Abstract
Prolonged dysregulation of the autonomic nervous system (ANS) may increase propensity for physical or psychiatric illness. The current study examined differences in respiratory sinus arrhythmia (RSA) regulation in 215 adolescents with or without autism spectrum disorder (ASD) at Time 1 (T1; 10–13 years old) and 1 year later (Time 2; T2). Linear mixed effects models demonstrated lower RSA regulation in ASD, and a small interaction effect, showing blunted change in RSA from T1 to T2. Developmental differences in RSA regulation were particularly notable in females with ASD and those taking psychotropic medications. Results expand previous findings of reduced parasympathetic regulation in ASD by revealing a blunted developmental slope, indicating diagnostic differences may persist or worsen over time, particularly in females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The parasympathetic nervous system (PNS), one of two branches of the autonomic nervous system (ANS), is involved in maintenance and regulation of unconscious, automatic bodily systems. The PNS regulates ‘rest and digest’ functions, such as lowering heart rate or blood pressure and increasing digestion. It largely serves to decrease physiological arousal, promote a state of relative calm, and conserve energy. In coordination with the sympathetic nervous system (SNS; ‘fight or flight’ branch), the PNS serves to regulate behavior and adaptive physiological response to changing environmental stimuli (Berntson et al., 2008). Consequences of proper or atypical functioning of the ANS thus go beyond physiology, as its moderating role in behavioral and emotional regulation may have significant implications for a broad range of psychopathologies.
The activity of the PNS can be measured by observing changes in visceral organ function such as heart rate, as the autonomic system innervates the sinoatrial node—the pacemaker—of the heart (Hamill et al., 2012; Longhurst & Fu, 2012). A common marker of autonomic function, heart rate variability (HRV), utilizes changes in the beat-to-beat intervals in heart rate to inform the relative contributions of both the PNS and the SNS. For example, changes in HRV within high frequency respiration ranges (Respiratory sinus arrhythmia; RSA) indicate changes in PNS regulation. Pharmacological blockade studies have shown that RSA is sensitive to cholinergic, but not adrenergic, receptor blockade, providing empirical support for its use as a parasympathetic marker (Berntson et al., 1993; Cacioppo et al., 1994). Furthermore, an extensive, interconnected neural network is responsible for regulating autonomic function, extending from reflexive or stimulus-specific control at the spinal- and brainstem-level to more integrated behavioral responses requiring cognitive processing at the forebrain level (Benarroch, 2012). While several brainstem regions coordinate physiological response by processing afferent stimuli, (e.g., Hamill et al., 2012) several major forebrain regions, collectively known as the Central Autonomic Network (Benarroch, 1993), exert additional top-down control over the system. For example, the amygdala can modulate autonomic response to emotional stimuli through direct connections with brainstem regions (Schwaber et al., 1982). The prefrontal cortex, which coordinates a number of cognitive control functions, projects to other major limbic regions (Thayer & Lane, 2000), including the amygdala and hippocampus, thereby positioning itself at the top of the response hierarchy (Ulrich-Lai & Herman, 2009).
Autonomic dysregulation has been implicated in autism spectrum disorder (ASD), a neurodevelopmental disorder characterized by impaired social communication and presence of restricted, repetitive behaviors and interests (APA, 2013). Current estimates suggest that one in every 54 children is diagnosed with ASD by the age of 8 years old (Maenner et al., 2020). Males are 4 times more likely to be diagnosed with ASD relative to females, although recent evidence suggests the ratio may be closer to 3:1 (Loomes et al., 2017). Individuals with ASD are at elevated risk for a number of comorbid conditions, including but not limited to epilepsy, sleep disorders, gastrointestinal dysfunction, attention deficit/hyperactivity disorder, anxiety disorders, and depression (e.g., Accardo & Malow, 2015; McElhanon et al., 2014; Simonoff et al., 2012). Comorbid rates of depression and anxiety are especially high, with lifetime anxiety prevalence recently estimated at 42% (Hollocks et al., 2019), and percentage of individuals diagnosed with a depressive disorder by adulthood estimated at 40% (Hollocks et al., 2019; Hudson et al., 2019), far surpassing global rates (Mayes et al., 2011). Notably, a dynamic equilibrium between the two branches of the ANS is said to be associated with fewer mental health problems (Nederhof et al., 2015). In contrast, an inflexible, invariable system is often characteristic of psychopathological states (Friedman, 2007; Friedman & Thayer, 1998; Thayer et al., 1996, 2000), highlighting the significance of identifying ANS dysfunction in autism and the extent to which it may contribute to psychopathologies in these individuals.
Previous research in ASD and ANS functioning often centers around the social deficits characteristic of the neurodevelopmental disorder, guided by the Polyvagal Theory (Porges, 1995, 2001, 2003a, 2007), which proposes that a parasympathetically-mediated default state regulates a ‘social engagement system’ (Porges, 2001, 2003b) of interconnected brainstem nuclei that regulate the myelinated vagus as well as cranial nerves directly involved in controlling muscles of the face and head. Therefore, in a parasympathetic-dominant system, calm visceral states may promote these pro-social behaviors such as eye contact and language production. However, if the PNS is poorly regulated and/or overridden by the SNS branch, this will block the social engagement system (Porges, 2003a, 2003b, 2007). Further, the opposite may be true, in which social difficulties contribute to autonomic dysfunction. For example, social impairments characteristic of ASD may predispose youth to difficult peer interactions, and over time older youth with ASD may become more aware of these social challenges and the effects on relationships (Knott et al., 2006; Kuusikko et al., 2008; Lopata et al., 2008). This may in turn shape future social anxiety (Bellini, 2006), driving limbic system regions to over-activate the ‘fight or flight’ sympathetic response and decrease parasympathetic regulation. As such, dysfunction within the vagal and social engagement system could be bidirectional with socially adaptive behaviors, signaling significant social and physiological implications for a number of clinical populations, including ASD.
Indeed, decreased parasympathetic regulation has been reported in individuals with ASD relative to those with typical development (TD) (Bal et al., 2010; Edmiston et al., 2016; Guy et al., 2014; Ming et al., 2005; Neuhaus et al., 2016; Vaughan Van Hecke et al., 2009). Further evidence notes atypical autonomic response to stressors, including reduced vagal parasympathetic flexibility (Muscatello et al., 2021a, 2021b; Neuhaus et al., 2016; Schaaf et al., 2015; Vaughan Van Hecke et al., 2009) as well as an atypically blunted sympathetic response (Edmiston et al., 2017). Nevertheless, it should be mentioned that several studies cite no significant autonomic differences in ASD, either during resting state (Kushki et al., 2014; Levine et al., 2012; Muscatello et al., 2020; Watson et al., 2012) or in response to a stressor (Hollocks et al., 2014; Kushki et al., 2014; Sheinkopf et al., 2013).
The variability in findings is likely a reflection of the heterogenous presentation of ASD, as well as contributions of factors contributing to vagal and autonomic functioning, including physical health, (e.g., Masi et al., 2007; Molfino et al., 2009; Shibao & Okamoto, 2012; Thayer & Sternberg, 2006), age, pubertal development (e.g., Eyre et al., 2014; Harteveld et al., 2021; Hinnant et al., 2011), and/or sex differences (e.g., Harteveld et al., 2021; Hinnant et al., 2011; Koenig et al., 2017). For example, there is evidence that resting-state HRV increases progressively from young- to middle-childhood, before reaching a relative plateau in late adolescence (Eyre et al., 2014; Harteveld et al., 2021), although stability may be affected by demographic factors such as race (Hinnant et al., 2011). For ASD, a recent study of autonomic reactivity during a social interaction task found that in school-aged youth (10–13 years) with ASD, age significantly moderated parasympathetic reactivity, with older youth demonstrating a particularly atypical reactivity pattern (Muscatello et al., 2021a, 2021b). In relation to psychopathologies, an examination of children 5–14 years with low- or high-risk of depression (based upon parent history of childhood-onset mood disorder) and without ASD demonstrated lower resting RSA in the high-risk group and a significant age by group effect, such that the developmental slope of resting RSA was blunted in the high-risk group (Gentzler et al., 2012).
As mentioned, effects of biological sex on RSA are notable, which may have implications for the variability of findings regarding ANS function in ASD. In non-clinical samples, it has been reported that male youth have greater parasympathetic regulation relative to females (see Koenig et al., 2017 for review). Yet, a recent large-scale analysis found females reached peak PNS regulation at an earlier age than males, likely due to hormonal and pubertal timing differences between sexes (Harteveld et al., 2021). Studies of adult females show strong associations between hormonal changes associated with menstruation and the ovulatory cycle and heart rate variability (Schmalenberger et al., 2019, 2020; Tenan et al., 2014; Vallejo et al., 2005). Considering the prevalence of males and historical under-representation of females in autism research, studies of ANS regulation in ASD have often been restricted to all-male samples, with little or no investigation of sex differences in this population. One recent study of adolescents noted significant variability within a small sample of females with ASD (Edmiston et al., 2016); however, the sample was under-powered to further examine these sex differences and thus included only males in final analyses. A greater understanding of sex-based autonomic differences and their relationship to symptoms of ASD is critical, particularly during the pubertal period, where females with ASD may be particularly impacted by atypical development (Corbett et al., 2020).
To address remaining questions regarding the role of development and biological sex in ANS functioning of youth with ASD, the current study aimed to examine diagnostic differences in resting parasympathetic regulation over the course of 2 years in adolescents with ASD or TD. The study sought to provide a preliminary characterization of the extent to which pubertal development and biological sex further contributed to differences in PNS functioning in adolescents with ASD. It was hypothesized that youth with ASD would demonstrate lower resting-state RSA across the two timepoints (1 year apart) (Hypothesis 1). Pubertal development was predicted to be positively related with RSA, where advanced pubertal status and older age correlate with higher resting state PNS (Hypothesis 2). Finally, preliminary analyses explored between- and within-sex differences. Particular focus was placed on females with and without ASD as previous research by the study team (Edmiston et al., 2016) cited significant variability within a small sample of females with ASD.
Materials and Methods
Participants
Participants were enrolled in a large, longitudinal study of pubertal development in youth with and without ASD (Corbett 2017). The current study includes data from Years 1 and 2 of the four-year study. At T1, 215 youth ages 10–13 years completed baseline RSA collection, including 123 youth with ASD (90 male, 33 female) and 92 TD (52 male, 40 female). Of these 215, a total of 184 participants (95 ASD, 89 TD) returned 1 year later for the T2 follow-up visits. Of those, 45 youth had incomplete data from an inability to complete in-lab visits, including RSA collection, due to the COVID-19 pandemic and associated shut-downs. There were no significant differences in age, IQ, T1 resting RSA, or BMI percentile between those who did and did not complete the full, in-person T2 study visit (all p > 0.05).
An estimated 42% of children with ASD have been reported to take at least one psychotropic medication (Mire et al., 2014), thus the current study did not include a completely medication-naïve sample to be more representative of the overall ASD population. At T1, In the ASD group, 49.6% of youth were taking at least one psychotropic medication, while 9.8% of TD participants reported taking a daily psychotropic. At T2, distribution of medicated vs. non-medicated were similar, with 48.9% of ASD participants on psychotropics compared to 10.2% of TD participants.
To be included in the study, participants were required to have an intelligence quotient (IQ) ≥ 70 as estimated by the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II, Wechsler, 2011). Participants in the ASD group had a confirmed diagnosis according to Diagnostic and Statistical Manual-5 criteria (APA, 2013). Diagnoses were confirmed by: (1) a previous diagnosis by a psychologist, psychiatrist, or behavioral pediatrician with autism expertise; (2) current clinical judgment, and (3) corroborated by the Autism Diagnostic Observation Schedule (ADOS-2; Lord et al., 2012), a semi-structured interview-based instrument administered by research-reliable personnel. The TD group was defined as adolescents without ASD or other developmental delay and/or neurodevelopmental disorder based on parent screening. Parents completed the Social Communication Questionnaires (SCQ; Rutter et al., 2003), a parent-report questionnaire used to screen for ASD symptoms. To be included in the TD group, youth had to score < 10 on the SCQ.
Procedures
All study procedures were approved by the Vanderbilt University Institutional Review Board (IRB) and were carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Parents/guardians and youth participants provided written consent and written and verbal assent, respectively.
Participation included two visits to the research laboratory at T1 and an additional visit 1 year later (T2). At the initial visit, all study procedures were discussed, and consent/assent was obtained from the guardian and child, respectively. Following consent, eligibility was confirmed by administration of the ADOS-2 (Lord et al., 2012) for ASD participants and the WASI-2 (Wechsler, 2011) for all participants. Following confirmation of eligibility, youth underwent a brief physical examination and completed the resting-state RSA collection (procedures described below). Approximately 12 months after completion of T1, participants returned for the T2 visit, when physical examination and resting RSA collection were repeated.
Pubertal Status
To obtain an objective measure of pubertal stage, participants completed a physical examination (PE), conducted by trained and licensed study physicians, to reliably identify pubertal development and determine Tanner stage (Marshall & Tanner, 1969, 1970). Tanner staging included two measures with 5 stages for Genitals (G1-G5 for boys) or Breasts (B1-B5 for girls; GB stage) and Pubic hair (P1-P5 for both genders; PH stage). Full procedures for the exam and reliability of the PE relative to parent- or self-reported measures have been previously described (Corbett et al. 2019). While GB staging is considered more reliable than PH staging (Emmanuel & Bokor, 2022), analyses were conducted for GB and PH scales separately for completeness and to be consistent with previous studies reporting on both staging categories (e.g., (Corbett et al., 2021b and Corbett et al., 2020)).
Body Mass Index (BMI)
During the physical exam, height (in.) and weight (lb.) were collected by study physicians using a calibrated stand-on Health-o-meter TM Professional 499KL Waist High Digital Scale with Height Rod (Hogentogler & Co., MD, USA). BMI was calculated according to the standard formula ([lb/in2] × 703) and converted into age- and sex-adjusted percentiles according to Center for Disease Control (CDC) growth charts for children and adolescents ages 2–19 years (https://www.cdc.gov/healthyweight/bmi/calculator.html).
Resting Respiratory Sinus Arrhythmia (RSA)
RSA was collected using MindWare Mobile Impedance Cardiograph units (MindWare Technologies LTD, Gahanna, OH) for synchronized electrocardiography (ECG) and respiration data collection using a seven-electrode configuration. To reduce potential anxiety surrounding the protocol, electrodes were described as ‘stickers’, and visual guides were available to demonstrate location of the electrodes on the torso. If participants expressed significant discomfort from the electrode placement, the protocol was discontinued. Of the 215 participants at T1, four participants with ASD were unable to complete the RSA collection procedures due to sensory sensitivities associated with the stickers. Additionally, 3 TD participants were missing baseline data due to equipment error or atypical heart rhythm. Data collection began following a 25-min acclimation period to the lab environment. Resting RSA was measured during a five-minute rest period in which all participants were instructed to sit quietly without engaging in any other tasks to reduce any potential confounding effects of movement or activity.
RSA was derived in accordance with guidelines set forth by the Society for Psychophysiological Research committee on heart rate variability (Task Force, 1996). ECG signal was sampled at 500 Hz and analyzed using the Heart Rate Variability Software Suite provided by MindWare Technologies (MindWare Technologies LTD, Gahanna, OH). RSA was quantified as the natural logarithm of integral power within the respiratory frequency (high frequency) band (0.12 to 0.42 Hz), and respiration was monitored by impedance cardiography. The respiration signal was displayed to ensure that values were within the designated frequency band. Respiratory frequency was confirmed to lie within the high frequency/RSA band (0.12–0.42 Hz) for all participants. Of the total processed data, 3.5% were excluded due to excessive motion artifact, equipment error, or cardiac arrhythmias. RSA was measured as ln ms2.
Statistical Analysis
Demographic variables were compared between ASD and TD groups using independent sample t-tests. If the assumption of equal variance was violated, the Welch degree of freedom approximation was used. RSA values were normally distributed and free of extreme outliers.
Hypotheses were tested using linear mixed effects models with a random intercept for subject to account for correlation within subjects. To test the hypothesis that resting RSA across T1 and T2 differed by diagnosis and sex, we fit a model of resting RSA with main effects for sex, diagnosis, and time. Psychotropic medication use was included as a covariate in all models. To test the extent to which change in RSA levels over time differed by diagnostic status, an interaction term between diagnosis and time was included. To examine hypothesized relationships between RSA and physical development, an additional model was fit including age, GB stage, and BMI as potential covariates or effect modifiers. The model was repeated using PH stage as well. Preliminary analyses were carried out in males and females separately to observe potential differences in resting RSA and development within-sex. Robust effect size index (S) (Vandekar et al., 2020) was computed, where S is equal to ½ Cohen’s d. Thresholds were interpreted according to Cohen (Cohen, 1988) for small (d = 0.2, S = 0.1), medium (d = 0.5, S = 0.25) and large (d = 0.8, S = 0.4) effects. All analyses were performed using IBM SPSS Statistics 28 (“IBM SPSS Statistics for Mac,” 2021).
Results
Demographic and diagnostic information is presented in Table 1. Groups did not differ on age, p > 0.05. While there was a significant difference between groups based upon IQ, t (211.88) = 7.07, p < 0.001, the ASD group fell well within the average range. As expected, ASD youth had significantly higher SCQ scores, t (150.98) = − 18.42, p < 0.001, and, consistent with previous reports (Corbett et al., 2021), average BMI percentile was significantly elevated in the ASD group, t (211) = − 2.61, p = 0.01.
Diagnosis and Developmental Effects on RSA
To test Hypothesis 1 anticipating diagnostic differences in resting RSA, we fit a model with main effects for sex, diagnosis, time, and an interaction term for diagnosis and time. To account for diagnostic differences in psychotropic medication use, this was included as a covariate of interest in the model. Results are presented in Table 2, and parameter estimates are available in Supplemental Table 1. As shown in Fig. 1, resting state RSA significantly increased from T1 to T2, p = 0.034, particularly within the TD group. There was a significant main effect for diagnosis, with lower RSA in youth with ASD, p = 0.046, as well as an interaction between diagnosis and time, p = 0.05, demonstrating a small effect size, S = 0.11. Specifically, the ASD group had a blunted change in RSA from T1 to T2 (see Fig. 1). Notably, psychotropic medication use was a significant covariate of interest, p = 0.02, where taking psychotropics was associated with lower RSA. There was no significant main effect for sex, p > 0.05.
Hypothesis 2 predicted positive associations with advanced physical development. Thus, a second model was fit with BMI, GB stage, and age. As shown in Table 3, age and GB stage did not contribute substantially over the effects of other covariates, p > 0.05. Therefore, we did not find sufficient evidence for an association between age and GB stage with RSA values. Consistent with previous reports (Muscatello et al., 2021a), there was a significant main effect for BMI, p = 0.017, with elevated BMI associated with lower resting RSA (see Supplemental Table 2 for parameter estimates). The main effect for diagnosis was no longer significant, p > 0.05, while psychotropic medication use remained a statistically significant predictor of RSA, p = 0.005 with a trend diagnosis and time interaction with small effect, p = 0.06, S = 0.12.
The model was repeated, including PH stage instead of GB stage. Similarly, PH stage was not associated with RSA, p > 0.05 (Supplemental Table 3).
Preliminary Within-Sex Analysis
As reported above, there were no significant between-sex differences in the primary models. However, the unequal distribution of males and females, with a notable male bias, may have left models underpowered to detect sex-based differences. To explore differences within males and females with or without ASD, preliminary analyses were conducted separately for males and for females. In males, there was neither a significant effect for diagnosis, p = 0.72, S = 0.0, nor for the diagnosis and time interaction, p = 0.11, S = 0.11 (See Table 4; Fig. 2). However, an increase in GB stage predicted an increase in RSA, p = 0.025, S = 0.18, while medications, p = 0.025, S = 0.18, and BMI, p = 0.01, S = 0.21, predicted lower RSA in males.
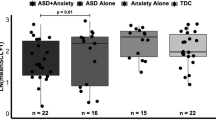
A different pattern of effects was observed in females. While diagnosis was similarly not statistically significant, it did maintain a small effect, S = 0.10 (Table 4). As shown in Fig. 3, females with ASD tended to have lower resting state RSA. Further, the diagnosis and time interaction did not reach the level of statistical significance, p > 0.05, but showed a small effect, S = 0.16. In contrast to males, BMI was not a significant predictor of RSA, p > 0.05. Notably, psychotropic medication use was a particularly strong predictor of lower RSA in females, p = 0.01, S = 0.32). Parameter estimates are presented in Supplemental Tables 4 and 5.
Discussion
To our knowledge, this study is the first to longitudinally examine resting state parasympathetic function in adolescents with ASD. Findings demonstrated youth with ASD have consistently lower resting RSA relative to their TD peers. Notably, the expected developmental increase in resting RSA may be slowed in ASD. Pilot analyses within males and females further suggest a unique phenotype characterized by lower RSA may be especially prevalent in females with ASD, particularly those taking psychotropic medications. The apparent developmental differences in PNS regulation over two timepoints, separated by 1 year, provide preliminary evidence for persistent autonomic dysfunction in ASD, which may worsen relative to TD peers throughout the adolescent period.
As predicted by Hypothesis 1, RSA in youth with ASD was significantly lower compared to youth with TD. Findings contribute to a growing literature citing reduced PNS functioning across the lifespan (e.g., Patriquin et al., 2019). This diminished parasympathetic regulation has been associated with more severe social symptoms in ASD (Edmiston et al., 2016; Neuhaus et al., 2014; Patriquin et al., 2013; Vaughan Van Hecke et al., 2009). Further, research in non-ASD populations cites atypical ANS regulation in psychopathological conditions, including, anxiety (e.g., Chalmers et al., 2014; Thayer et al., 1996), depression (e.g., Koenig et al., 2016; Rottenberg et al., 2007), and Post-Traumatic Stress Disorder (e.g., Campbell et al., 2019). Given the prevalence of both physiological dysfunction and psychological comorbidities, ongoing efforts have sought to determine whether the two are related in ASD. Some findings have reported correlations between autonomic regulation and response with internalizing symptoms (e.g., anxiety, depression) in ASD (Kushki et al., 2013; Muscatello et al., 2020; Neuhaus et al., 2014; Panju et al., 2015), while other studies in ASD have been unable to demonstrate a link between autonomic regulation and anxiety or depression (Hollocks et al., 2014, 2016; Kushki et al., 2014). Considering the current findings of lower RSA in ASD throughout the early adolescent years, it will be important to examine the trajectory of autonomic functioning as a potential marker of risk for, or resilience to, later internalizing diagnoses.
In the current study, a diagnosis and time interaction was observed, in which youth with ASD had a blunted slope in RSA change from T1 to T2. Research suggests that normative development of the PNS includes a gradual increase in regulation throughout childhood, with a plateauing around late-childhood and adolescence (e.g., Gatzke-Kopp & Ram, 2018; Harteveld et al., 2021; Hinnant et al., 2011). Moreover, at least one study in typically developing individuals demonstrated differential parasympathetic reactivity to stress in younger versus older youth (El-Sheikh, 2005). Cross-sectional analyses of physiological systems support age and developmental differences in ASD. For example, studies of school-aged youth found evidence of decreasing heart rate in older youth both with and without ASD (Harder et al., 2016; Kushki et al., 2014). However, there were no age effects in cardiac autonomic function, particularly parasympathetic-mediated RSA (Harder et al., 2016). In stress reactivity, recent evidence reported that youth with ASD had lower RSA response to social interaction relative to same-aged TD peers, and with diagnostic differences especially prevalent in the older ASD youth (Muscatello et al., 2021a, 2021b). Furthermore, examination of related stress systems, such as the hypothalamic–pituitary–adrenal axis, have reported diagnostic differences in physiological functioning to be positively associated with older age in ASD children and adolescents (e.g., Corbett et al., 2010, 2021a, 2021b; Muscatello & Corbett, 2018; Schupp et al., 2013). The current study suggests a delayed developmental slope may be associated with an autism diagnosis, emphasizing the need for future research into whether atypical developmental trajectories persist throughout later stages of puberty and are associated with physical, behavioral, and/or psychological outcomes.
Despite the within-individual change in RSA over the two timepoints, neither pubertal development (Tanner stage) nor age was significantly associated with average resting RSA in the full sample. However, genital/breast stage was associated with elevated RSA in males and was at trend in females. It may be that the differences in timing and tempo between males and females (Biro et al., 2001; Marceau et al., 2011; Mendle et al., 2010) are important for considering developmental influence on physiology, such as RSA, particularly in ASD (Corbett et al., 2020). It should also be acknowledged that developmental change in ANS functioning is complex, as a number of contextual factors (e.g., family stress, poverty) may contribute to individual differences and variability (Gatzke-Kopp & Ram, 2018; Hinnant et al., 2011). Furthermore, despite the longitudinal nature of the study, the total age range of the current study sample remained relatively narrow at ages 10–14 years. During the 1 year especially, the majority of participants were at the earlier stages of pubertal development (Tanner 1 and 2). The current sample will be followed longitudinally for an additional 2 years, and it is expected that pubertal and age effects will become evident as the participants progress into mid- to late-adolescence. A more intensive investigation of pubertal timing and tempo in ASD from early to late puberty, considering other contextual factors such as sex, race, and trauma, among others, will more completely outline the extent to which development of the parasympathetic system is related to the broader autism phenotype.
Lastly, the current study sought to examine between- and within-sex differences in autonomic function, particularly in ASD. Primary models were not significant for a main effect of sex (male vs. female). However, the distribution of males versus females was heavily biased towards males, likely hindering power for detecting differences in females. As such, preliminary analyses were carried out separately for males and for females to examine diagnostic effects within sex. As reported and displayed in Figs. 2 and 3, the diagnostic effect for lower RSA in ASD was more pronounced in females, though neither group reached the level of statistical significance. Nevertheless, this finding may be consistent with previous research noting significant variability in RSA in female adolescents with ASD (Edmiston et al., 2016).
Evidence in typically developing youth and adults supports sex-based differences in autonomic functioning. For example, females tend to demonstrate reduced vagal activity and increased heart rate compared to their male peers (Koenig et al., 2017). Hormonal influences are particularly notable for females, with reported differences in HRV according to the phase of the menstrual cycle (Schmalenberger et al., 2019, 2020). As Koenig and Thayer (2016) explain, lower vagal activity in females, particularly during the adolescent period, may have significant clinical implications. Namely, reduced PNS activity is associated with psychopathologies, including depression (e.g., Koenig et al., 2016), with higher prevalence rates in females relative to males (Kessler, McGonagle, Swartz, Blazer, & Nelson, 1993). Concerningly, an estimated 40% of adults with ASD will be diagnosed with a depressive disorder in their lifetime (Hollocks et al., 2019; Hudson et al., 2019), well-above the global population average (Mayes et al., 2011). The combination of increased prevalence based upon female sex and an autism diagnosis suggests an additive effect, which leaves females with ASD particularly susceptible (Schwartzman et al., 2022). As such, a close examination of sex differences in parasympathetic regulation and implications for later development of internalizing conditions may be especially critical in female adolescents with ASD.
Lastly, the contribution of psychotropic medication use cannot be ignored. As previously described, reductions in RSA have been frequently cited within several psychiatric diagnoses, such as anxiety and depression (Koenig et al., 2016; Thayer et al., 1996). It has been hypothesized that use of psychotropic medication, particularly antidepressants, moderates the observed relationship between psychiatric disorders and reduced parasympathetic regulation (Licht et al., 2010). However, a recent large meta-analysis of 170 studies concluded that medications had only a small impact on autonomic function in otherwise healthy participants with a psychiatric disorder (Alvares et al., 2016). In our study, we did find psychotropic medication use to be predictive of RSA. Considering the large number of youth with ASD reported to be taking psychotropics (Mire et al., 2014), it will be important for future studies to closely examine the extent to which medication use and other health factors contribute to the lower RSA reported across numerous studies of ASD youth.
Limitations
The current study was strengthened by the longitudinal examination of resting RSA to examine changes in PNS function over 2 years. Pubertal development was measured using a gold-standard physician-conducted physical examination. Despite these strengths, limitations do exist. First, the sample was relatively limited to early adolescence, whereas important differences may emerge later in development. Future studies following the current sample over a total of 4 years will ultimately provide a more nuanced overview of parasympathetic development in ASD throughout a broader range of puberty and adolescence. Relatedly, the two assessments were one-year apart, and it remains unclear the extent to which group differences may change, if at all, over the course of several years. Secondly, the total number of females in the current sample limited our ability to closely examine sex differences within diagnoses. Preliminary findings noted lower PNS regulation in ASD, particularly ASD females. Thus, future studies in a much larger sample of females will provide insight into the effects of sex on PNS development. Additionally, the sample was limited to individuals with an IQ > 70 and who predominantly identified as White. While the sample included youth on medications to be more inclusive and representative of the population, the current study did not examine the possible effects of different types of medications on physiological functioning. Future efforts may aim to include more diverse samples with a broad range of cognitive functioning and from under-represented groups to expand conclusions across the full range of the autism spectrum and account for potential RSA development differences based upon IQ or race. It will also be important to further consider the role of possible confounding variables, including medication use, BMI changes, or occurrence of mood and other comorbid conditions in expanded longitudinal studies with a diverse sample. In addition to attrition, a common challenge in longitudinal studies, the T2 sample was affected by the early months of the COVID-19 pandemic and associated shutdowns. We were unable to collect second timepoint RSA data for several participants as all research visits were moved to a virtual format and limited to psychological testing and behavioral reports. Finally, the current study relied on a sole measure of parasympathetic function, namely, RSA; however, some research suggests RSA may not be a perfect metric of pure cardiac vagal tone (Grossman & Taylor, 2007). Future studies will be pursued in which multiple autonomic measures are included, such as those indexing both parasympathetic and sympathetic function, to provide a more comprehensive understanding of autonomic function or dysfunction in ASD. Nevertheless, the current study provides critical preliminary evidence for an extended difference in parasympathetic influence within ASD, which warrants further exploration.
Conclusion
The current study provides preliminary support for atypical developmental trajectories of parasympathetic regulation in ASD. Over a two-timepoint period (Years 1 and 2 of a longitudinal study), adolescents with ASD demonstrated lower RSA, with differences in the 2nd year exacerbated by an apparent blunted trajectory in ASD. While no direct evidence was observed for either pubertal stage or age as a predictor of resting RSA across the full sample, participants will be followed for a total of 4 years, so that relationships across the broader developmental range may be revealed. Finally, preliminary evidence of within-sex differences suggest diagnostic effects may be especially apparent for females with ASD. It will be necessary to consider potential clinical implications of prolonged ANS dysfunction, and future studies will continue to elucidate the impact of physiological functioning on sociobehavioral outcomes as well as co-occurring psychopathological conditions in individuals with ASD.
References
Accardo, J. A., & Malow, B. A. (2015). Sleep, epilepsy, and autism. Epilepsy & Behavior, 47, 202–206. https://doi.org/10.1016/j.yebeh.2014.09.081
Alvares, G. A., Quintana, D. S., Hickie, I. B., & Guastella, A. J. (2016). Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: A systematic review and meta-analysis. Journal of Psychiatry and Neuroscience, 41(2), 89–104. https://doi.org/10.1503/jpn.140217
APA. (2013). Diagnostic and statistical manual of mental disorders (DSM-5) (5th ed.). American Psychiatry Association.
Bal, E., Harden, E., Lamb, D., Van Hecke, A. V., Denver, J. W., & Porges, S. W. (2010). Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders, 40(3), 358–370. https://doi.org/10.1007/s10803-009-0884-3
Bellini, S. (2006). The development of social anxiety in adolescents with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities, 21(3), 138–145. https://doi.org/10.1177/10883576060210030201
Benarroch, E. E. (1993). The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clinic Proceedings, 68(10), 988–1001. https://doi.org/10.1016/s0025-6196(12)62272-1
Benarroch, E. E. (2012). Central autonomic control. In D. Robertson, I. Biaggioni, G. Burnstock, P. A. Low, & J. F. R. Paton (Eds.), Primer on the autonomic nervous system (3rd ed., pp. 9–12). Academic Press.
Berntson, G. G., Cacioppo, J. T., & Quigley, K. S. (1993). Cardiac psychophysiology and autonomic space in humans: Empirical perspectives and conceptual implications. Psychological Bulletin, 114(2), 296–322. https://doi.org/10.1037/0033-2909.114.2.296
Berntson, G. G., Norman, G. J., Hawkley, L. C., & Cacioppo, J. T. (2008). Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology, 45(4), 643–652. https://doi.org/10.1111/j.1469-8986.2008.00652.x
Biro, F. M., McMahon, R. P., Striegel-Moore, R., Crawford, P. B., Obarzanek, E., Morrison, J. A., & Falkner, F. (2001). Impact of timing of pubertal maturation on growth in black and white female adolescents: The national heart, lung, and blood institute growth and health study. Journal of Pediatrics, 138(5), 636–643. https://doi.org/10.1067/mpd.2001.114476
Cacioppo, J. T., Uchino, B. N., & Berntson, G. G. (1994). Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology, 31(4), 412–419. https://doi.org/10.1111/j.1469-8986.1994.tb02449.x
Campbell, A. A., Wisco, B. E., Silvia, P. J., & Gay, N. G. (2019). Resting respiratory sinus arrhythmia and posttraumatic stress disorder: A meta-analysis. Biological Psychology, 144, 125–135. https://doi.org/10.1016/j.biopsycho.2019.02.005
Chalmers, J. A., Quintana, D. S., Abbot, M.J.-A., & Kemp, A. H. (2014). Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Frontiers in Psychiatry. https://doi.org/10.3389/fpsyt.2014.00080/abstract
Cohen, J. (1988). Statisical Power Analysis for the Behavioral Sciences (2nd ed.). Lawrence Erlbaum Associates.
Corbett, B. A. (2017). Examining stress and arousal across pubertal development in ASD. National Institute of Mental Health.
Corbett, B. A., Muscatello, R. A., Horrocks, B. K., Klemencic, M. E., & Tanguturi, Y. (2021a). Differences in body mass index (bmi) in early adolescents with autism spectrum disorder compared to youth with typical development. Journal of Autism and Developmental Disorders, 51(8), 2790–2799. https://doi.org/10.1007/s10803-020-04749-0
Corbett, B. A., Muscatello, R. A., Kim, A., Patel, K., & Vandekar, S. (2021b). Developmental effects in physiological stress in early adolescents with and without autism spectrum disorder. Psychoneuroendocrinology, 125, 105115. https://doi.org/10.1016/j.psyneuen.2020.105115
Corbett, B. A., Muscatello, R. A., Tanguturi, Y., McGinn, E., & Ioannou, S. (2019). Pubertal development measurement in children with and without autism spectrum disorder: A comparison between physical exam, parent- and self-report. Journal of Autism and Developmental Disorders, 49(12), 4807–4819. https://doi.org/10.1007/s10803-019-04192-w
Corbett, B. A., Schupp, C. W., Simon, D., Ryan, N., & Mendoza, S. (2010). Elevated cortisol during play is associated with age and social engagement in children with autism. Molecular Autism, 1(1), 13. https://doi.org/10.1186/2040-2392-1-13
Corbett, B. A., Vandekar, S., Muscatello, R. A., & Tanguturi, Y. (2020). Pubertal timing during early adolescence: Advanced pubertal onset in females with autism spectrum disorder. Autism Research. https://doi.org/10.1002/aur.2406
Edmiston, E. K., Jones, R. M., & Corbett, B. A. (2016). Physiological response to social evaluative threat in adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders, 46(9), 2992–3005. https://doi.org/10.1007/s10803-016-2842-1
Edmiston, E. K., Muscatello, R. A., & Corbett, B. A. (2017). Altered pre-ejection period response to social evaluative threat in adolescents with autism spectrum disorder. Research in Autism Spectrum Disorders, 36, 57–65. https://doi.org/10.1016/j.rasd.2017.01.008
El-Sheikh, M. (2005). Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental Psychobiology, 46(1), 66–74. https://doi.org/10.1002/dev.20036
Emmanuel, M., & Bokor, B. R. (2022). Tanner stages. StatPearls.
Eyre, E. L., Duncan, M. J., Birch, S. L., & Fisher, J. P. (2014). The influence of age and weight status on cardiac autonomic control in healthy children: A review. Autonomic Neuroscience, 186, 8–21. https://doi.org/10.1016/j.autneu.2014.09.019
Friedman, B. H. (2007). An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology, 74(2), 185–199. https://doi.org/10.1016/j.biopsycho.2005.08.009
Friedman, B. H., & Thayer, J. F. (1998). Anxiety and autonomic flexibility: A cardiovascular approach. Biological Psychology, 49(3), 303–323. https://doi.org/10.1016/s0301-0511(98)00051-9
Gatzke-Kopp, L., & Ram, N. (2018). Developmental dynamics of autonomic function in childhood. Psychophysiology, 55(11), e13218. https://doi.org/10.1111/psyp.13218
Gentzler, A. L., Rottenberg, J., Kovacs, M., George, C. J., & Morey, J. N. (2012). Atypical development of resting respiratory sinus arrhythmia in children at high risk for depression. Developmental Psychobiology, 54(5), 556–567. https://doi.org/10.1002/dev.20614
Grossman, P., & Taylor, E. W. (2007). Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology, 74(2), 263–285. https://doi.org/10.1016/j.biopsycho.2005.11.014
Guy, L., Souders, M., Bradstreet, L., DeLussey, C., & Herrington, J. D. (2014). Brief report: Emotion regulation and respiratory sinus arrhythmia in autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(10), 2614–2620. https://doi.org/10.1007/s10803-014-2124-8
Hamill, R. W., Shapiro, R. E., & Vizzard, M. A. (2012). Peripheral autonomic nervous system. In D. Robertson, I. Biaggioni, G. Burnstock, P. A. Low, & J. F. R. Paton (Eds.), Primer on the autonomic nervous system (3rd ed., pp. 17–26). Academic Press.
Harder, R., Malow, B. A., Goodpaster, R. L., Iqbal, F., Halbower, A., Goldman, S. E., & Diedrich, A. (2016). Heart rate variability during sleep in children with autism spectrum disorder. Clinical Autonomic Research, 26(6), 423–432. https://doi.org/10.1007/s10286-016-0375-5
Harteveld, L. M., Nederend, I., Ten Harkel, A. D. J., Schutte, N. M., de Rooij, S. R., Vrijkotte, T. G. M., & Femc, N. A. T. C. D. (2021). Maturation of the cardiac autonomic nervous system activity in children and adolescents. Journal of the American Heart Association, 10(4), e017405. https://doi.org/10.1161/JAHA.120.017405
Hinnant, J. B., Elmore-Staton, L., & El-Sheikh, M. (2011). Developmental trajectories of respiratory sinus arrhythmia and preejection period in middle childhood. Developmental Psychobiology, 53(1), 59–68. https://doi.org/10.1002/dev.20487
Hollocks, M. J., Howlin, P., Papadopoulos, A. S., Khondoker, M., & Simonoff, E. (2014). Differences in HPA-axis and heart rate responsiveness to psychosocial stress in children with autism spectrum disorders with and without co-morbid anxiety. Psychoneuroendocrinology, 46, 32–45. https://doi.org/10.1016/j.psyneuen.2014.04.004
Hollocks, M. J., Lerh, J. W., Magiati, I., Meiser-Stedman, R., & Brugha, T. S. (2019). Anxiety and depression in adults with autism spectrum disorder: A systematic review and meta-analysis. Psychological Medicine, 49(4), 559–572. https://doi.org/10.1017/S0033291718002283
Hollocks, M. J., Pickles, A., Howlin, P., & Simonoff, E. (2016). Dual cognitive and biological correlates of anxiety in autism spectrum disorders. Journal of Autism and Developmental Disorders, 46(10), 3295–3307. https://doi.org/10.1007/s10803-016-2878-2
Hudson, C. C., Hall, L., & Harkness, K. L. (2019). Prevalence of depressive disorders in individuals with autism spectrum disorder: A meta-analysis. Journal of Abnormal Child Psychology, 47(1), 165–175. https://doi.org/10.1007/s10802-018-0402-1
IBM. (2021). SPSS Statistics for Mac (Version Version 28.0). IBM Corp.
Kessler, R. C., McGonagle, K. A., Swartz, M., Blazer, D. G., & Nelson, C. B. (1993). Sex and depression in the national comorbidity survey. I: Lifetime prevalence, chronicity and recurrence. Journal of Affective Disorders, 29(2–3), 85–96. https://doi.org/10.1016/0165-0327(93)90026-g
Knott, F., Dunlop, A.-W., & Mackay, T. (2006). Living with ASD: How do children and their parents assess their difficulties with social interaction and understanding? Autism, 10(6), 609–617. https://doi.org/10.1177/1362361306068510
Koenig, J., Kemp, A. H., Beauchaine, T. P., Thayer, J. F., & Kaess, M. (2016). Depression and resting state heart rate variability in children and adolescents—A systematic review and meta-analysis. Clinical Psychology Review, 46, 136–150. https://doi.org/10.1016/j.cpr.2016.04.013
Koenig, J., Rash, J. A., Campbell, T. S., Thayer, J. F., & Kaess, M. (2017). A meta-analysis on sex differences in resting-state vagal activity in children and adolescents. Frontiers in Physiology, 8, 582. https://doi.org/10.3389/fphys.2017.00582
Koenig, J., & Thayer, J. F. (2016). Sex differences in healthy human heart rate variability: A meta-analysis. Neuroscience and Biobehavioral Reviews, 64, 288–310. https://doi.org/10.1016/j.neubiorev.2016.03.007
Kushki, A., Brian, J., Dupuis, A., & Anagnostou, E. (2014). Functional autonomic nervous system profile in children with autism spectrum disorder. Molecular Autism, 5(1), 39. https://doi.org/10.1186/2040-2392-5-39
Kushki, A., Drumm, E., Pla Mobarak, M., Tanel, N., Dupuis, A., Chau, T., & Anagnostou, E. (2013). Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS ONE, 8(4), e59730. https://doi.org/10.1371/journal.pone.0059730
Kuusikko, S., Pollock-Wurman, R., Jussila, K., Carter, A. S., Mattila, M.-L., Ebeling, H., & Moilanen, I. (2008). Social anxiety in high-functioning children and adolescents with Autism and Asperger syndrome. Journal of Autism and Developmental Disorders, 38(9), 1697–1709. https://doi.org/10.1007/s10803-008-0555-9
Levine, T. P., Sheinkopf, S. J., Pescosolido, M., Rodino, A., Elia, G., & Lester, B. (2012). Physiologic arousal to social stress in children with autism spectrum disorders: A pilot study. Research in Autism Spectrum Disorders, 6(1), 177–183. https://doi.org/10.1016/j.rasd.2011.04.003
Licht, C. M., de Geus, E. J., van Dyck, R., & Penninx, B. W. (2010). Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biological Psychiatry, 68(9), 861–868. https://doi.org/10.1016/j.biopsych.2010.06.032
Longhurst, J. C., & Fu, L.-W. (2012). Cardiac and other visceral afferents. In D. Robertson, I. Biaggioni, G. Burnstock, P. A. Low, & J. F. R. Paton (Eds.), Primer on the automatic nervous system Academic Press (3rd ed., pp. 171–175). Academic Press.
Loomes, R., Hull, L., & Mandy, W. P. L. (2017). What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 56(6), 466–474. https://doi.org/10.1016/j.jaac.2017.03.013
Lopata, C., Volker, M. A., Putnam, S. K., Thomeer, M. L., & Nida, R. E. (2008). Effect of social familiarity on salivary cortisol and self-reports of social anxiety and stress in children with high functioning autism spectrum disorders. Journal of Autism and Developmental Disorders, 38(10), 1866–1877. https://doi.org/10.1007/s10803-008-0575-5
Lord, C., Rutter, M., DiLavore, P. C., Risi, S., Gotham, K., & Bishop, S. L. (2012). Autism diagnostic observation schedule, 2nd edition. Western Psychological Services.
Maenner, M. J., Shaw, K. A., Baio, J., & Alovel, E. (2020). Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR. Surveillance summaries, 69(S4), 1–12.
Marceau, K., Ram, N., Houts, R. M., Grimm, K. J., & Susman, E. J. (2011). Individual differences in boys’ and girls’ timing and tempo of puberty: Modeling development with nonlinear growth models. Developmental Psychology, 47(5), 1389–1409. https://doi.org/10.1037/a0023838
Marshall, W. A., & Tanner, J. M. (1969). Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood, 44(235), 291–303. https://doi.org/10.1136/adc.44.235.291
Marshall, W. A., & Tanner, J. M. (1970). Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood, 45(239), 13–23. https://doi.org/10.1136/adc.45.239.13
Masi, C. M., Hawkley, L. C., Rickett, E. M., & Cacioppo, J. T. (2007). Respiratory sinus arrhythmia and diseases of aging: Obesity, diabetes mellitus, and hypertension. Biological Psychology, 74(2), 212–223. https://doi.org/10.1016/j.biopsycho.2006.07.006
Mayes, S. D., Calhoun, S. L., Murray, M. J., Ahuja, M., & Smith, L. A. (2011). Anxiety, depression, and irritability in children with autism relative to other neuropsychiatric disorders and typical development. Research in Autism Spectrum Disorders, 5(1), 474–485. https://doi.org/10.1016/j.rasd.2010.06.012
McElhanon, B. O., McCracken, C., Karpen, S., & Sharp, W. G. (2014). Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics, 133(5), 872–883. https://doi.org/10.1542/peds.2013-3995
Mendle, J., Harden, K. P., Brooks-Gunn, J., & Graber, J. A. (2010). Development’s tortoise and hare: Pubertal timing, pubertal tempo, and depressive symptoms in boys and girls. Developmental Psychology, 46(5), 1341–1353. https://doi.org/10.1037/a0020205
Ming, X., Julu, P. O. O., Brimacombe, M., Connor, S., & Daniels, M. L. (2005). Reduced cardiac parasympathetic activity in children with autism. Brain & Development, 27(7), 509–516. https://doi.org/10.1016/j.braindev.2005.01.003
Mire, S. S., Nowell, K. P., Kubiszyn, T., & Goin-Kochel, R. P. (2014). Psychotropic medication use among children with autism spectrum disorders within the Simons simplex collection: Are core features of autism spectrum disorder related? Autism, 18(8), 933–942. https://doi.org/10.1177/1362361313498518
Molfino, A., Fiorentini, A., Tubani, L., Martuscelli, M., Rossi Fanelli, F., & Laviano, A. (2009). Body mass index is related to autonomic nervous system activity as measured by heart rate variability. European Journal of Clinical Nutrition, 63(10), 1263–1265. https://doi.org/10.1038/ejcn.2009.35
Muscatello, R. A., Andujar, J., Taylor, J. L., & Corbett, B. A. (2020). Exploring key physiological system profiles at rest and the association with depressive symptoms in autism spectrum disorder. Journal of Autism and Developmental Disorders. https://doi.org/10.1007/s10803-020-04516-1
Muscatello, R. A., & Corbett, B. A. (2018). Comparing the effects of age, pubertal development, and symptom profile on cortisol rhythm in children and adolescents with autism spectrum disorder. Autism Research, 11(1), 110–120. https://doi.org/10.1002/aur.1879
Muscatello, R. A., Kim, A., Vandekar, S., & Corbett, B. A. (2021a). Diagnostic and physical effects in parasympathetic response to social evaluation in youth with and without autism spectrum disorder. Journal of Autism and Developmental Disorders. https://doi.org/10.1007/s10803-021-05224-0
Muscatello, R. A., Vandekar, S. N., & Corbett, B. A. (2021b). Evidence for decreased parasympathetic response to a novel peer interaction in older children with autism spectrum disorder: A case-control study. Journal of Neurodevelopmental Disorders, 13(1), 6. https://doi.org/10.1186/s11689-020-09354-x
Nederhof, E., Marceau, K., Shirtcliff, E. A., Hastings, P. D., & Oldehinkel, A. J. (2015). Autonomic and adrenocortical interactions predict mental health in late adolescence: The TRAILS study. Journal of Abnormal Child Psychology, 43(5), 847–861. https://doi.org/10.1007/s10802-014-9958-6
Neuhaus, E., Bernier, R., & Beauchaine, T. P. (2014). Brief report: Social skills, internalizing and externalizing symptoms, and respiratory sinus arrhythmia in autism. Journal of Autism and Developmental Disorders, 44(3), 730–737. https://doi.org/10.1007/s10803-013-1923-7
Neuhaus, E., Bernier, R. A., & Beauchaine, T. P. (2016). Children with autism show altered autonomic adaptation to novel and familiar social partners. Autism Research, 9(5), 579–591. https://doi.org/10.1002/aur.1543
Panju, S., Brian, J., Dupuis, A., Anagnostou, E., & Kushki, A. (2015). Atypical sympathetic arousal in children with autism spectrum disorder and its association with anxiety symptomatology. Molecular Autism, 6(1), 64. https://doi.org/10.1186/s13229-015-0057-5
Patriquin, M. A., Hartwig, E. M., Friedman, B. H., Porges, S. W., & Scarpa, A. (2019). Autonomic response in autism spectrum disorder: Relationship to social and cognitive functioning. Biological Psychology, 145, 185–197. https://doi.org/10.1016/j.biopsycho.2019.05.004
Patriquin, M. A., Lorenzi, J., & Scarpa, A. (2013). Relationship between respiratory sinus arrhythmia, heart period, and caregiver-reported language and cognitive delays in children with autism spectrum disorders. Applied Psychophysiology and Biofeedback, 38(3), 203–207. https://doi.org/10.1007/s10484-013-9225-6
Porges, S. W. (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology, 32(4), 301–318. https://doi.org/10.1111/j.1469-8986.1995.tb01213.x
Porges, S. W. (2001). The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology, 42(2), 123–146. https://doi.org/10.1016/s0167-8760(01)00162-3
Porges, S. W. (2003a). The Polyvagal Theory: Phylogenetic contributions to social behavior. Physiology & Behavior, 79(3), 503–513. https://doi.org/10.1016/s0031-9384(03)00156-2
Porges, S. W. (2003b). Social engagement and attachment: A phylogenetic perspective. Annals of the New York Academy of Sciences, 1008(1), 31–47. https://doi.org/10.1196/annals.1301.004
Porges, S. W. (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. https://doi.org/10.1016/j.biopsycho.2006.06.009
Rottenberg, J., Clift, A., Bolden, S., & Salomon, K. (2007). RSA fluctuation in major depressive disorder. Psychophysiology, 44(3), 450–458. https://doi.org/10.1111/j.1469-8986.2007.00509.x
Rutter, M., Bailey, A., & Lord, C. (2003). Social communication questionnaire. Western Psychological Services.
Schaaf, R. C., Benevides, T. W., Leiby, B. E., & Sendecki, J. A. (2015). Autonomic dysregulation during sensory stimulation in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(2), 461–472. https://doi.org/10.1007/s10803-013-1924-6
Schmalenberger, K. M., Eisenlohr-Moul, T. A., Jarczok, M. N., Eckstein, M., Schneider, E., Brenner, I. G., & Ditzen, B. (2020). Menstrual cycle changes in vagally-mediated heart rate variability are associated with progesterone: Evidence from two within-person studies. Journal of Clinical Medicine. https://doi.org/10.3390/jcm9030617
Schmalenberger, K. M., Eisenlohr-Moul, T. A., Wurth, L., Schneider, E., Thayer, J. F., Ditzen, B., & Jarczok, M. N. (2019). A systematic review and meta-analysis of within-person changes in cardiac vagal activity across the menstrual cycle: Implications for female health and future studies. Jornal of Clinical Medicine. https://doi.org/10.3390/jcm8111946
Schupp, C. W., Simon, D., & Corbett, B. A. (2013). Cortisol responsivity differences in children with autism spectrum disorders during free and cooperative play. Journal of Autism and Developmental Disorders, 43(10), 2405–2417. https://doi.org/10.1007/s10803-013-1790-2
Schwaber, J. S., Kapp, B. S., Higgins, G. A., & Rapp, P. R. (1982). Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. Journal of Neuroscience, 2(10), 1424–1438.
Schwartzman, J. M., Williams, Z. J., & Corbett, B. A. (2022). Diagnostic- and sex-based differences in depression symptoms in autistic and neurotypical early adolescents. Autism, 26(1), 256–269. https://doi.org/10.1177/13623613211025895
Sheinkopf, S. J., Neal-Beevers, A. R., Levine, T. P., Miller-Loncar, C., & Lester, B. (2013). Parasympathetic response profiles related to social functioning in young children with autistic disorder. Autism Research and Treatment, 2013, 868396. https://doi.org/10.1155/2013/868396
Shibao, C., & Okamoto, L. (2012). Agents potentiating sympathetic tone. In D. Robertson, I. Biaggioni, G. Burnstock, P. A. Low, & J. F. R. Paton (Eds.), Primer on the autonomic nervous system Acadmic Press (3rd ed., pp. 627–630). Academic Press.
Simonoff, E., Jones, C. R. G., Pickles, A., Happé, F., Baird, G., & Charman, T. (2012). Severe mood problems in adolescents with autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 53(11), 1157–1166. https://doi.org/10.1111/j.1469-7610.2012.02600.x
Task Force of the European Society of Cardiology and The North American Society of Pacing and Electrophysiology. (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. European Heart Journal, 17, 354–381. https://doi.org/10.1161/01.cir.93.5.1043
Tenan, M. S., Brothers, R. M., Tweedell, A. J., Hackney, A. C., & Griffin, L. (2014). Changes in resting heart rate variability across the menstrual cycle. Psychophysiology, 51(10), 996–1004. https://doi.org/10.1111/psyp.12250
Thayer, J. F., Friedman, B. H., & Borkovec, T. D. (1996). Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry, 39(4), 255–266. https://doi.org/10.1016/0006-3223(95)00136-0
Thayer, J. F., Friedman, B. H., Borkovec, T. D., Johnsen, B. H., & Molina, S. (2000). Phasic heart period reactions to cued threat and nonthreat stimuli in generalized anxiety disorder. Psychophysiology, 37(3), 361–368.
Thayer, J. F., & Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. https://doi.org/10.1016/s0165-0327(00)00338-4
Thayer, J. F., & Sternberg, E. (2006). Beyond heart rate variability: vagal regulation of allostatic systems. Annals of the New York Academy of Sciences, 1088(1), 361–372. https://doi.org/10.1196/annals.1366.014
Ulrich-Lai, Y. M., & Herman, J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10(6), 397–409. https://doi.org/10.1038/nrn2647
Vallejo, M., Marquez, M. F., Borja-Aburto, V. H., Cardenas, M., & Hermosillo, A. G. (2005). Age, body mass index, and menstrual cycle influence young women’s heart rate variability—a multivariable analysis. Clinical Autonomic Research, 15(4), 292–298. https://doi.org/10.1007/s10286-005-0272-9
Vandekar, S., Tao, R., & Blume, J. (2020). A robust effect size index. Psychometrika, 85(1), 232–246. https://doi.org/10.1007/s11336-020-09698-2
Vaughan Van Hecke, A., Lebow, J., Bal, E., Lamb, D., Harden, E., Kramer, A., & Porges, S. W. (2009). Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Development, 80(4), 1118–1133. https://doi.org/10.1111/j.1467-8624.2009.01320.x
Watson, L. R., Roberts, J. E., Baranek, G. T., Mandulak, K. C., & Dalton, J. C. (2012). Behavioral and physiological responses to child-directed speech of children with autism spectrum disorders or typical development. Journal of Autism and Developmental Disorders, 42(8), 1616–1629. https://doi.org/10.1007/s10803-011-1401-z
Wechsler, D. (2011). Wechsler abbreviated scale of intelligence, 2nd edition (WASI-II) (2nd ed.). Pearson.
Funding
This study was funded by the National Institute of Mental Health (MH111599 PI: Corbett). Core support was provided by the National Institute of Child Health and Human Development (U54 HD083211, PI: Neul) and the National Center for Advancing Translational Sciences (CTSA UL1 TR000445). None of the funding sources were involved in the study design, collection, analysis and interpretation of the data, writing of the report, or the decision to submit the article for publication. We are grateful to the children and families who participated and continue to support our research.
Author information
Authors and Affiliations
Contributions
RAM contributed to the design of the study, led data acquisition, analyzed physiological data, analyzed and interpreted the data, and wrote the initial draft of the manuscript. AP contributed to data acquisition, scheduling of protocols, physiological data analysis, and editing of the final manuscript. AR and IS contributed to collection and analysis of physiological data and editing of the final manuscript. BAC designed the study, oversaw data acquisition, analysis, and interpretation, and contributed to writing the initial draft of the manuscript. All authors participated in the preparation and editing of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical stands of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed written consent and assent was obtained from all parents and study participants, respectively, prior to inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muscatello, R.A., Pachol, A., Romines, A. et al. Development and Parasympathetic Regulation in Male and Female Adolescents with Autism Spectrum Disorder: A Two-Timepoint Longitudinal Study. J Autism Dev Disord 53, 3613–3626 (2023). https://doi.org/10.1007/s10803-022-05664-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-022-05664-2