Abstract
Comparative studies of closely related species with similar ecological requirements are essential to understand the behavioral adaptations that allow them to live in sympatry. We investigated the mechanisms that enable the coexistence of two congeneric macaques—the Assamese macaque (Macaca assamensis) and rhesus macaque (M. mulatta)—in the Western Himalaya along the India–Nepal border in the State of Uttarakhand, India. For five months from December 2016, we collected scan samples of the behavior of one Assamese macaque group (N = 9975 samples) and two rhesus macaque groups (N = 14,402). Activity budget comparisons revealed that the former spent more time on feeding and the latter on resting and moving. Although the two species had 29 (37%) of 78 food items in common, only Mallotus philippensis and agricultural crops formed a major part of their shared diet (contributing to >1% of feeding scans). The Assamese macaque fed predominantly on leaves and had a broader niche than did the rhesus macaque, which fed mostly on fruits. We also observed differences in feeding schedules and feeding heights of the two species. The two species showed variation in home range, daily movement patterns, habitat use, and sleeping sites that need further investigation. The two species exhibited a lower dietary and spatial niche overlap in winter, a period of relatively low resource abundance than in spring. The observed differences in diet and space use suggest that the niches of the two macaques are separated in several dimensions, which may have promoted their coexistence in this region. Similar long-term studies across different habitats and seasons can improve our understanding of resource use by primates in sympatry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concept of niche differentiation has been widely used to understand how sympatric species limit competition over resources (Holt 2009). Closely related sympatric species acquire distinct niches through behavioral and ecological adaptations that promote coexistence (Hutchinson 1959). Three major theories have been put forward to explain the causes and consequences of coexistence: 1) the competitive exclusion principle (Gause 1934), 2) the theory of limiting similarity (Abrams 1983), and 3) ecological character displacement (Brown and Wilson 1956). Among these theories, ecological character displacement takes into consideration phylogenetic relationships and predicts that competition for limited resources drives closely related species to diverge adaptively when in sympatry (Stuart and Losos 2013). Diet, ranging, and activity patterns have been identified as three major dimensions along which sympatric species most often diverge (Schoener 1974; Schreier et al.2009).
Primates are a widely studied group with regard to their adaptations when in sympatry. Most studies of closely related sympatric primates have identified diet as the primary factor enabling coexistence. To minimize conflict, primates consume different plant species or parts and even plants in different phenophases (Ganzhorn 1989; Ruslin et al.2019; Yamagiwa and Basabose 2006). Habitat differentiation, in terms of horizontal distribution, vertical stratification, or use of different microhabitats, has also been identified as a factor governing niche separation among primates (Lahann 2008; Nadjafzadeh and Heymann 2008; Rakotondranary and Ganzhorn 2011). In addition, scheduling activities differently has been explored as another way to reduce competition, allowing species to coexist (Dunbar and Dunbar 1974; Snodderly et al.2019). Of the studies of the possible modes of coexistence and niche partitioning between primates (Schreier et al.2009), few have taken into consideration the phylogenetic distances between the species involved (Houle 1997). Nevertheless, existing data suggest an apparently lower limit to the phylogenetic distance among species that exist in sympatry, and that the closest, coexisting phylogenetic taxa are usually kin species rather than sister species (Houle 1997).
The genus Macaca is the most diverse of all primate genera, with 22 species presently known (Thierry 2007). Morphological and molecular evidence classifies species in this genus into three or four lineages or species groups, which separated less than three million years ago (Fooden 1980; Tosi et al.2003; Zhang and Shi 1993). Dispersal of these species groups is thought to be closely related to changing climatic conditions and repeated cycles of habitat shrinkage and expansion (Fooden 1976). The distribution of these species groups indicates a pattern in which geographical ranges of species within a lineage are broadly allopatric while those of different species group are partially sympatric (Fooden 1976). Despite their morphological and anatomical similarities, competition appears to have resulted in the geographical segregation of Macaca species throughout South and Southeast Asia. However, eight species are partly sympatric in the heartland of the genus, indicating some form of ecological segregation (Fooden 1982). This pattern of ecological separation suggests that, along with Pleistocene climate changes (Eudey 1980), interspecific competition and the resultant differential adaptations (Pianka 2011) may have played a major role in the evolutionary development of their present-day distribution. Sympatric macaque species include bonnet macaques (Macaca radiata) and lion-tailed macaques (M. silenus) in the Western Ghats (Sushma and Singh 2006; Singh et al.2011) and the rhesus macaque, Assamese macaque, northern pig-tailed macaque (M. leonina), and the stump-tailed macaque (M. arctoides) in the lowland rainforests of northeastern India (Sharma et al.2012). In each case, these sympatric species belong to different species groups.
Assamese macaques of the sinica and rhesus macaques of the fascicularis lineage exist in sympatry in many parts of East and Southeast Asia (Fooden 1982). The fragmented distribution of the sinica lineage suggests that it dispersed earlier compared to the broadly distributed fascicularis lineage. The recent dispersal of the latter probably brought it into contact with other lineages, suggesting a competitive relationship, which contributed to the reduction and disjunction of ranges of species groups that dispersed earlier (Delson 1980). In areas where the distribution ranges of these closely related species still overlap they may avoid direct competition by small-scale spatial avoidance or by adapting to distinct niches to coexist (Houle 1997). For example, differences in diet and habitat use enable the coexistence of Assamese and rhesus macaques in a seasonal limestone rainforest of China (Zhou et al.2014).
We compared the diet, ranging patterns, and activity budget of the Assamese macaque with that of the sympatric rhesus macaque in the Himalaya, the westernmost limit of the Assamese macaque distribution (Chandola et al.2006; Verma and Verma 2011). We describe the ecological niches of the two macaque species and assess variation in niche overlap across winter and spring seasons. We test the following hypotheses: 1) The two study species coexist by partitioning their resources. If this is the case, we predict that the two species differ in diet or space use. 2) Resource availability plays a major role in determining niche overlap. If this is the case, we predict niche differentiation to be more prominent in the cold winter months: a period of relatively low resource abundance in the Himalaya.
Methods
Study Site and Subjects
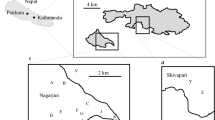
We conducted the study from December 2016 to April 2017 in the Askot landscape, a part of the Middle and Upper Himalayas, in the Pithoragarh district of Uttarakhand state in northern India. The area is demarcated by Tibet to its north, the river Kali separates it from Nepal in the southeast, and the western boundary is formed by eastern banks of the Gori river (Samant et al.1998). Elements of the Western Himalaya, the Central Himalaya, and the Tibetan Plateau converge in this landscape, making it a regionally important site for its species richness and biological distinctiveness (Dhar et al.1997).
We selected a 7-km2 area (29°48′ to 29°46′N and 80°21′ to 80°23′E) in the southern end of the Askot landscape close to the confluence of the rivers Kali and Gori (Fig. 1). This area has steep slopes, with elevations ranging from 645 to 1740 m. The temperature during the study varied from a minimum of 2 °C in winter to a maximum of 42 °C in spring, with a mean of 16.5 °C. The area has patches of dense forest: the Montane Valley Sal Forest, Moist Mixed Deciduous Forest, Himalayan Chir Pine Forest, and Banj Oak Forest (Champion and Seth 1968). There are also seven hamlets, where agriculture is the primary source of livelihood for the local communities. The main crops planted by the villagers during the study period were wheat (Triticum aestivum), masoor (Lens culinaris), barley (Hordeum vulgare), potato (Solanum tuberosum), onion (Allium cepa), gram (Cicer arietinum), pea (Pisum sativum), radish (Raphanus raphanistrum), and cauliflower (Brassica oleracea). The remaining part of the study area was typically constituted by degraded lands and abandoned fallow fields. In this area, we selected and habituated one group of Assamese and two groups of rhesus macaques for study (Table I).
Data Collection
We followed each of the macaque groups for 5 days (one session) every month from dawn until dusk. We carried out four such sessions for each group. We segregated these data into winter from December to late February (mean temperature 14 °C) and spring, from March to April (mean temperature 21 °C). To capture seasonal differences, we waited for 20 days between subsequent sessions on the same group.
We conducted behavioral observations on the selected groups using scan sampling (Altmann 1974). Each scan lasted 5 min, with scans being repeated at 10-min intervals. During each scan, we recorded the age, sex, and activity of all visible individuals. We grouped activities into five categories: feeding, resting, moving, social interactions, and others (including autogrooming, vigilance, urinating, defecating, vocalizing, yawning, and sneezing). In addition, when an individual was feeding, we noted the plant species and the parts eaten, along with the individual’s foraging height, categorized as ground, low (<3 m), mid (3–6 m), and high (>6 m). We classified the plant parts eaten as leaf (young/mature), fruit and seed (ripe/semiripe), flower and bud (young/mature), and stem and root (young/mature). We collected plant species that could not be identified in the field and identified them later at the herbarium at the Wildlife Institute of India, Dehradun.
We obtained 9975 individual samples in 201 contact hours for Assamese macaques and 14,402 samples in 348 contact hours for rhesus macaques, distributed across winter and spring (Table II). Of these, we obtained 2797 samples from adult Assamese and 3034 samples from adult rhesus macaques (Table III).
To determine the ranging patterns of the macaque groups, we collected GPS locations at 30-min intervals. We also collected information on the habitat, categorized as cliff, mixed broadleaf forest, open forest, scrub, or agricultural field. At the end of the day, when the individuals retired to their sleeping sites, we recorded information regarding the type of sleeping site used (cliff/tree species) and its elevation.
Data Analysis
We describe the activity budget as the percentage of data points from scan sampling falling into each activity. We calculated separate activity budgets for winter and spring. We examined the differences in feeding schedules of the two species between 07:00 and 17:00 h using a two-sample χ2 test.
To describe diet and use of forest strata, we computed the percentages of scans attributed to particular plant parts consumed by the two species and their feeding heights across the two seasons. We used Levin’s index (Hurlbert 1978; Krebs 1989) to calculate the niche breadth of the two species: Levin’s standardized niche breadth (BA) =\( \frac{B-1}{N-1} \); B is Levin’s measure of niche breadth \( \Big(\ \left(B=\frac{1}{\sqrt{P_{j^2}}}\right) \), where Pj is the proportion of individuals found in or using resource state j, and N is the number of possible resource states. We used the total count of all food items that a macaque species ate as the number of possible resource states (N) and the proportion of scanned individuals feeding on a plant species j as Pj.
To calculate food niche overlap between the two macaque species, we used Pianka’s index, with the formula:
where Ojk is Pianka’s index of niche overlap between species j and k, while Pij andPik are the proportions of use of resource category i by species j and k respectively. We took plant species to be a resource category. Pianka’s index varies between 0 (total separation) and 1 (total overlap) (Krebs 1989; Pianka 1973).
We used Arc GIS 10.5 to map daily movement and home range of the study groups. We calculated home range using the minimum convex polygon (MCP) method (Börger et al.2006) to allow comparison with other studies. We also generated 95% and 50% kernel density contours using a reference bandwidth to determine the centers of activity (Worton 1989). We created MCP and kernel density contours using the HRT tool in ARC GIS 10.5 (Rodgers et al.2007). We used the percentage of locations in various habitat types to describe habitat use.
Ethical Note
All research reported in this article adhered to the legal requirements of India, with our field research being conducted with permission granted by the Chief Wildlife Warden of the Uttarakhand State Forest Department. Our research adhered to the principles for the ethical treatment of primates, in accordance with the IPS Code of Best Practices for Field Primatology. We did not handle any live or dead animals during this study. To the best of our knowledge, there is no conflict of interest, financial or otherwise, with regard to the research reported here.
Results
Activity Budgets
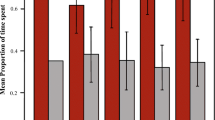
A comparison of the activity budgets of the study groups showed that Assamese macaques spent more time in feeding (38%) and social interactions (19%), than the rhesus macaques, which spent more time in resting (25%) and moving (24%). The feeding schedules of the two species differed both in winter (χ2 = 19, df = 9, P < 0.05) and in spring (χ2 = 39, df = 9, P < 0.05). The differences, however, were less prominent in winter than in spring, when we observed clear feeding peaks in the Assamese macaques during the afternoon, whereas rhesus macaque feeding peaked around morning and evening (Fig. 2).
Diet
Food Items, Niche Breadth and Niche Overlap
The two macaque species consumed 78 food plants during the study, 37% of which were eaten by both species, accounting for 50% of the diet of the Assamese macaques and 58% of the diet of the rhesus macaques. In winter, there was a dietary overlap of 35% of the 60 plant species fed on, accounting for 50% of the diet of the Assamese macaques and 54% of that of the rhesus macaque. In spring, the macaques fed on 51 plant species, with a dietary overlap of 31% of food plants, constituting 43% of the diet of the Assamese macaques and 53% of that of the rhesus macaques.
When we limited the comparison to major food items (plants that accounted for >1% of the total feeding scans), we saw a marked difference between the two species. Combining the two seasons, the two species shared only two food items: agricultural crops and Mallotus philippensis (Table IV). The macaques shared only one food item in winter (agricultural crops) and four in spring (agricultural crops, Mallotus philippensis, Pyrus pashia, and Woodfordia fruticosa; Table V).
The standardized niche breadth was 0.12 for the Assamese and 0.09 for the rhesus macaque, ranging from 0.13 in winter to 0.10 in spring for the Assamese and from 0.14 in winter to 0.07 in spring for the rhesus macaque. The food niche overlap between the two species across the two seasons was 0.45, and increased from 0.22 in winter to 0.51 in spring.
Plant Parts Used
In winter, leaves (64%) were the dominant plant part in the Assamese macaque diet, which changed to flower and bud (41%) in spring. For rhesus macaques, fruit and seed were the dominant plant parts in both winter (66%) and spring (37%; Fig. 3).
Feeding Height
Although the feeding scans revealed that both study species spent most of their time either on the ground or 3–5 m above ground, Assamese macaques generally fed in the middle canopy (winter 39%; spring 40%) while rhesus macaques tended to feed on the ground (winter 48%; spring 49%; Fig. 4).
Ranging Patterns
Day Range
All three macaque groups had variable day ranges across the two seasons (Fig. 5). The Assamese macaques had a median (± SE) day range of 0.89 (± 0.15) km in winter and 1.26 (± 0.15) km in spring while that of the rhesus macaques varied from 1.10 (± 0.12) km in winter to 1.66 (± 0.11) km in spring.
Variation in the day range of Assamese and rhesus macaque groups in winter and spring at Uttarakhand, India, December 2016–April 2017. Boxes represent the 25th and 75th percentiles. The horizontal bar in the middle of the box represents the median value. Bars indicate minimum and maximum distance traveled per day.
Home Range
The Assamese macaque group had the largest home range (Tables VI and VII). During the study, the home range size plateaued at a mean of 215 (± SD 7.50) fixes for the three study groups. The home ranges of all three groups overlapped partially (Table VIII; Fig. 6).
Habitat Use
We often saw the Assamese macaques either on cliffs (37% of observation time) or in mixed broadleaf forests (29%) and rhesus macaques in open forests (46%) or agricultural fields (22%; Fig. 7).
Sleeping Sites
We located eight sleeping sites for the Assamese macaques and all were on steep cliffs. On 12 of 20 sampling days, the species used the same sleeping site for three or four consecutive days. Rhesus macaques, however, used tall trees of Shorea robusta, Pinus roxburghii, Engelhardtia spicata, Bischofia javanica, Toona ciliata, Quercus glauca, and Bauhinia variegata as sleeping sites on 33 of 35 sampling days and used cliffs only twice. Unlike the Assamese macaques, rhesus macaques never stayed at the same sleeping site for two consecutive days. The Assamese macaque sleeping sites were located at a mean distance of 502 (± SE 40) m from human habitation, compared with 280 (± 26) m for the rhesus macaques.
Discussion
We found that Assamese and the rhesus macaques coexisted in the mountainous landscape of the Western Himalaya by occupying different niches with respect to their activity budgets, diet, and space use.
The two macaque species differed in their activities, with the Assamese macaques feeding more and the rhesus macaques resting and moving more. These results reflect those for the same species at other study sites (Jaman and Huffman 2013; Sarkar et al.2012). Folivore primates often need diverse food resources to obtain the best complement of nutrients (Westoby 1978). This may explain why the Assamese macaques, which are predominantly folivores in the area, required more feeding time than rhesus macaques. Compared to leaves, which are abundant and evenly dispersed, fruits tend to be more patchily distributed, and therefore require relatively greater travel to find them (Sarkar et al.2012). The frugivore rhesus macaques in our study may have traveled more than Assamese macaques to locate fruiting trees. The relatively greater time spent moving by this species could also be attributed to their frequent crop-foraging behavior because they were often chased away by the local people. Such extensive locomotion usually implies greater energy costs (Sarkar et al.2012), which might explain the observed prolonged resting phases followed by movement bouts of the rhesus macaques, which typically occurred after feeding spells in the agricultural fields.
Food resources, often considered to be limited, typically constitute a commodity over which closely related, sympatric species are most likely to compete (Ganzhorn 1999). In this study, Assamese and rhesus macaques probably limited conflict over food by feeding exclusively on particular plant species, limiting their food niche overlap. Although a total of 78 food species were consumed by the two macaque species, they shared only 29 species (37%). In limestone habitats of China these macaque species shared even fewer food species: 15 (11%) of the 131 plant species consumed by both macaque species. This finding might be related to the differential use of various limestone zones by these macaque species, with zones having distinct plant species. Unlike in our study, Assamese macaques in China concentrated on few plant species, especially Indocalamus calcicolus (62% of diet), decreasing the number of foods shared by the species (Zhou et al.2014).
The two macaque species also differed in their dietary niche breadth. The Assamese had a larger niche breadth than the rhesus macaques, which might have primarily been due to the Assamese macaque feeding on leaves, the consumption of which is positively related to diet diversity (Dunn et al.2010). The rhesus macaque’s smaller niche breadth might similarly be ascribed to its frugivorous diet, along with the fact that a relatively high proportion (~30%) of its diet came from agricultural crops. The Assamese and the rhesus macaques have previously been described to be predominantly folivore and frugivore, respectively, in limestone habitat of China (Zhou et al.2011). The crop foraging we observed by rhesus macaques reflects findings elsewhere (Lee and Priston 2005; Saraswat et al.2015). The dispersal of the fascicularis species group, including rhesus macaques, appears to have historically corresponded with the expansion of human settlements (Fooden 1976), which might have helped rhesus macaques, in particular, to adapt and take advantage of resources associated with human communities (Richard et al.1989).
An examination of the seasonal differences in feeding patterns of the study macaques revealed their dietary niche overlap increases from 0.22 in the fruit-scarce winter season to 0.51 in spring, when fruits became relatively abundant. Such a trend, where niche overlap increases with increase in food resources, is consistent with that shown by many other sympatric primates (Marshall et al.2009; Singh et al.2011; Stevenson et al.2000). Such patterns are, however, not invariable and occasionally there may be higher diet overlaps during periods of relatively low fruit availability. For example, dietary overlap between Assamese and rhesus macaques in the limestone hills of China increased in the dry season, as fruit scarcity during this time forced the latter to consume leaves (Zhou et al.2014). We found that an increase in dietary overlap was complemented by a variation in the scheduling of feeding activity, as reported by other studies (Dunbar and Dunbar 1974; Schreier et al.2009).
In spring the two species showed a noticeably greater convergence in the plant parts they consumed when compared to winter. At this time, the dominant plant part in the diet of the Assamese macaque changed from leaves to fruit, which was a major plant part in the diet of rhesus macaques, both in winter and in spring. The results contrast with an increase in the contribution of leaves from 13% in winter to 60% in spring in the diet of a closely related species, Arunachal macaque (Macaca munzala), in the Eastern Himalaya (Mendiratta et al.2009). The diet composition of our study species varies greatly across different habitats and seasons. Assamese macaques, for example, prefer flowers and fruits when these are available (Chalise 2003; Huang et al.2015). In the temperate forests of Pakistan, leaves, stems, and other vegetative plant parts make up 84% of the diet of rhesus macaques, whereas fruits contribute less than nine percent of their feeding records (Goldstein and Richard 1989). Whether rhesus macaques chose or were forced to feed on such a diet due to the scarcity of fruits in the disturbed habitats studied is unclear, though their flexibility may underpin the ability of this species to spread extensively despite forest disappearance and agricultural expansion, resulting in its remarkably wide distribution (Richard et al.1989).
Along with dietary differences, the two macaque species in our study differed from one another with respect to the habitat and substratum heights used during foraging. The Assamese macaque was relatively more arboreal, feeding mostly at the middle-canopy layer, probably because it spent a large proportion of its time in mixed broadleaf forest habitats. Rhesus macaques, in contrast, depended largely on agricultural crops and therefore spent a majority of their time feeding at the ground level. Such vertical separation has also been observed in lion-tailed macaque, bonnet macaque, and gray langur (Semnopithecus entellus) in the Western Ghats (Singh et al.2011) and in other sympatric primates (Feeroz 2011; Hadi et al.2012; Lahann 2008, 2012). Differential habitat use may be a mechanism to reduce interspecific competition (Ganzhorn 1989; Hadi et al.2012; Schwab and Ganzhorn 2004).
The selection of different sleeping sites by the two macaque species might be another factor responsible for segregating these species in horizontal space in our study area. While rhesus macaques used trees, spread extensively over an area, to rest, Assamese macaques used cliffs, as has also been reported by Chalise (2003). Selection of cliffs as a sleeping site has also been reported for the Arunachal macaque, a species closely related to the Assamese macaque (Kumar et al.2008). As cliffs were available in the home ranges of both species, the selection of different sleeping sites might be explained by different requirements. For example, an Assamese macaque group may prefer to sleep in a single tree and may therefore require larger trees, as in Thailand, where an entire group used a single tree to sleep (Richter et al.2016).
The day range and the seasonal home range—key measures of space use by primates—also differed between the study species. Folivores typically have shorter day ranges than frugivores (Carbone et al.2004). Our study illustrated this well, with Assamese macaques tending to move less in both the study seasons when compared to the rhesus macaques. Many species often travel less and rest more during periods of fruit scarcity, presumably to save energy (Stevenson et al.2000). For both our study species, we found the daily movement of the groups to be less in winter and more in spring, when the abundance of fruiting trees clearly increased in the study area.
In summary, sympatric Assamese and rhesus macaques appear to exhibit prominent ecological and behavioral differences in the subtropical Western Himalayan Mountains of northern India. The observed species-specific distinction in diets, feeding schedules, and spatial use apparently allowed these species to coexist in their often resource-limited habitats, especially in winter. We observed relatively low dietary and spatial niche overlaps between the two macaque species during winter, a period of relatively low resource abundance; this overlap, however, increased noticeably during the spring season, a period marked by high abundance of shared resources. Similar studies over longer durations across different habitats and seasons can improve our understanding of resource use by sympatric primates.
In addition to the presence of conspecifics, anthropogenic environmental impacts may also lead to niche modification in primates. The ability of Assamese and rhesus macaques to share resources will depend largely on their habitats remaining intact. Continued loss and degradation of broadleaf forests due to land use practices, such as agricultural expansion and developmental activities including hydroelectric power projects and road construction, pose a significantly greater risk to the less adaptable Assamese macaques than to the rhesus macaques. Further, during our fieldwork, the local people reported that large numbers of rhesus macaque groups had been translocated to their villages from the cities, to reduce urban human–macaque conflict (see also Kumar et al.2011). The increasing numbers of highly adaptable rhesus macaques may outcompete the Assamese macaques in the region, where from there habitats are already restricted (Sharma et al.2012). Given that human impacts continue to grow, studies of niche seggregation should consider how closely related species differ in exploiting these anthropogenic resources.
Data Availability
All data analyzed in this study are included in the article.
References
Abrams, P. (1983). The theory of limiting similarity. Annual Review of Ecology and Systematics, 14, 359–376.
Altmann, J. (1974). Observational study of behavior: Sampling methods. Behaviour, 49, 227–266.
Börger, L., Franconi, N., De Michele, G., Gantz, A., Meschi, F., et al (2006). Effects of sampling regime on the mean and variance of home range size estimates. Journal of Animal Ecology, 75, 1393–1405.
Brown, W. L., & Wilson, E. O. (1956). Character displacement. Systematic Zoology, 5, 49–64.
Carbone, C., Cowlishaw, G., Isaac, N. J., & Rowcliffe, J. M. (2004). How far do animals go? Determinants of day range in mammals. The American Naturalist, 165, 290–297.
Chalise, M. K. (2003). Assamese macaques (Macaca assamensis) in Nepal. Primate Conservation, 19, 99–107.
Champion, S. H., & Seth, S. K. (1968). A revised survey of the forest types of India. In Nasik. India: Government of India Press.
Chandola, S., Rawat, G. S., & Naithani, H. B. (2006). On the occurrence of a little known macaque in Uttaranchal. Indian Forester, 132, 885–886.
Delson, E. (1980). Fossil macaques, phyletic relationships and a scenario of deployment. In Lindberg, D. G. (ed), The macaques. Studies in ecology, behavior and evolution. Van Nostrand Reinhold, New York, pp. 10–30.
Dhar, U., Rawal, R. S., & Samant, S. S. (1997). Structural diversity and representativeness of forest vegetation in a protected area of Kumaun Himalaya, India: Implications for conservation. Biodiversity and Conservation, 6, 1045–1062.
Dunbar, R. I. M., & Dunbar, E. P. (1974). Ecological relations and niche separation between sympatric terrestrial primates in Ethiopia. Folia Primatologica, 21, 36–60.
Dunn, J. C., Cristóbal-Azkarate, J., & Veà, J. J. (2010). Seasonal variations in the diet and feeding effort of two groups of howlers in different sized forest fragments. International Journal of Primatology, 31, 887–903.
Eudey, A. A. (1980). Pleistocene glacial phenomena and the evolution of Asian macaques. In D. G. Lindburg (Ed.), The macaques: Studies in ecology, behavior and evolution (pp. 52–83). New York: Van Nostrand Reinhold.
Feeroz, M. M. (2011). Resource partitioning among the sympatric primate species of West Bhanugach forest reserve of Bangladesh. In E. Røskaft & D. J. Chivers (Eds.), Proceedings of the International Conference on Biodiversity—Present state, problems and prospects of its conservation (pp. 33–43). Chittagong: University of Chittagong.
Fooden, J. (1976). Provisional classification and key to living species of macaques (Primates: Macaca). Folia Primatologica, 25, 225–236.
Fooden, J. (1980). Classification and distribution of living macaques (Macaca Laćepède, 1799). In D. G. Lindburg (Ed.), The macaques: Studies in ecology, behavior and evolution (pp. 1–9). New York: Van Nostrand Reinhold.
Fooden, J. (1982). Ecogeographic segregation of macaque species. Primates, 23, 574–579.
Ganzhorn, J. U. (1989). Niche separation of seven lemur species in the eastern rainforest of Madagascar. Oecologia, 79, 279–286.
Ganzhorn, J. U. (1999). Body mass, competition and the structure of primate communities. In J. G. Fleagle, C. Janson, & K. E. Reed (Eds.), Primate communities (pp. 141–157). Cambridge: Cambridge University Press.
Gause, G. F. (1934). Experimental analysis of Vito Volterra’s mathematical theory of the struggle for existence. Science, 79, 16–17.
Goldstein, S. J., & Richard, A. F. (1989). Ecology of rhesus macaques (Macaca mulatta) in Northwest Pakistan. International Journal of Primatology, 10, 531–567.
Hadi, S., Ziegler, T., Waltert, M., Syamsuri, F., Mühlenberg, M., & Hodges, J. K. (2012). Habitat use and trophic niche overlap of two sympatric colobines, Presbytis potenziani and Simias concolor, on Siberut Island, Indonesia. International Journal of Primatology, 33, 218–232.
Holt, R. D. (2009). Bringing the Hutchinsonian niche into the 21st century: Ecological and evolutionary perspectives. Proceedings of the National Academy of Sciences of the USA, 106, 19659–19665.
Houle, A. (1997). The role of phylogeny and behavioral competition in the evolution of coexistence among primates. Canadian Journal of Zoology, 75, 827–846.
Huang, Z., Huang, C., Tang, C., Huang, L., Tang, H., Ma, G., & Zhou, Q. (2015). Dietary adaptations of Assamese macaques (Macaca assamensis) in limestone forests in Southwest China. American Journal of Primatology, 77, 171–185.
Hurlbert, S. H. (1978). The measurement of niche overlap and some relatives. Ecology, 59, 67–77.
Hutchinson, G. E. (1959). Homage to Santa Rosalia or why are there so many kinds of animals? The American Naturalist, 93, 145–159.
Jaman, M. F., & Huffman, M. A. (2013). The effect of urban and rural habitats and resource type on activity budgets of commensal rhesus macaques (Macaca mulatta) in Bangladesh. Primates, 54, 49–59.
Krebs, C. J. (1989). Ecological methodology. New York: Harper & Row.
Kumar, R. S., Gama, N., Raghunath, R., Sinha, A., & Mishra, C. (2008). In search of the munzala: Distribution and conservation status of the newly-discovered Arunachal macaque Macaca munzala. Oryx, 42, 360–366.
Kumar, R., Radhakrishna, S., & Sinha, A. (2011). Of least concern? Range extension by the rhesus macaque Macaca mulatta threatens long-term survival of the bonnet macaque M. radiata in peninsular India. International Journal of Primatology, 32, 945–959.
Lahann, P. (2008). Habitat utilization of three sympatric cheirogaleid lemur species in a littoral rain forest of southeastern Madagascar. International Journal of Primatology, 29, 117–134.
Lee, P. C., & Priston, N. E. C. (2005). Human attitudes to primates: Perceptions of pests, conflict and consequences for conservation. In J. D. Paterson & J. Wallis (Eds.), Commensalism and conflict: The human–primate interface (pp. 1–23). Norman: American Society of Primatologists.
Marshall, A. J., Cannon, C. H., & Leighton, M. (2009). Competition and niche overlap between gibbons (Hylobates albibarbis) and other frugivorous vertebrates in Gunung Palung National Park, West Kalimantan, Indonesia. In D. Whattaker & S. Lappan (Eds.), The gibbons Developments in primatology: Progress and prospects (pp. 161–188). New York: Springer Science+Business Media.
Mendiratta, U., Kumar, A., Mishra, C., & Sinha, A. (2009). Winter ecology of the Arunachal macaque Macaca munzala in Pangchen Valley, western Arunachal Pradesh, northeastern India. American Journal of Primatology, 71, 939–947.
Nadjafzadeh, M. N., & Heymann, E. W. (2008). Prey foraging of red titi monkeys, Callicebus cupreus, in comparison to sympatric tamarins, Saguinus mystax and Saguinus fuscicollis. American Journal of Physical Anthropology, 135, 56–63.
Pianka, E. R. (1973). The structure of lizard communities. Annual Review of Ecology and Systematics, 4, 53–74.
Pianka, E. R. (2011). Evolutionary ecology. 7th edition- eBook. Available at: http://www.zo.utexas.edu/courses/bio373/ERP-EvolEcol.html.
Rakotondranary, S. J., & Ganzhorn, J. U. (2011). Habitat separation of sympatric Microcebus spp. in the dry spiny forest of South-Eastern Madagascar. Folia Primatologica, 82, 212–223.
Richard, A. F., Goldstein, S. J., & Dewar, R. E. (1989). Weed macaques: The evolutionary implications of macaque feeding ecology. International Journal of Primatology, 10, 569–594.
Richter, C., Heesen, M., Nenadić, O., Ostner, J., & Schülke, O. (2016). Males matter: Increased home range size is associated with the number of resident males after controlling for ecological factors in wild Assamese macaques. American Journal of Physical Anthropology, 159, 52–62.
Rodgers, A. R., Carr, A. P., Beyer, H. L., Smith, L., & Kie, J. G. (2007). HRT: Home range tools for ArcGIS. Version 1.1. Thunder Bay, Ontario: Ontario Ministry of Natural Resources, Centre for Northern Forest Ecosystem Research.
Ruslin, F., Matsuda, I., & Md-Zain, B. M. (2019). The feeding ecology and dietary overlap in two sympatric primate species, the long-tailed macaque (Macaca fascicularis) and dusky langur (Trachypithecus obscurus obscurus), in Malaysia. Primates, 60, 41–50.
Samant, S. S., Dhar, U., & Rawal, R. S. (1998). Biodiversity status of a protected area in west Himalaya: Askot wildlife sanctuary. The International Journal of Sustainable Development and World Ecology, 5, 194–203.
Saraswat, R., Sinha, A., & Radhakrishna, S. (2015). A god becomes a pest? Human-rhesus macaque interactions in Himachal Pradesh, northern India. European Journal of Wildlife Research, 61, 435–443.
Sarkar, P., Srivastava, A., Dasgupta, S., & Bhattacharjee, P. C. (2012). Activity profile of free ranging forest group of Assamese macaque. The Clarion, 1, 59–67.
Schoener, T. W. (1974). Resource partitioning in ecological communities. Science, 185, 27–39.
Schreier, B. M., Harcourt, A. H., Coppeto, S. A., & Somi, M. F. (2009). Interspecific competition and niche separation in primates: A global analysis. Biotropica, 41, 283–291.
Schwab, D., & Ganzhorn, J. U. (2004). Distribution, population structure and habitat use of Microcebus berthae compared to those of other sympatric cheirogalids. International Journal of Primatology, 25, 307–330.
Sharma, N., Madhusudan, M. D., Sarkar, P., Bawri, M., & Sinha, A. (2012). Trends in extinction and persistence of diurnal primates in the fragmented lowland rainforests of the upper Brahmaputra Valley, northeastern India. Oryx, 46, 308–311.
Singh, M., Roy, K., & Singh, M. (2011). Resource partitioning in sympatric langurs and macaques in tropical rainforests of the Central Western Ghats, South India. American Journal of Primatology, 73, 335–346.
Snodderly, D. M., Ellis, K. M., Lieberman, S. R., Link, A., Fernandez-Duque, E., & Di Fiore, A. (2019). Initiation of feeding by four sympatric Neotropical primates (Ateles belzebuth, Lagothrix lagotricha poeppigii, Plecturocebus (Callicebus) discolor, and Pithecia aequatorialis) in Amazonian Ecuador: Relationships to photic and ecological factors. PLoS One, 14, e0210494. https://doi.org/10.1371/journal.pone.0210494.
Stevenson, P. R., Quinones, M. J., & Ahumada, J. A. (2000). Influence of fruit availability on ecological overlap among four neotropical primates at Tinigua National Park, Colombia. Biotropica, 32, 533–544.
Stuart, Y. E., & Losos, J. B. (2013). Ecological character displacement: glass half full or half empty?. Trends in ecology & evolution, 28, 402–408.
Sushma, H. S., & Singh, M. (2006). Resource partitioning and interspecific interactions among sympatric rain forest arboreal mammals of the Western Ghats, India. Behavioral Ecology, 17, 479–490.
Thierry, B. (2007). Unity in diversity: Lessons from macaque societies. Evolutionary Anthropology: Issues, News, and Reviews, 16, 224–238.
Tosi, A. J., Morales, J. C., & Melnick, D. J. (2003). Paternal, maternal, and biparental molecular markers provide unique windows onto the evolutionary history of macaque monkeys. Evolution, 57, 1419–1435.
Verma, A., & Verma, N. (2011). Kala bandar: The mystery macaque of Uttarakhand. Sanctuary Asia, 31, 38–41. http://www.sanctuaryasia.com/component/content/article/7880-kala-bandar-the-mystery-macaque-of-uttarakhand.html.
Westoby, M. (1978). What are the biological bases of varied diets? The American Naturalist, 112, 627–631.
Worton, B. J. (1989). Kernel Methods for Estimating the Utilization Distribution in Home-Range Studies. Ecology, 70, 164–168.
Yamagiwa, J., & Basabose, A. K. (2006). Diet and seasonal changes in sympatric gorillas and chimpanzees at Kahuzi–Biega National Park. Primates, 47, 74–90.
Zhang, Y. P., & Shi, L. M. (1993). Phylogenetic relationships of macaques as inferred from restriction endonuclease analysis of mitochondrial DNA. Folia Primatologica, 60, 7–17.
Zhou, Q., Wei, H., Huang, Z., & Huang, C. (2011). Diet of the Assamese macaque Macaca assamensis in limestone habitats of Nonggang, China. Current Zoology, 57, 18–25.
Zhou, Q., Wei, H., Tang, H., Huang, Z., Krzton, A., & Huang, C. (2014). Niche separation of sympatric macaques, Macaca assamensis and M. mulatta, in limestone habitats of Nonggang, China. Primates, 55, 125–137.
Acknowledgments
We thank the Director and the Dean at the Wildlife Institute of India for providing all logistical support and funding to carry out this research. We are grateful for the field assistance provided by Ishwar Singh Dhami, Janki Devi, Nanda Devi, Bhavana Panday and Uttam Singh, without whom this research would not have been possible. We express our sincere gratitude to the Uttarakhand Forest Department for granting permission for this work. We also acknowledge Editor-in-Chief Joanna Setchell, Associate Editor Oliver Schuelke, and two anonymous reviewers whose detailed and helpful comments greatly improved this manuscript.
Author information
Authors and Affiliations
Contributions
This research formed part of the Master’s dissertation work of PJ. PJ and SK formulated the study with active inputs from AS and GT; PJ conducted the study under the guidance and support of SK, GT, and AS.
Corresponding author
Additional information
Handling Editor: Joanna M. Setchell
Rights and permissions
About this article
Cite this article
Justa, P., Kumar, R.S., Talukdar, G. et al. Sharing from the Same Bowl: Resource Partitioning between Sympatric Macaque Species in the Western Himalaya, India. Int J Primatol 40, 356–373 (2019). https://doi.org/10.1007/s10764-019-00092-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-019-00092-z