Abstract
Periprosthetic osteolysis is a serious complication of total hip replacement (THR) in the medium to long term. Although often asymptomatic, osteolysis can lead to prosthesis loosening and periprosthetic fracture. These complications cause significant morbidity and require complex revision surgery. Here, we review advances in our understanding of the cell and tissue response to particles produced by wear of the articular and non-articular surfaces of prostheses. We discuss the molecular and cellular regulators of osteoclast formation and bone resorptive activity, a better understanding of which may lead to pharmacological treatments for periprosthetic osteolysis. We describe the development of imaging techniques for the detection and measurement of osteolysis around THR prostheses, which enable improved clinical management of patients, provide a means of evaluating outcomes of non-surgical treatments for periprosthetic osteolysis, and assist in pre-operative planning for revision surgery. Finally, there have been advances in the materials used for bearing surfaces to minimise wear, and we review the literature regarding the performance of these new materials to date.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The problem of periprosthetic osteolysis
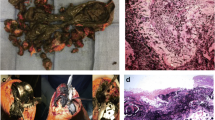
Loosening of hip replacement prostheses due to loss of adjacent bone, known as periprosthetic osteolysis, is the most common reason for revision of total hip replacements in the medium to long term (Harris 1995; Maloney et al. 1997; Chiang et al. 2003). An example of a total hip replacement, showing osteolysis adjacent to the acetabular cup, is shown in Fig. 1. Non-linear periprosthetic osteolysis is characterised by localised and often ballooning lesions in bone adjacent to prostheses and is often first noted around stable prostheses before the bone loss leads to implant loosening (Zicat et al. 1995). Even when this type of osteolysis is progressive and results in major bone loss, patients may remain asymptomatic until the bone fails to support the prosthetic implant, at which time major revision surgery is required.
CT image showing a total hip replacement (THR) in situ. The image shows the femoral component, with the stem inserted into the proximal femur and the prosthetic femoral head sitting in the acetabular implant. The latter comprises a metal acetabular shell or cup inserted into the acetabulum of the pelvis and a polyethylene-bearing surface, which is X-ray transparent. In this example, the femoral head is eccentric in the acetabular component because the liner is worn. Osteolysis can be seen in the pelvis, corresponding to the screw and the empty screw holes
Fluid pressure and wear particles at the bone-prosthesis interface cause osteolysis
The mechanism of periprosthetic osteolysis is likely to be multifactorial. While factors such as prosthesis design, surgical technique and quality of fixation are known to be important for early loosening of prostheses, loosening of prostheses due to osteolysis in the medium to long term is related more to prosthesis materials and the type and volume of wear particles generated, and the resultant tissue reaction. Osteolysis around long-term implanted prostheses has been attributed to both the tissue response to wear particles derived from both the articular and non-articular interfaces of prostheses and to fluid movement and pressure at the prosthesis-bone interface.
Histological examination of tissue retrieved from the joint capsule and from the prosthesis-bone interface around hip replacements revised for loosening and osteolysis revealed large numbers of prosthesis-derived particles and an inflammatory response. This was characterised by the presence of macrophages, multinucleated foreign body giant cells containing engulfed particles, lymphocytes, fibroblasts and osteoclasts on bone surfaces (Vernon-Roberts and Freeman 1977; Willert 1977). We reported a direct association between wear particles and osteoclastic bone resorption in 1988 (Howie et al. 1988). In an in vivo rat model of joint replacement, which allowed movement of fluid and wear particles to the periprosthetic bone, the presence of wear particles led to the formation of a connective tissue layer of variable thickness at the prosthesis-bone interface and osteolytic lesions.
Animal studies and studies examining retrieved interface tissue have determined that a number of particle-associated factors influence the extent and type of chronic inflammatory tissue response and the extent of osteolysis (Howie and Vernon-Roberts 1988a, b; Howie 1990; Howie et al. 1990; Howie et al. 1993; Green et al. 1998; Koseki et al. 2005). These include the chemical composition of the prosthesis, the size, shape and surface area of the particles generated, as well as the rate of production and thus concentration of particles present. While wear resulting in excessive numbers of particles being shed from any of the components of a hip replacement will initiate an inflammatory response leading to osteolysis, irrespective of the material used, the large numbers of wear particles generated by wear of the polyethylene liner are the most common cause (Haynes et al. 1993; Schmalzried and Callaghan 1999; Neale et al. 2000). Wear of metal components resulting in the generation of metal particles and metal ions is usually related to poor prosthesis design or occurs as a result of wear-through of the polyethylene liner leading to metal on metal wear.
Identification of bone resorbing mediators
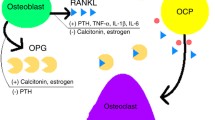
Phagocytosis of these wear particles by macrophages is now known to initiate a cascade of events that leads to osteolysis and prosthesis loosening. The particle-stimulated macrophages express a number of inflammatory mediators, including cytokines (IL-1β, TNFα, IL-6, IL-8 and IL-11), colony stimulating factors (M-CSF and GM-CSF), prostaglandins (PGE2), matrix metalloproteinases and chemokines (Haynes et al. 1993; Jiranek et al. 1993; Xu et al. 1997; Nakashima et al. 1998; Haynes et al. 2004; Talmo et al. 2006). The release of these mediators stimulates the recruitment and activity of more macrophages and other cells such as fibroblasts to the prosthesis-bone interface. Mononuclear precursors of osteoclasts are known to be present within the macrophage infiltrate, which are capable of differentiating into functional bone resorbing osteoclasts in the presence of inflammatory mediators (Sabokbar et al. 1997; Neale et al. 1999; Haynes et al. 2001).
In addition to these inflammatory mediators, we have shown that an accumulation of wear particles is also frequently associated with a marked increase in expression of receptor activator NF-κB ligand (RANKL) and its receptor, RANK (Crotti et al. 2004; Holding et al. 2006). It is well established that the activated RANKL/RANK ligand-receptor complex is central to the differentiation and activity of osteoclasts, and therefore bone resorption (Yasuda et al. 1998; Nakagawa et al. 1998; Asagiri and Takayanagi 2007). The expression and activity of RANKL is known to be induced by a number of pro-inflammatory cytokines and we and others have shown that TNFα can greatly enhance RANKL activity (Holding et al. 2006; Lam et al. 2000; Lee et al. 2001; Fuller et al. 2002).
Immunohistochemical and in situ hybridisation studies of periprosthetic tissue from sites adjacent to osteolytic lesions have revealed that macrophages, multinucleated giant cells and, to a lesser extent, fibroblasts express high levels of RANK, RANKL and TNFα (Haynes et al. 2001; Holding et al. 2006; Gehrke et al. 2003). Our studies have found strong correlations between osteolytic lesion size, the concentration of polyethylene particles, and RANK, RANKL and TNFα expression (Holding et al. 2006). We have investigated the direct effect of prosthesis-derived polyethylene particles on differentiating human osteoblasts, using a 3D collagen gel culture system (Atkins et al. 2009). This system provides the necessary architecture and microenvironment to allow normal human osteoblastic cells to undergo differentiation into a mature osteocyte-like phenotype over a 21- to 28-day culture period, indicated by increased mRNA expression of osteocyte markers such as E11, DMP1 and SOST, and adoption of a stellate morphology (Atkins et al. 2009). In the presence of polyethylene particles, the osteocyte-like cells increase their expression of mRNA species that are associated with the promotion of osteoclast formation and activity (RANKL, IL-8 and M-CSF) and a concomitantly decreased expression of the osteoclast antagonist, osteoprotegerin. Qualitatively similar results were found after exposure of the mouse MLO-Y4 osteocyte-like cell line to polyethylene particles. We have also shown that inflammatory mediators can upregulate the expression, by human osteocyte-like cells, of the negative regulator of bone formation, sclerostin (Vincent et al. 2009). These results suggested that polyethylene particles might directly or indirectly influence the behaviour in bone of osteocytes, now increasingly recognised as directing both bone formation and bone resorption (Atkins and Findlay 2012). In support of this, we have shown expansion of the osteocyte lacunae adjacent to polyethylene particles in the mouse calvarial osteolysis model (Atkins et al., unpublished). Direct effects were also found on osteoclast differentiation and activity after exposure to polyethylene particles in the collagen gel model. Osteoclasts generated by treatment with RANKL had increased resorptive activity if they were concurrently exposed to polyethylene particles, as well as increased expression of the immunoreceptor tyrosine-based activation motif (ITAM)-related molecules OSCAR, FcRγ, TREM2 and DAP12, which are important for osteoclast formation (Alias et al. 2012).
These findings from in vitro and in vivo studies, and studies examining interface tissue directly link polyethylene particles to the key mediators of osteoclast formation and bone resorption activity in periprosthetic osteolysis, and suggest that treatment with inhibitors of these mediators may be useful in preventing or delaying periprosthetic osteolysis.
Non-surgical treatment of osteolysis
A better understanding of the biology of periprosthetic osteolysis may lead to pharmacological approaches to its treatment when prostheses remain well fixed in situ. Studies with anti-resorptive agents in animal models have shown promise (Millett et al. 2002; von Knoch et al. 2005) and a small number of studies have been carried out in patients with periprosthetic osteolysis using anti-resorptive drugs, including bisphosphonates and the anti-TNFα inhibitor, etanercept (Arabmotlagh et al. 2009; Schwarz et al. 2003). Gene therapy has also been proposed for the treatment of periprosthetic osteolysis (Goater et al. 2002; Ulrich-Vinther 2007; Zhang et al. 2010). However, although agents such as bisphosphonates and a human monoclonal antibody to RANKL, denosumab, have shown efficacy in reducing the systemic bone loss of osteoporosis (McClung et al. 2013), an evidence base for using these agents clinically in established periprosthetic osteolysis is so far lacking. To evaluate the role of these and other potential treatments for periprosthetic osteolysis, treatment protocols will need to be based on the severity of the osteolysis and its rate of progression and, importantly, accurate measurement of osteolysis will be required for such treatments to be properly evaluated.
Detection, assessment, measurement and monitoring of osteolysis
It is now generally accepted that plain radiography is not sufficiently sensitive for the reliable detection of the presence or extent of periprosthetic osteolysis (Engh et al. 2002; Leung et al. 2005; Walde et al. 2005). High resolution multi-slice or helical CT with metal artefact reduction protocols have been developed to provide a sensitive and accurate measure of the volume of osteolytic lesions close to metal prostheses (Leung et al. 2005; Walde et al. 2005; Link et al. 2000; Puri et al. 2002; Looney et al. 2002; Stamenkov et al. 2003). We and others have evaluated the accuracy of CT to detect osteolytic lesions (Leung et al. 2005; Walde et al. 2005; Stamenkov et al. 2003), and have used it to identify patient and prosthesis-related factors that may influence development of osteolysis (Puri et al. 2002; Looney et al. 2002; Kitamura et al. 2005; Howie et al. 2007; Stamenkov et al. 2010).
Our clinical CT studies to date have focussed on periacetabular osteolysis around cementless acetabular components in the medium to long-term post-implantation (Howie et al. 2007; Stamenkov et al. 2010; Howie et al. 2012). Periacetabular osteolysis is the major long-term complication of these prostheses, and is often seen in the presence of well-fixed components. The aim of our studies has been to understand the natural history of these lesions and the factors that promote their formation and progression. Specifically, we have sought to obtain accurate data on the size and progression of periprosthetic lesions in patients suspected of having osteolysis, so as to improve patient management and to assist in planning for revision surgery.
The progression of periacetabular osteolysis over prolonged periods of up to 9 years was monitored using CT, in a cohort of patients with cementless acetabular components who were suspected of having periacetabular osteolysis. We found that patients either developed low volumes of osteolytic lesions that were relatively quiescent, even after long periods of implantation, or patients had extensive, progressive periacetabular osteolytic lesions after a similar post-operative period (Howie et al. 2007; Howie et al. 2012). The latter group is therefore likely to be at higher risk of acetabular component loosening, component migration and acute periprosthetic bone fracture. Several factors were found to be good predictors of progression of osteolysis, particularly the volume of the osteolytic lesions at initial CT and patient activity (Howie et al. 2012). The strongest predictor of progression was obtained by combining these two risk factors. Thus, patients with a high volume of osteolysis at the initial CT and those who were most active had the largest increases in osteolytic lesion size over the monitoring period. These data have significant implications for monitoring patients over time, and will potentially identify which patients might best be targeted for novel treatments or early surgical intervention.
Surgical treatment of osteolysis
The decision to revise patients with asymptomatic periacetabular osteolysis adjacent to radiographically stable acetabular components is complex. Important factors to consider include impending wear-through of the polyethylene liner or large, rapidly progressing osteolytic lesions, particularly if fixation of the cup is threatened (Naudie and Engh 2004; Stulberg et al. 2002), as well as life expectancy and comorbidities of the patient, and prosthesis type (Maloney et al. 1997; Chiang et al. 2003; Stulberg et al. 2002). The loss of bone due to periprosthetic osteolysis can compromise the outcome of revision joint replacement and multiple revisions on the same joint are not uncommon, with a reduction in average prosthesis survival for each subsequent revision procedure (Garcia-Cimbrelo et al. 2007). When revision surgery is indicated in the presence of significant periprosthetic osteolysis, the planning of that surgery is facilitated by the use of CT to identify the location and extent of osteolysis (Saleh et al. 2003).
With the introduction of modular hip components, liner exchange surgery, without removal of the metal shell, has emerged as a surgical treatment option in certain circumstances in the presence of well-fixed acetabular components. During this surgery, if osteolytic lesions can be accessed through empty screw holes in the metal shell or by cortical ‘windows’, debridement and bone grafting of the lesions can be undertaken to replace bone lost in the osteolytic process. The alternative surgical treatment option is revision of the entire acetabular component. However, removal of a well-fixed acetabular component could potentially result in significant loss of acetabular bone stock, thereby increasing the risk of insufficient bone ingrowth, and hence subsequent loosening of the new acetabular component.
Using serial CT scans, it is now possible to monitor and subsequently compare the progression of individual osteolytic lesions prior to and after liner exchange surgery, thereby enabling assessment of the effect on osteolytic lesion progression of removing the source of polyethylene particles as well as being able to monitor the integration of the bone graft (Engh et al. 2007). An example of longitudinal monitoring of periacetabular osteolytic lesions pre- and post-liner exchange surgery/grafting using serial CT scans is shown in Fig. 2. Despite this patient maintaining his activity levels following liner exchange, cemented liner exchange surgery appeared to halt the progression in size of the osteolytic lesions.
a Longitudinal monitoring of periacetabular osteolytic lesions pre- and post-cemented liner exchange surgery, using serial CT scans. Total osteolytic lesion volumes are shown for one patient prior to (open diamond) and following (filled diamond) liner exchange surgery. b, c Selected sagittal images from the CT scans of this patient, which show an osteolytic lesion adjacent to the acetabular component (arrows) increasing in size from 20.1 cm3 (b) to 29.7 cm3 (c) 3 years later. d. Sagittal CT image of the same osteolytic lesion 3 years after liner exchange surgery showing fill of the lesion with bone graft (arrow) with good graft incorporation and no evidence of ongoing bone loss
Advances in orthopaedic materials
The orthopaedic device industry continues to develop materials with purportedly improved wear properties. Polyethylene, the polymer most commonly used in articulations, has undergone evolution over the five decades of its use. Most recently, the ongoing problem of wear of the conventional ultra high molecular weight polyethylene in bearing surfaces prompted the development of highly cross-linked polyethylenes. Increasing the cross-linking of the polymer has been shown in vitro to result in significantly lower polyethylene wear rates in hip simulator studies (McKellop et al. 1999, 2000; Muratoglu et al. 2001). Over the last decade, highly cross-linked polyethylenes have largely replaced conventional polyethylene as the polyethylene of choice in acetabular liners, and their lower wear has been confirmed clinically using sensitive radiographic in vivo measures of wear (Kurtz et al. 2011; Thomas et al. 2011; Engh et al. 2012). Polyethylene cross-linking is achieved with the use of 5–10 Mrad of gamma or electron-beam irradiation. Early cross-linked polyethylenes were manufactured using 5 Mrad irradiation and were known as moderately cross-linked polyethylenes. Currently, most polyethylenes are highly cross-linked, achieved through the use of 9.5–10 Mrad irradiation.
The decreased wear rate has, however, been identified as a trade-off against reduced mechanical properties of the highly cross-linked polyethylenes, compared to the previous conventional polyethylenes (Ries and Pruitt 2005). A small number of cases of rim cracking and rim fractures have been reported (Bradford et al. 2004; Tower et al. 2007). Most of these appear related to excess loading on unsupported thinner polyethylene in malpositioned acetabular components. This has led to the development of a new generation of polyethylenes, namely, highly cross-linked polyethylenes stabilised with vitamin E to reduce oxidation, which appear to provide improved mechanical properties in vitro (Oral et al. 2006; Oral and Muratoglu 2011). Long-term results of these materials are not yet available.
Laboratory studies have suggested that wear particles generated from highly cross-linked polyethylene may cause an increased biological response, which may in turn lead to osteolysis despite a low wear rate (Endo et al. 2002; Ingram et al. 2004; Illgen et al. 2009). Specifically, highly cross-linked polyethylene particles were found to be significantly more inflammatory than conventional polyethylene particles, based on the relative cytokine release from macrophages in vitro (Ingram et al. 2004; Illgen et al. 2009). Furthermore, although laboratory wear of highly cross-linked polyethylene particles produces fewer particles overall, the relative percentage of small wear particles, namely those in the 0.1–1.0 μm size range, is higher, compared to that found with conventional polyethylene particles (Endo et al. 2002).
Importantly, there is little clinical evidence to date that the reduction in wear of highly cross-linked polyethylene translates to a decrease in periprosthetic osteolysis. A number of studies of highly cross-linked polyethylenes have reported a low incidence of osteolysis on plain radiographs (Kurtz et al. 2011; Thomas et al. 2011; Engh et al. 2012), but the sensitivity of plain radiographs in detecting osteolytic lesions is poor. In two small studies using CT, the reported incidence of osteolysis at 5–6 years ranged from 2 to 8 % in patients with cross-linked polyethylene liners (Leung et al. 2007; Mall et al. 2011). Using CT, we have also identified a number of cases of osteolytic lesions exceeding 1 cm3 at 7 years, following total hip replacement with highly cross-linked polyethylene liners (Howie et al. unpublished). The concern arising from these studies is that osteolysis was detected in the absence of significant wear of the polyethylene liner. Reduced wear of cross-linked polyethylene may therefore not correspond to a similar level of reduction in the incidence of osteolysis.
Concluding remarks
An integrated approach to understanding periprosthetic osteolysis has identified particles resulting from wear of the prosthetic materials, especially polyethylene, as essential drivers of this process. Knowledge of the cellular and molecular mechanisms for periprosthetic osteolysis may lead to non-surgical approaches to inhibiting bone loss and thereby prolonging the useful life of prostheses. Improved imaging of osteolytic lesions through the use of CT is providing new insights into the natural history of periprosthetic osteolysis and more informative ways to monitor osteolytic lesions in patients. Imaging will also assist not only in identifying patients who may benefit from drug therapy, but also in determining clinically relevant outcomes. Finally, although it is now recognised that cross-linking reduces polyethylene wear, more clinical studies are needed to determine if cross-linking will also reduce the incidence of periprosthetic osteolysis and hence significantly improve the long-term outcomes of total hip replacement.
References
Alias E, Dharmapatni AS, Holding AC, Atkins GJ, Findlay DM, Howie DW, Crotti TN, Haynes DR (2012) Polyethylene particles stimulate expression of ITAM-related molecules in peri-implant tissues and when stimulating osteoclastogenesis in vitro. Acta Biomater 8:3104–3112
Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M (2009) Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty. J Orthop Res 27:183–188
Asagiri M, Takayanagi H (2007) The molecular understanding of osteoclast differentiation. Bone 40:251–264
Atkins GJ, Findlay DM (2012) Osteocyte regulation of bone mineral: a little give and take. Osteoporos Int 23:2067–2079
Atkins GJ, Welldon KJ, Holding CA, Haynes DR, Howie DW, Findlay DM (2009) The induction of a catabolic phenotype in human primary osteoblasts and osteocytes by polyethylene particles. Biomaterials 30:3672–3681
Bradford L, Baker DA, Graham J, Chawan A, Ries MD, Pruitt LA (2004) Wear and surface cracking in early retrieved highly cross-linked polyethylene acetabular liners. J Bone Joint Surg Am 86:1271–1282
Chiang PP, Burke DW, Freiberg AA, Rubash HE (2003) Osteolysis of the pelvis. Evaluation and treatment. Clin Orthop Relat Res 417:164–174
Crotti TN, Smith MD, Findlay DM, Zreiqat H, Ahern MJ, Weedon H, Hatzinikolous G, Capone M, Holding C, Haynes DR (2004) Factors regulating osteoclast formation in human tissues adjacent to peri-implant bone loss: expression of receptor activator NFkappaB, RANK ligand and osteoprotegerin. Biomaterials 25:565–573
Endo M, Tipper JL, Barton DC, Stone MH, Ingham E, Fisher J (2002) Comparison of wear, wear debris and functional biological activity of moderately crosslinked and non-crosslinked polyethylenes in hip prostheses. **Proc Instn Mech Engrs Part H 216:111–122
Engh AC Jr, Sychterz CJ, Young AM, Pollock DC, Toomey SD, Engh CA Sr (2002) Interobserver and intraobserver variability in radiographic assessment of osteolysis. J Arthroplasty 17:752–759
Engh CA Jr, Egawa H, Beykirch SE, Hopper RH, Engh CA (2007) The quality of osteolysis grafting with cementless acetabular component retention. Clin Orthop Relat Res 465:150–154
Engh CA Jr, Hopper RH Jr, Huynh C, Ho H, Sritulanondha S, Engh CA Sr (2012) A prospective, randomized study of cross-linked and non cross-linked polyethylene for total hip arthroplasty at 10-year follow-up. J Arthroplasty 27:2–7
Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ (2002) TNFα potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology 143:1108–1118
Garcia-Cimbrelo E, Tapia M, Martin-Hervas C (2007) Multislice computed tomography for evaluating acetabular defects in revision THA. Clin Orthop Relat Res 463:138–143
Gehrke T, Sers C, Morawietz L, Fernahl G, Neidel J, Frommelt L, Krenn V (2003) Receptor activator of nuclear factor kappaB ligand is expressed in resident and inflammatory cells in aseptic and septic prosthesis loosening. Scand J Rheumatol 32:287–294
Goater JJ, O’Keefe RJ, Rosier RN, Puzas JE, Schwarz EM (2002) Efficacy of ex vivo OPG gene therapy in preventing wear debris induced osteolysis. J Orthop Res 20:169–173
Green TR, Fisher J, Stone M, Wroblewski BM, Ingham E (1998) Polyethylene particles of a “critical size” are necessary for the induction of cytokines by macrophages in vitro. Biomaterials 19:2297–2302
Harris WH (1995) The problem is osteolysis. Clin Orthop Relat Res 311:46–53
Haynes DR, Rogers SD, Hay S, Pearcy MJ, Howie DW (1993) The differences in toxicity and release of bone-resorbing mediators induced by titanium and cobalt-chromium-alloy wear particles. J Bone Joint Surg Am 75:825–834
Haynes DR, Crotti TN, Potter AE, Loric M, Atkins GJ, Howie DW, Findlay DM (2001) The osteoclastogenic molecules RANKL and RANK are associated with periprosthetic osteolysis. J Bone Joint Surg Br 83:902–911
Haynes DR, Crotti TN, Zreiqat H (2004) Regulation of osteoclast activity in peri-implant tissues. Biomaterials 25:4877–4885
Holding CA, Findlay DM, Stamenkov R, Neale SD, Lucas H, Dharmapatni AS, Callary SA, Shrestha KR, Atkins GJ, Howie DW, Haynes DR (2006) The correlation of RANK, RANKL and TNFalpha expression with bone loss volume and polyethylene wear debris around hip implants. Biomaterials 27:5212–5219
Howie DW (1990) Tissue response in relation to type of wear particles around failed hip arthroplasties. J Arthroplasty 5:337–348
Howie DW, Vernon-Roberts B (1988a) The synovial response to intraarticular cobalt-chrome wear particles. Clin Orthop Relat Res 232:244–254
Howie DW, Vernon-Roberts B (1988b) Long term effects of intraarticular cobalt-chrome alloy wear particles in rats. J Arthroplasty 3:327–336
Howie DW, Vernon-Roberts B, Oakeshott R, Manthey B (1988) A rat model of resorption of bone at the cement-bone interface in the presence of polyethylene wear particles. J Bone Joint Surg Am 70:257–263
Howie DW, Cornish BL, Vernon-Roberts B (1990) Resurfacing hip arthroplasty: classification of loosening and the role of prosthesis wear particles. Clin Orthop Relat Res 255:144–159
Howie DW, Manthey B, Hay S, Vernon-Roberts B (1993) The synovial response to intraarticular injection in rats of polyethylene wear particles. Clin Orthop Relat Res 292:352–357
Howie DW, Neale SD, Stamenkov R, McGee MA, Taylor DJ, Findlay DM (2007) Progression of acetabular periprosthetic osteolytic lesions measured by computed tomography. J Bone Joint Surg Am 89:1818–1825
Howie DW, Neale SD, Martin W, Costi K, Kane T, Stamenkov R, Findlay DM (2012) Progression of periacetabular osteolytic lesions. J Bone Joint Surg Am 94:e1171–e1176
Illgen RL 2nd, Bauer LM, Hotujec BT, Kolpin SE, Bakhtiar A, Forsythe TM (2009) Highly crosslinked vs conventional polyethylene particles: relative in vivo inflammatory response. J Arthroplasty 24:117–124
Ingram JH, Stone M, Fisher J, Ingham E (2004) The influence of molecular weight, crosslinking and counterface roughness on TNF-alpha production by macrophages in response to ultra high molecular weight polyethylene particles. Biomaterials 25:3511–3522
Jiranek WA, Machado M, Jasty M, Jevsevar D, Wolfe HJ, Goldring SR, Goldberg MJ, Harris WH (1993) Production of cytokines around loosened cemented acetabular components. J Bone Joint Surg Am 75:863–884
Kitamura N, Leung SB, Engh CA Sr (2005) Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop Relat Res 441:291–297
Koseki H, Matsumoto T, Ito S, Doukawa H, Enomoto H, Shindo H (2005) Analysis of polyethylene particles isolated from periprosthetic tissue of loosened hip arthroplasty and comparison with radiographic appearance. J Orthop Sci 10:284–290
Kurtz SM, Gawel HA, Patel JD (2011) History and systemic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clin Orthop Relat Res 469:2262–2277
Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL (2000) TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 106:1481–1488
Lee SE, Chung WJ, Kwak HB, Chung CH, Kwak KB, Lee ZH, Kim HH (2001) Tumor necrosis factor-α supports the survival of osteoclasts through the activation of Akt and ERK. J Biol Chem 276:49343–49490
Leung S, Naudie D, Kitamura N, Walde T, Engh CA (2005) Computed tomography in the assessment of periacetabular osteolysis. J Bone Joint Surg Am 87:592–597
Leung SB, Egawa H, Stepniewski A, Beykirch S, Engh CA Jr, Engh CA Sr (2007) Incidence and volume of pelvic osteolysis at early follow-up with highly cross-linked and noncross-linked polyethylene. J Arthroplasty 22:134–139
Link TM, Berning W, Scherf S, Joosten U, Joist A, Engelke K, Daldrup-Link HE (2000) CT of metal implants: reduction of artefacts using an extended CT scale technique. J Comput Assist Tomogr 24:165–172
Looney R, Boyd A, Totterman S, Seo G, Tamez-Pena J, Campbell D, Novotny L, Olcott C, Martell J, Hayes FA, O’Keefe RJ, Schwarz EM (2002) Volumetric computerized tomography as a measurement of peri-prosthetic acetabular osteolysis and its correlation with wear. Arthr Res 4:59–63
Mall NA, Nunley RM, Zhu JJ, Maloney WJ, Barrack RL, Clohisy JC (2011) The incidence of acetabular osteolysis in young patients with conventional versus highly crosslinked polyethylene. Clin Orthop Relat Res 469:372–381
Maloney WJ, Herzwurm P, Paprosky W, Rubash HE, Engh C (1997) Treatment of pelvic osteolysis associated with a stable acetabular component inserted without cement as part of a total hip replacement. J Bone Joint Surg Am 79:1628–1634
McClung MR, Lewiecki EM, Geller ML, Bolognese MA, Peacock M, Weinstein RL, Ding B, Rockabrand E, Wagmar RB, Miller PD (2013) Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporosis Int 24:227–235
McKellop H, Shen FW, Lu B, Campbell P, Salovey R (1999) Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res 17:157–167
McKellop H, Shen FW, Lu B, Campbell P, Salovey R (2000) Effect of sterilization method and other modifications on the wear resistance of acetabular cups made of ultra-high molecular weight polyethylene. J Bone Joint Surg Am 82:1708–1725
Millett PJ, Allen MJ, Bostrom MP (2002) Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am 84:236–249
Muratoglu OK, Bragdon CR, O’Connor DO, Jasty M, Harris WH (2001) A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. J Arthroplasty 16:149–160
Nakagawa N, Yamaguchi K, Shima N, Yasuda H, Yano K, Morinaga T, Higashio K (1998) RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun 253:395–400
Nakashima Y, Sun DH, Maloney WJ, Goodman SB, Schurman DJ, Smith RL (1998) Induction of matrix metalloproteinase expression in human macrophages by orthopaedic particulate debris in vitro. J Bone Joint Surg Br 80:694–700
Naudie DDR, Engh CA Sr (2004) Surgical management of polyethylene wear and pelvic osteolysis with modular uncemented acetabular components. J Arthroplasty 19:124–129
Neale SD, Sabokbar A, Howie DW, Murray DW, Athanasou NA (1999) Macrophage colony-stimulating factor and interleukin-6 release by periprosthetic cells stimulates osteoclast formation and bone resorption. J Orthop Res 17:686–694
Neale SD, Haynes DR, Howie DW, Murray DW, Athanasou NA (2000) The effect of particle phagocytosis and metallic wear particles on osteoclast formation and bone resorption in vitro. J Arthroplasty 15:654–662
Oral E, Muratoglu OK (2011) Vitamin E diffused, highly crosslinked UHMWPE: a review. Int Orthop 35:215–223
Oral E, Christensen SD, Malhi AS, Wannomae KK, Muratoglu OK (2006) Wear resistance and mechanical properties of highly cross-linked, ultrahigh-molecular weight polyethylene doped with vitamin E. J Arthroplasty 21:580–591
Puri L, Wixson R, Stern S, Kohli J, Hendrix R, Stulberg D (2002) Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am 84:609–614
Ries MD, Pruitt L (2005) Effect of cross-linking on the microstructure and mechanical properties of ultra-high molecular weight polyethylene. Clin Orthop Relat Res 440:149–156
Sabokbar A, Fujikawa Y, Neale S, Murray DW, Athanasou NA (1997) Human arthroplasty derived macrophages differentiate into osteoclastic bone resorbing cells. Ann Rheum Dis 56:414–420
Saleh KJ, Celebrezze M, Kassim R, Dykes DC, Gioe TJ, Callaghan JJ, Salvati EA (2003) Functional outcome after revision hip arthroplasty. A metaanalysis. Clin Orthop Relat Res 416:254–264
Schmalzried TP, Callaghan JJ (1999) Current concepts review. Wear in total hip and knee replacements. J Bone Joint Surg Am 81:115–136
Schwarz EM, Campbell D, Totterman S, Boyd A, O’Keefe RJ, Looney RJ (2003) Use of volumetric computerized tomography as a primary outcome measure to evaluate drug efficacy in the prevention of peri-prosthetic osteolysis: a 1-year clinical pilot of etanercept vs. placebo. J Orthop Res 21:1049–1055
Stamenkov R, Howie D, Taylor J, Findlay D, McGee M, Kourlis G, Carbone A, Burwell M (2003) Measurement of bone defects adjacent to acetabular components of hip replacements. Clin Orthop Relat Res 412:117–124
Stamenkov RB, Howie DW, Neale SD, McGee MA, Taylor DJ, Findlay DM (2010) Distribution of periacetabular osteolytic lesions varies according to component design. J Arthroplasty 25:913–919
Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB (2002) Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am 84:116–122
Talmo CT, Shanbhag A, Rubash HE (2006) Nonsurgical management of osteolysis. Challenges and opportunities. Clin Orthop Relat Res 453:254–264
Thomas GER, Simpson DJ, Mehmood S, Taylor A, McLardy-Smith P, Gill HS, Murray DW, Glyn-Jones S (2011) The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty. J Bone Joint Surg Am 93:716–722
Tower SS, Currier JH, Currier BH, Lyford KA, van Citters DW, Mayor MB (2007) Rim cracking of the cross-linked longevity polyethylene acetabular liner after total hip arthroplasty. J Bone Joint Surg Am 89:2212–2217
Ulrich-Vinther M (2007) Gene therapy methods in bone and joint disorders. Evaluation of the adeno-associated virus vector in experimental models of articular cartilage disorders, periprosthetic osteolysis and bone healing. Acta Orthop Suppl 78:1–64
Vernon-Roberts B, Freeman MAR (1977) The tissue response to total joint replacement prostheses. In: The scientific basis of joint replacement. Pitman Medical, London, pp 86–129
Vincent C, Findlay DM, Welldon KJ, Wijenayaka AR, Zheng TS, Haynes DR, Fazzalari NL, Evdokiou A, Atkins GJ (2009) Pro-inflammatory cytokines TNF-related weak inducer of apoptosis (TWEAK) and TNFalpha induce the mitogen-activated protein kinase (MAPK)-dependent expression of sclerostin in human osteoblasts. J Bone Miner Res 24:1434–1449
von Knoch F, Heckelei A, Wedemeyer C, Saxler G, Hilken G, Brankamp J, Sterner T, Landgraeber S, Henschke F, Loer F, von Knoch M (2005) Suppression of polyethylene particle-induced osteolysis by exogenous osteoprotegerin. J Biomed Mater Res A 75:288–294
Walde TA, Weiland DE, Leung SB, Kitamura N, Sychterz CJ, Engh CA Jr, Claus AM, Potter HG, Engh CA Sr (2005) Comparison of CT, MRI, and radiographs in assessing pelvic osteolysis: a cadaveric study. Clin Orthop Relat Res 437:138–144
Willert HG (1977) Reactions of the articular cartilage to wear products of artificial joint prostheses. J Biomed Mater Res 11:157–164
Xu JW, Konttinen YT, Waris V, Patiala H, Sorsa T, Santavirta S (1997) Macrophage-colony stimulating factor (M-CSF) is increased in the synovial-like membrane of the prosthetic tissues in the aseptic loosening of total hip replacement (THR). Clin Rheumatol 16:244–248
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S-I, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Moringa T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclast inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602
Zhang L, Jia TH, Chong AC, Bai L, Yu H, Gong W, Wooley PH, Yang SY (2010) Cell-based osteoprotegerin therapy for debris-induced aseptic prosthetic loosening on a murine model. Gene Ther 17:1262–1269
Zicat B, Engh CA, Gokcen E (1995) Patterns of osteolysis around total hip components inserted with and without cement. J Bone Joint Surg Am 77:432–439
Acknowledgments
The authors acknowledge the profound influence of Professor Barrie Vernon-Roberts on the elucidation of the cause of periprosthetic osteolysis, and on their own research careers and research directions. His remarkable powers of observation, important mentoring activities and scientific insights, support and encouragement have directly and indirectly benefited us all greatly. We thank Dr. Roumen Stamenkov for his contributions to the work. We acknowledge the support of the Australian National Health and Medical Research Council (NHMRC), the Australian Orthopaedic Association Research Foundation, the Royal Adelaide Hospital, the University of Adelaide and the medical and nursing staff of the Royal Adelaide Hospital.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was intended for inclusion in the Special Issue dedicated to the life and work of Professor Barrie Vernon-Roberts [Inflammopharmacology 2013;21(4):269 et seq.], but was inadvertently omitted.
Rights and permissions
About this article
Cite this article
Howie, D.W., Neale, S.D., Haynes, D.R. et al. Periprosthetic osteolysis after total hip replacement: molecular pathology and clinical management. Inflammopharmacol 21, 389–396 (2013). https://doi.org/10.1007/s10787-013-0192-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-013-0192-6