an age × sex interaction. When such a wave-like change was present, values decreased at age 7–8 and increased again afterwards. This pattern was never seen in girls. The wave-like change of LF in boys versus almost no increase in girls, could explain the absence of an age effect in the two-way ANOVA for LF. This highlights the importance of considering sex-specific analyses and using non-linear reference curves. Next to the general age-related increase, decreases were seen for LF/HF ratio and LFnu. Indeed, decreases in LF/HF could be caused by decreases in LFnu concordant with increases in HFnu.
Comparison of our reference values with those previously reported would be not straightforward because of methodological and population differences. Nonetheless, we tried to compare them with the large study of Galeev et al. giving age- and sex-specific short-term reference values in 6- to 16-year-old Russian children. They showed overall higher LF/HF values, higher LF values and slightly lower HF values than our study, but the time domain parameters (mRR, SDNN and RMSSD) showed the same trend as ours (Galeev et al. 2002). Also, our values very much resembled the reported RMSSD in German children (Longin et al. 2009) and LF and HF in Dutch children (Bosch et al. 2009).
Determinants of HRV parameters
Our results confirm the sex differences as found in adults (Umetani et al. 1998) and adolescents (Faulkner et al. 2003) i.e. lower values in females. Nevertheless, no sex differences were seen in the sympathovagal balance (LF/HF), as was also shown in an adolescent study (Fukuba et al. 2009). Previous studies with young children as in our sample, showed only sex differences from 9 years onwards (Galeev et al. 2002) or not at all (Goto et al. 1997).
Age differences were already discussed in the reference values section giving overall wave-like increasing trends (especially in boys) except for decreases in LFnu and LF/HF ratio without wave-like patterns. Most child studies found a HRV increase until 6 or 9 years old with a decrease or stagnation afterwards (Goto et al. 1997; Finley and Nugent 1995; Silvetti et al. 2001; Massin and vonBernuth 1997). Our findings confirm this increasing trend although we also detected in boys the wave-like changes as found in a large sample of 6- to 16-year-old children (Galeev et al. 2002). The observed HRV age changes may reflect the development of vagal and sympathetic control of the heart with regulatory shifts. Indeed, neural autonomic functions mature over childhood attaining peak levels in adolescence with an improved cardiovagal autonomic function (Lenard et al. 2004). Previously, increasing cholinergic and decreasing adrenergic modulation was seen over the first 14 years of child development, resulting in a decreasing LF/HF (Massin and vonBernuth 1997).
Circadian HRV rhythms in children exist with a midnight peak of time domain and LF and HF values and a trough for LF/HF (Massin et al. 2000). In our study, the time point (daytime) showed only a small influence on PA and only in boys, with lower values later on the day. If possible, recordings have to be done in a restricted time frame. Nevertheless, large-scale studies are not inevitably of lower quality by measuring over a larger time frame of the day.
Both decreased PA and increased SA are risk markers predicting morbidity (Thayer et al. 2010). Consequently, we examined the influence of lifestyle-related parameters such as physical activity, physical fitness and body composition on PA and SA.
Physical activity has been related to better HRV indices, especially a higher PA (Gutin et al. 2005; Krishnan et al. 2009), although sometimes only found with vigorous activity (Buchheit et al. 2007). Furthermore, physical training has improved HRV in children with low baseline HRV (Nagai and Moritani 2004) (increased LF and HF). In our study, higher physical activity was associated with higher PA (RMSSD, pNN50 and HF) and consequently higher values in the dually innervated LF.
Apart from physical activity, also physical fitness should be considered. After all, only low-to-moderate correlations have been detected between children’s objectively measured activity and fitness (Dencker and Andersen 2011) and individual differences exist in the response to physical activity (Bouchard and Rankinen 2001). While activity is a lifestyle factor, physical fitness is a physiological condition and could as such be more related to physiological markers as HRV, as we have seen. Previously, physical fitness was also shown to be better related to cardiovascular risk factors than physical activity in children (Hurtig-Wennlof et al. 2007). HRV associations with physical fitness have rarely been published and were limited to VO2max measurement. Higher RMSSD had previously been found in more fit children (Gutin et al. 2005), while no effect of fitness was seen in an adolescent group (Brunetto et al. 2005). We observed beneficial effects: higher mRR and PA (HF, RMSSD, pNN50) with increasing fitness and with the sprint time as most influencing determinant. In girls, arm strength was the only determinant. Taken together, quite similar associations were seen for physical fitness and physical activity. Nevertheless, physical fitness was a more powerful determinant because of the abundant and higher coefficients that remained significant even after correction for physical activity.
We tested the effect of body composition in our low-overweight population (7 %), but no effect of fatness was seen. Beneficial associations of HRV variables for FFM (the counterpart of fat) were mainly present in girls and FFM-mRR was the only association remaining significant after correction for physical fitness. As HRV associations of physical fitness and activity remained significant after correction for body composition and FFM could be causally linked to physical activity, the fitness determinants might be more potent. There is ample evidence from literature that HRV differs between obese and lean children (Kaufman et al. 2007; Gutin et al. 2005) with an overall lower PA, independent of physical activity (Nagai and Moritani 2004). Changes in SA were more likely to depend on obesity duration (Rabbia et al. 2003). This could explain the lack of associations in a low-overweight population. In another low-to-moderate-overweight sample, no differences were seen except for lower HR with higher FFM in girls (Krishnan et al. 2009).
Apart from sex differences in the HRV parameters, sex differences were also seen in the associations with determinants. Previously, similar sex differences were seen with physical activity associations in boys only and FFM associations in girls (Krishnan et al. 2009). These sex differences could perhaps be partially explained by our population characteristics: a higher prevalence of overweight in girls and higher fitness in boys. The sexes were equally distributed over age and time point, so this could not explain the sex differences in the associations. Moreover, the physical fitness tests were previously shown reliable and no sex difference in reliability exists (Ortega et al. 2008).
Strengths and limitations
Standardized HRV measurements with an extra manual quality control were executed in a large healthy child population of 460 subjects. Current published reference values for children have often been based on smaller samples (Faulkner et al. 2003; Goto et al. 1997; Massin and vonBernuth 1997; Longin et al. 2009; Finley and Nugent 1995), except for the large Russian study of Galeev et al. (2002). The LMS method allowed for non-linear HRV changes with age. It was a major strength as wave-like changes were indeed seen in boys.
Next to age and sex differences, we also examined lifestyle factors. Therefore, standardized techniques have been implemented: objectively measured physical activity instead of the frequently used subjective questionnaires, a well-known physical fitness test battery with proven reliability and body fat percentage measured with specialized technology. Examining physical fitness was a major asset as it has rarely been studied in relation to HRV and as it was a more potent determinant than physical activity in our study. Furthermore, physical fitness and activity were related to HRV independent of other determinants and HR.
Nevertheless, some limitations are connected to these determinants. (1) We had only a low percentage of overweight in our sample (7 %). Although we found no influence of body fatness on HRV, there might be an influence in high-overweight populations. (2) Another checked determinant was the time point of registration, with almost no influence on HRV. To ascertain this statement, future studies should measure the same child on several different time points to examine the influence of time more directly. Alternatively, we could recommend 24 h HRV recordings if this is logistical feasible as these take into account the diurnal rhythm and the other spectral components ‘very low frequency’ and ‘ultra low frequency’. (3) Finally, two measurement limitations related to the used software should be considered. (3a) Non-stationarity was only manually inspected (no outliers, an equidistance, minimal variation, stable mean and Gaussians distributions), increasing the likelihood to overestimate the contribution of the sympathetic activity (Magagnin et al. 2011). (3b) Although visual respiratory observation was done during the registration, breathing rate was not measured with an extra channel, possibly confounding our frequency domain parameters. Breathing rate was previously shown to be negatively related with LF and HF power, but had no influence on time domain parameters (Brown et al. 1993; Penttila et al. 2001). Nonlinearities are more likely to happen with a low breathing rate (e.g. at 10/min breathing rate), a long RRI and in the presence of important respiratory sinus arrhythmia (Porta et al. 2000). Nevertheless, the influence of nonlinearities will be minimal in our population as children have a general high breathing rate (18–21/min mean in our age range) (Wallis et al. 2005) and short RR interval; and respiratory sinus arrhythmia induced by the Valsalva manoeuvre was most likely abolished.
Conclusions
Performing short-term HRV measurements with a polar chest belt was easily feasible as part of a large epidemiological study in children. Our sex- and age-specific reference values can be used in the future and highlighted the importance of considering sex differences and using non-linear reference curves. Overall, girls had lower HRV and age-related parasympathetic increases and sympathetic decreases were seen. Apart from physical activity and fat-free mass, in particular fitness (sprint time) influenced HRV in a favourable way by increased parasympathetic activity, especially in boys and independent of body composition, physical activity and heart rate. Consequently, future HRV studies in children should consider age, sex and physical fitness.
Abbreviations
- BF%:
-
Body fat percentage
- ChiBS:
-
Children’s body composition and stress
- FFT:
-
Fast Fourier transform
- FM:
-
Fat mass
- FFM:
-
Fat-free mass
- HRV:
-
Heart rate variability
- HF:
-
High frequency
- ICC:
-
Intraclass correlation
- LOA:
-
Limits of agreement
- LF:
-
Low frequency
- VO2max:
-
Maximal oxygen uptake
- mRR:
-
Mean RRI
- MVPA:
-
Moderate-to-vigorous activity
- Nu:
-
Normalized units
- PA:
-
Parasympathetic activity
- pNN50:
-
Percentage of consecutive normal RRI differing more than 50 ms
- RMSSD:
-
Root mean square of successive differences
- RRI:
-
RR intervals
- SDNN:
-
Standard deviation of the normal RRI
- SA:
-
Sympathetic activity
References
Bosch NM, Riese H, Dietrich A, Ormel J, Verhulst FC, Oldehinkel AJ (2009) Preadolescents’ somatic and cognitive-affective depressive symptoms are differentially related to cardiac autonomic function and cortisol: the TRAILS study. Psychosom Med 71(9):944–950
Bouchard C, Rankinen T (2001) Individual differences in response to regular physical activity. Med Sci Sports Exerc 33(6 Suppl):S446–S451 discussion S452–453
Brown TE, Beightol LA, Koh J, Eckberg DL (1993) Important influence of respiration on human R–R interval power spectra is largely ignored. J Appl Physiol 75(5):2310–2317
Brunetto AF, Roseguini BT, Silva BM, Hirai DM, Guedes DP (2005) Effects of gender and aerobic fitness on cardiac autonomic responses to head-up tilt in healthy adolescents. Pediatr Cardiol 26(4):418–424
Buchheit M, Platat C, Oujaa M, Simon C (2007) Habitual physical activity, physical fitness and heart rate variability in preadolescents. Int J Sports Med 28(3):204–210
Chandola T, Heraclides A, Kumari M (2010) Psychophysiological biomarkers of workplace stressors. Neurosci Biobehav Rev 35(1):51–57
Cole TJ, Green PJ (1992) Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 11(10):1305–1319
Council of Europe (1988) Eurofit. Handbook for the EUROFIT tests of physical fitness. Council of Europe, Committee for the Development of Sport, Rome
Dencker M, Andersen LB (2011) Accelerometer-measured daily physical activity related to aerobic fitness in children and adolescents. J Sports Sci 29(9):887–895
Dietrich A, Rosmalen JGM, Althaus M, van Roon AM, Mulder LJM, Minderaa RB, Oldehinkel AJ, Riese H (2010) Reproducibility of heart rate variability and baroreflex sensitivity measurements in children. Biol Psychol 85(1):71–78
Faulkner MS, Hathaway D, Tolley B (2003) Cardiovascular autonomic function in healthy adolescents. Heart Lung 32(1):10–22
Finley JP, Nugent ST (1995) Heart-rate-variability in infants, children and young-adults. J Auton Nerv Syst 51(2):103–108
Fukuba Y, Sato H, Sakiyama T, Yamaoka Endo M, Yamada M, Ueoka H, Miura A, Koga S et al (2009) Autonomic nervous activities assessed by heart rate variability in pre- and post-adolescent Japanese. J Physiol Anthropol 28(6):269–273
Galeev AR, Igisheva LN, Kazin EM (2002) Heart rate variability in healthy six- to sixteen-year-old children. Hum Physiol 28(4):428–432
Gamelin FX, Baquet G, Berthoin S, Bosquet L (2008) Validity of the polar S810 to measure R–R intervals in children. Int J Sports Med 29(2):134–138
Goto M, Nagashima M, Baba R, Nagano Y, Yokota M, Nishibata K, Tsuji A (1997) Analysis of heart rate variability demonstrates effects of development on vagal modulation of heart rate in healthy children. J Pediatr 130(5):725–729
Gutin B, Howe C, Johnson MH, Humphries MC, Snieder H, Barbeau P (2005) Heart rate variability in adolescents: relations to physical activity, fitness, and adiposity. Med Sci Sports Exerc 37(11):1856–1863
Hurtig-Wennlof A, Ruiz JR, Harro M, Sjostrom M (2007) Cardiorespiratory fitness relates more strongly than physical activity to cardiovascular disease risk factors in healthy children and adolescents: the European Youth Heart Study. Eur J Cardiovasc Prev Rehabil 14(4):575–581
Kaufman CL, Kaiser DR, Steinberger J, Kelly AS, Dengel DR (2007) Relationships of cardiac autonomic function with metabolic abnormalities in childhood obesity. Obesity 15(5):1164–1171
Krishnan B, Jeffery A, Metcalf B, Hosking J, Voss L, Wilkin T, Flanagan DE (2009) Gender differences in the relationship between heart rate control and adiposity in young children: a cross-sectional study (EarlyBird 33). Pediatr Diabetes 10(2):127–134
Lenard Z, Studinger P, Mersich B, Kocsis L, Kollai M (2004) Maturation of cardiovagal autonomic function from childhood to young adult age. Circulation 110(16):2307–2312
Longin E, Dimitriadis C, Shazi S, Gerstner T, Lenz T, Konig S (2009) Autonomic nervous system function in infants and adolescents: impact of autonomic tests on heart rate variability. Pediatr Cardiol 30(3):311–324
Magagnin V, Bassani T, Bari V, Turiel M, Maestri R, Pinna GD, Porta A (2011) Non-stationarities significantly distort short-term spectral, symbolic and entropy heart rate variability indices. Physiol Meas 32(11):1775–1786
Massin M, vonBernuth G (1997) Normal ranges of heart rate variability during infancy and childhood. Pediatr Cardiol 18(4):297–302
Massin M, Maeyns K, Withofs N, Ravet F, Gerard P (2000) Circadian rhythm of heart rate and heart rate variability. Arch Dis Child 83(2):179–182
Michels N, Vanaelst B, Vyncke K, Sioen I, Huybrechts I, De Vriendt T, De Henauw S (2012) Children’s body composition and stress—the ChiBS study: aims, design, methods, population and participation characteristics. Arch Public Health 70(1):17
Nagai N, Moritani T (2004) Effect of physical activity on autonomic nervous system function in lean and obese children. Int J Obes Relat Metab Disord 28(1):27–33
Niskanen JP, Tarvainen MP, Ranta-Aho PO, Karjalainen PA (2004) Software for advanced HRV analysis. Comput Methods Programs Biomed 76(1):73–81
Ortega FB, Artero EG, Ruiz JR, Vicente-Rodriguez G, Bergman P, Hagstromer M, Ottevaere C, Nagy E, Konsta O, Rey-Lopez JP, Polito A, Dietrich S, Plada M, Beghin L, Manios Y, Sjostrom M, Castillo MJ, Group HS et al (2008) Reliability of health-related physical fitness tests in European adolescents. The HELENA Study. Int J Obes 32(Suppl 5):S49–S57
Penttila J, Helminen A, Jartti T, Kuusela T, Huikuri HV, Tulppo MP, Coffeng R, Scheinin H (2001) Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: effects of various respiratory patterns. Clin Physiol 21(3):365–376
Porta A, Baselli G, Guzzetti S, Pagani M, Malliani A, Cerutti S (2000) Prediction of short cardiovascular variability signals based on conditional distribution. IEEE Trans Biomed Eng 47(12):1555–1564
Rabbia F, Silke B, Conterno A, Grosso T, De Vito B, Rabbone I, Chiandussi L, Veglio F (2003) Assessment of cardiac autonomic modulation during adolescent obesity. Obes Res 11(4):541–548
Silvetti MS, Drago F, Ragonese P (2001) Heart rate variability in healthy children and adolescents is partially related to age and gender. Int J Cardiol 81(2–3):169–174
Task Force of ESC/NASPE (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93(5):1043–1065
Thayer JF, Yamamoto SS, Brosschot JF (2010) The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 141(2):122–131
Trost SG, Loprinzi PD, Moore R, Pfeiffer KA (2011) Comparison of accelerometer cut points for predicting activity intensity in youth. Med Sci Sports Exerc 43(7):1360–1368
Umetani K, Singer DH, McCraty R, Atkinson M (1998) Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol 31(3):593–601
Wallis LA, Healy M, Undy MB, Maconochie I (2005) Age related reference ranges for respiration rate and heart rate from 4 to 16 years. Arch Dis Child 90(11):1117–1121
Wells JC, Williams JE, Chomtho S, Darch T, Grijalva-Eternod C, Kennedy K, Haroun D, Wilson C, Cole TJ, Fewtrell MS (2010) Pediatric reference data for lean tissue properties: density and hydration from age 5 to 20 y. Am J Clin Nutr 91(3):610–618
Acknowledgments
This work was done as part of the IDEFICS Study (http://www.idefics.eu). We gratefully acknowledge the financial support of the European Community within the Sixth RTD Framework Programme Contract No. 016181 (FOOD). The information in this document reflects the author’s view and is provided as is. Nathalie Michels is financially supported by the research council of Ghent University (Bijzonder Onderzoeksfonds). Isabelle Sioen and Barbara Vanaelst are financially supported by the Research Foundation—Flanders. The results of the present study do not constitute endorsement by ACSM. The authors want to thank the ChiBS children and their parents for their voluntary participation.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Massimo Pagani.
Rights and permissions
About this article
Cite this article
Michels, N., Clays, E., De Buyzere, M. et al. Determinants and reference values of short-term heart rate variability in children. Eur J Appl Physiol 113, 1477–1488 (2013). https://doi.org/10.1007/s00421-012-2572-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2572-9