Abstract
Knowledge about the feeding ecology and dietary overlap of sympatric primates is essential for understanding how animals avoid or reduce interspecific competition. From April 2014 to March 2015, we investigated the feeding ecologies of two sympatric primates, a hindgut fermenter, the long-tailed macaque (Macaca fascicularis) and a foregut fermenter, the dusky langur (Trachypithecus obscurus obscurus), in a mixed landscape consisting of urban and agro-forested areas and forest fragments in Malaysia. We collected a total of 5570 and 4029 of feeding records for M. fascicularis and T. o. obscurus, respectively, using the 10-min scan sampling method. Food availability and seasonal changes in plant species consumed by both study groups were determined by vegetation surveys carried out across an area of 1.6 ha. A total of 113 and 130 plant species were consumed by M. fascicularis and T. o. obscurus, respectively. Leaves (51%) and fruits (40%) accounted for the majority of the feeding records in T. o. obscurus, whereas fruits (32%) and anthropogenic foods (27%) together with leaves (15%) and insects (6%) accounted for the majority of the feeding records for M. fascicularis. Throughout the year, there were 59 consumed plant species common to both species, and the dietary overlap was the highest for fruits. Although leaves were always more abundant than fruits in our study site, the amount of monthly fruit eating by the two species showed a significant correlation with that of fruit availability. Monthly fruit availability had a positive effect on overall monthly dietary overlap while flower and leaf availability had a negative effect. We showed that fruit was the preferred food resource of two sympatric species with different digestive systems. This could have implications for resource competition, interspecific competition, and niche separation, which should be investigated in more detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In both old and new-world forests, many primate species commonly occur sympatrically (Reed and Bidner 2004). An understanding of interspecific competition and niche separation is indispensable for explaining how these primates can coexist (Fleagle et al. 1999). In Southeast Asian forests, foregut- and hindgut-fermenting primates commonly coexist within the same habitats (e.g., MacKinnon and MacKinnon 1980; Matsuda et al. 2016; Singh et al. 2011; Ungar 1995). This may be due to the specialized stomachs of foregut fermenters (i.e., colobine monkeys), which are complex and multi-chambered, allowing them to process foods, where bacteria detoxify defensive chemicals and digest cellulose, allow them to exploit a diet of leaves in greater quantities than other sympatric hindgut-fermenting primates (Chivers 1994). However, contrary to earlier assumptions that colobines mostly exploit ubiquitous food sources such as leaves (Davies and Oates 1994; Kirkpatrick 1999), recent colobine studies have reported high levels of fruit and/or seed consumption (Koenig and Borries 2001; Vandercone et al. 2012), which may cause high interspecific competition for foods and niche overlap between foregut- and hindgut-fermenting primates. This indicates that dietary studies on coexisting primates with different physiological systems (foregut vs. hindgut fermenters) are important for further understanding the underlying mechanisms, e.g., resource competition and/or niche separation, in coexisting primates.
MacKinnon and MacKinnon (1980) focused on the effects of diet and seasonality on feeding behavior in coexisting populations of M. fascicularis and T. o. obscurus in secondary dipterocarp forest, finding a limited niche overlap in diet between the species. Another comparative study on the feeding ecologies of sympatric macaques (M. silenus and M. radiata) and colobines (Semnopithecus entellus) living in evergreen forests also showed little dietary overlap (Singh et al. 2011). In addition, Ungar (1995), who focused on the fruit preferences of four sympatric primates inhabiting a primary lowland rain forest showed a clear difference between colobines (Presbytis thomasi) and other monogastric primates, including Hylobates lar, M. fascicularis and Pongo pygmaeus; the colobines mostly consumed the seeds of dry fruits, whereas the other primates preferred consuming fruit flesh. In contrast, very little information is available about the coexisting mechanism such as resource competition and/or niche separation, in sympatric primates inhabiting the forest edge near adjacent urban areas, where both anthropogenic foods and cultivated plants with natural foods are available. Since colobines that inhabit such fragmented habitats are known to consume a high amount of both natural and cultivated plant fruits (Dela 2012), it is ecologically important to understand the dietary flexibility of coexisting macaques and colobines in such habitat.
We examined the feeding ecology of two sympatric hindgut- and foregut-fermenting primates, long-tailed macaques (M. fascicularis) and dusky langurs (T. o. obscurus), in the mixed landscape consisting of urban and agro-forested areas and forest fragments of Bangi Permanent Forest Reserve, Selangor, Malaysia (Md-Zain et al. 2010). M. fascicularis is one of the most widespread primate species, occurring in a broad variety of habitats, and has been well studied not only in Malaysia but also in other countries, where they are highly opportunistic omnivores/frugivores (Aldrich-Blake 1980; Gumert et al. 2011; Md-Zain et al. 2011; Sha and Hanya 2013a). Ecological knowledge of T. o. obscurus, with a distribution restricted to peninsular Malaysia, is less complete (Md-Zain and Ch’ng 2011). A single study in a secondary dipterocarp forest described them as a folivore with a high preference for leaves from the upper forest canopy (Curtin 1980). To gain a more complete picture of the coexisting mechanisms in primates with different physiological systems (i.e., M. fascicularis and T. o. obscurus), with special reference to the forest edge habitat near adjacent urban areas (where it is ecologically important to understand primate dietary flexibility but has never been studied), we sought to (1) describe the basic feeding ecologies and monthly differences in diets between the species, (2) describe the relationship between resource availability and the feeding behavior of each species, and (3) compare diet composition and dietary overlap between the two species. Finally, we discussed the dietary flexibility of coexisting macaques and colobines in this type of forest.
Methods
Study site and animals
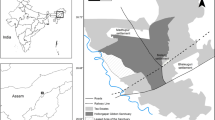
This study was conducted in mixed landscape and forest fragments of the Bangi Permanent Forest Reserve, Selangor, Malaysia (Fig. 1, 101°45′E, 2°54′N), continuously from April 2014 to March 2015, except in August. The study area is hilly, with the highest point reaching 80 m above sea level, and includes the main campus of the Universiti Kebangsaan Malaysia (UKM), intercity roads, foothill oil palm cultivations, and human premises. The monthly rainfall distribution and temperatures were obtained from a nearby weather station at the UKM (station name: Pekan Bangi Lama, station ID: 2817180). The mean minimum and maximum temperatures were approximately 24.7 and 32.0 °C, respectively, and total annual precipitation at the site was 2157 mm. The UKM Permanent Forest Reserve, although fragmented, still harbors important plant resources, including over 500 species of seed plants (Mat Salleh 1999). M. fascicularis and T. o. obscurus commonly inhabit the area with pig-tailed macaques (M. nemestrina) and pale-thighed langurs (Presbytis siamensis siamensis) encountered more infrequently (Md-Zain and Abdullah 2008).
One multimale–multifemale group of M. fascicularis and a one-male–multifemale group of T. o. obscurus were habituated to observers, and some members of the groups with distinctive features such as unusually short tail lengths, missing and deformed hand digits, a hunchback, and distinct skin color were identified individually. The group of M. fascicularis included 49 individuals: 12 adult males, 14 adult females, 11 subadults, eight juveniles, and four infants. The group of T. o. obscurus included 18 individuals: one adult male, seven adult females, four subadults, three juveniles, and three infants.
Behavioral data collection
We collected behavioral data from the adults and subadults in the M. fascicularis and T. o. obscurus groups continuously during our daily observation period, which lasted from 08:00 (or the time when the groups were found) until 18:30 (or the time when the groups were lost). We followed each focal group alternately for at least 3 days a month. During the observation periods, we conducted scan sampling at 10-min intervals (Altmann 1974). The total observation times for M. fascicularis and T. o. obscurus were 406.5 h and 373.1 h, respectively, and the monthly observation times periods were 29.5–59.0 h (mean, 37.0 h) and 29.9–42.4 h (mean, 34.5 h), respectively. The observation periods per day for M. fascicularis and T. o. obscurus were 10.2 ± SD 0.32 h and 10.4 ± SD 0.5 h, respectively. We recorded the activity (feeding, moving, and resting) of all visible adults and subadults. Feeding included handling, masticating, or swallowing food items. The food items consisted of leaves, fruits, flowers, anthropogenic foods (i.e., food waste), and others. Food plants were taxonomically identified in situ. If a food plant species was unknown, leaves, fruits, and/or flowers were collected for identification at the UKM by Mr. Ahmad Fitri Zohari.
Vegetational and phenological survey
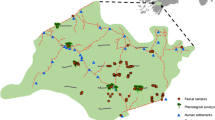
We set up ten quadrats of 40 × 40 m (i.e., 1.6 ha) in our study site. The vegetative plots were set up as follows: seven of the quadrats were in the edge of secondary forest while three were set up in agroforest for timber and bird food (Idilfitri and Mohamad 2012), as it is known to form part of home ranges of the focal groups according to a previous study by Md-Zain et al. (2011). In each quadrat, we marked trees with a DBH (diameter at breast height) ≥ 10 cm. We taxonomically identified all marked trees to species level if possible with the support of the Herbarium Universiti Kebangsaan Malaysia. At the end of each monthly survey, except for August 2014, we recorded the phenology of the 349 marked plants in the ten plots by examining each plant for the presence of leaves, fruits, and flowers. In the monthly phenological survey, food availability was determined as the leafing, fruiting, and flowering ratio (number of leafing, fruiting, and flowering plants/total number of monitored plants).
Data analysis
We tested for differences in the time spent feeding (%) on plant parts by M. fascicularis and T. o. obscurus and the mean monthly dietary overlap for each plant part (i.e., leaves, fruits, and flowers) using a Mann–Whitney U test with Bonferroni correction (p = 0.05/N; where N is the number of comparisons made). The dietary overlap of the primate species was calculated using the Holmes–Pitelka index, Di = ∑Si, where Di is the total percent overlap and Si is the percent overlap between shared food items (Holmes and Pitelka 1968). We tested for a correlation between the monthly availability of each plant part (leaves, fruits, and flowers) for the two primate species, using Spearman’s rank correlation coefficient. A linear model was used to examine whether the monthly mean proportion of time spent feeding on each plant part (i.e., leaves, fruits, and flowers) was affected by the monthly food availability of each plant part. The proportion of the monthly mean time spent feeding on each plant part was logit transformed [log (p/1−p)] and treated as a normally distributed response variable. The other factors (monthly availability of fruit, leaves, and flowers) were treated as explanatory variables. We examined a set of models with all possible combinations of the explanatory variables and ranked them by the corrected version of the Akaike information criterion (AIC) for small sample sizes, called the AICc (Burnham and Anderson 2002). Following guidelines published for wildlife research (Johnson and Omland 2004), we selected as the best-supported models those with a ΔAIC(c) score ≤ 2, where ΔAIC(c) = AIC(c)−minimum AIC(c) within the candidate model set (Burnham and Anderson 2002). In other words, if the AIC(c) in a model is less than two units larger than in the best model, it was also discussed and reported. The same procedure (i.e., linear model) was used to examine whether the monthly proportion of dietary overlap (but logit transformed) between two primate species was affected by the monthly food availability of each plant part. Since logit transformation is not applicable to zero values, we did not perform an analysis on the food availability of flowers, including seven data points in which the proportion of the dietary overlap was zero. These analyses were performed in R ver. 3.1.0 (R Development Core Team 2014) using the dredge function in the MuMIn package, ver. 1.9.13 (Bartoń 2013).
Behavioral data collection is non-invasive, and our research was conducted following the protocols approved by the Department of Wildlife and National Park Malaysia. In addition, the protocols adhere to all the legal requirements of Malaysia.
Results
Vegetation characteristics and food availability
We marked 349 trees (119 species, 82 genera, 40 families) within our vegetation plots. The five most abundant families were Meliaceae (13.4% of all plants), Leguminosae (4.4%), Moraceae (3.7%), Myrtaceae (3.5%), and Euphorbiaceae (3.4%). The five most abundant tree species were Azadirachta excelsa (26.7% of all trees), S. koetjape (3.4%), Aidia densiflora (3.4%), Macaranga gigantea (2.3%), and Archidendron jiringa (2.3%). The vegetation survey area covered 0.16 ha, or approximately 0.45% and 1.14% of the study groups’ home ranges for M. fascicularis and T. o. obscurus, respectively. The cumulative number of plant species sampled did not reach an asymptote (Fig. 2), indicating that the edges of secondary forest are still growing and have high plant species diversity. Moreover, it was clear that the sampling effort would not be adequate to cover all the species.
Of the 349 trees marked within the plots, 238 (68.2%) and 167 (47.9%) were plant species that we observed M. fascicularis and T. o. obscurus to use as food sources, respectively. This included 211 (M. fascicularis) and 165 (T. o. obscurus) trees containing leaves (60.5 and 47.3%), 189 and 134 containing fruits/seeds (54.2 and 38.4%), and 150 and 131 containing flowers (43.0 and 37.5%), respectively. Food availability (potential food plants) for M. fascicularis and T. o. obscurus, determined as the leafing, fruiting, and flowering ratio (number of leafing, fruiting, and flowering plants/total number of monitored plants) in the monthly phenological survey fluctuated seasonally, with a similar pattern (Fig. 3a, b). The monthly availability of leaves, fruits, and flowers of M. fascicularis was significantly correlated with that of T. o. obscurus (leaves: rs = 0.97 and p < 0.01; fruits: rs = 0.98 and p < 0.01; flowers: rs = 0.98 and p < 0.01). Leaves were abundant throughout the year but with two peaks in July 2014 and March 2015. The availability of flowers was always lower than that of leaves and fruits, except in January and March 2015. The availability of fruits was generally higher from May to July 2014 and lower from January to February than in the remaining months.
Food habits
We collected a total of 5570 and 4029 feeding records for M. fascicularis and T. o. obscurus, respectively. The total number of plant species consumed within the feeding records was 113 (82 genera, 43 families; Appendix I) for M. fascicularis. The five most consumed species were Elaeis guineensis (11%), Ficus variegate (4%), Melastoma malabathricum (3.1%), Schizostachyum jaculans (3.1%), and Acacia mangium (2.7%). In comparison, a total of 130 plant species (85 genera, 43 families; Appendix II) were consumed by T. o. obscurus: the five most consumed species were Adenanthera pavovina (9.9%), Archidendron dulce (5.7%), Sandoricum koetjape (4.9%), F. variegate (4.7%), and Endospermum diadenum (4.4%). Both M. fascicularis and T. o. obscurus frequently consumed leaves, fruits/seeds, and flowers, although only M. fascicularis fed on anthropogenic foods and insects. Overall, for M. fascicularis, 31.9% of the feeding records were for fruits/seeds (27.8%/4.1%), 26.9% were for anthropogenic foods, 16.1% were for leaves (young leaf: 40.2%; mature leaf: 12.6%; both young and mature leaf: 47.1), 15.3% were for insects, 5.5% were for flowers, and 4.3% were for other food items, including red soil and fungi (Table 1). The number of plant species providing leaves, fruits, and flowers were 48, 57, and 32, respectively. Meanwhile, for T. o. obscurus, 51.2% of the feeding records were for leaves (young leaf: 36.1%; mature leaf: 4.7%; both young and mature leaf: 56.1; unknown: 3.1), 40.1% were for fruits/seeds (34.6%/5.5%), 7.8% were for flowers, and < 1% were for other food items, including red soil and unknown food items (Table 1). The number of plant species providing leaves, fruits, and flowers were 104, 45, and 34, respectively.
We collected a total of 1,184 and 856 fruit eating records of M. fascicularis and T. o. obscurus, respectively. The exploitation of ripe fruits, both ripe and unripe fruits, and unripe fruits accounted for 90.8, 5.4, and 3.8% of the fruit-eating records of M. fascicularis, respectively. M. fascicularis consumed only fruit flesh during 60.7% of fruit eating while consuming both fruit flesh and seeds during 39.3% and only seeds during 0% (see Table 2 in details). On the other hand, the exploitation of ripe fruits, both ripe and unripe fruits, unripe fruits, and unknown accounted for 67.5, 18.0, 13.7, and 0.8% in T. o. obscurus, respectively. T. o. obscurus consumed only fruit flesh during 41.08% of fruit eating while consuming both fruit flesh and seeds during 42.3% and only seeds during 15.8% (see Table 2 in details).
Comparison between two primate species throughout the year
T. o. obscurus generally spent more time consuming fruits/seeds, leaves, and flowers than did M. fascicularis, though M. fascicularis spent much more time feeding on insect and anthropogenic foods. The mean time spent feeding on leaves differed significantly between the two species (Z = 7.00, p < 0.001), although there was no significant differences in terms of eating flowers (Z = 0.72, p = 0.468); it should be noted that the difference in the time spent feeding on fruits/seeds tended towards significance (Z = 1.96, p = 0.050). Throughout the year, the number of shared items and the absolute percentage dietary overlap between T. o. obscurus and M. fascicularis were 59 (45 genera, 32 families) and 24.3%, respectively. Overall, the dietary overlap was the highest for fruits. The mean monthly dietary overlap index for consumed fruits (mean, 16.9%, SD, 10.08) was significantly higher than that for leaves (mean, 8.30%, SD, 4.58) and flowers (mean 1.05, SD 2.26), and that for leaves was significantly higher than that for flowers: fruits vs. leaves: Z = 2.73 and p < 0.01; fruits vs. flowers: Z = 4.04 and p < 0.001; leaves vs. flowers: Z = 3.70 and p < 0.001.
Monthly variation in diet
We found that flower eating by both M. fascicularis and T. o. obscurus was constantly low throughout the study period (Fig. 4). On the other hand, leaf and fruit eating was almost always higher in T. o. obscurus than in M. fascicularis, and insect and anthropogenic food was only consumed by M. fascicularis. The best-fit model predicting fruit eating by M. fascicularis showed only a positive effect of fruit availability (y = − 0.6507 + 1.319x); the ΔAICc score of the second model was > 2. The best-fit models predicting leaf and flower eating by M. fascicularis were both null models. Similar to M. fascicularis, the best-fit model predicting fruit eating by T. o. obscurus included only a positive effect of fruit availability (y = − 0.5706 + 1.656x); it should be noted that the second model was a null model. Indeed, the best-fitting models predicting leaf eating and flower eating by T. o. obscurus were both null models.
Comparison between two primate species
The amount of monthly overlap for three plant parts (i.e., fruit, flowers, and leaves) fluctuated seasonally. The dietary overlap for leaves and flowers was small and showed a relatively even distribution across the seasons, although that for fruits was unevenly distributed, with clear peaks in June/July 2014, (63.4 and 41.4%, respectively; Fig. 5). In June and July, when the dietary overlap of fruits was extremely high, both primate species consumed the cultivated fruits Nephelium lappaceum and Sandoricum koetjape except Ochanostachys amentacea. The best-fit model predicting the amount of monthly dietary overlap for the consumed fruits included only a positive effect of fruit availability (y = − 0.7541 + 1.295x); the second model was a null model (Fig. 6). Conversely, the best-fitting model to explain the dietary overlap of leaves, which was evaluated using the AIC(c) criterion, included fruit and flower availabilities (Table 3), although the ΔAIC(c) values of some other models were also < 2.0. The dietary overlap of leaves increased with decreasing fruit and flower availabilities (Fig. 6).
Discussion
This study described the feeding ecologies of T. o. obscurus and M. fascicularis inhabiting a forest edge adjacent to an urban area. Our findings on the dietary composition of T. o. obscurus are similar to those found in a lowland evergreen dipterocarp forest, Kuala Lompat, Peninsular Malaysia—leaves: 58%, fruits: 32%, flowers: 7%, and others: 3% (Curtin 1980)—although our T. o. obscurus study group consumed more plant species (130 vs. 87) than reported by Curtin (1980). However, it would be impossible to compare the number of consumed plant species directly between the two studies because the sampling methods differed.
On the other hand, our M. fascicularis study group showed a typical dietary composition, as reported in Singapore (Sha and Hanya 2013a) and Bali, Indonesia (Brotcorne 2014), where the animals live on the forest edge with an adjacent urban area; fruits, anthropogenic foods/food waste, and insects were the most important food sources. In this study, M. fascicularis fed on 17 of the same genus of plant species reported by Sha and Hanya (2013b), with > 70% of species (25 out of 36 species) recorded available or consumed by M. fascicularis. Distinctive diet choices by M. fascicularis in forest edges are evident, as some fruits are mostly derived from cultivated and herbaceous plants. The sympatric T. o. obscurus did not consume the anthropogenic food sources, although they also consumed leaves and seeds derived from urban/cultivated plants, suggesting different nutritional benefits for hindgut- and foregut-fermenting primates. Higher sugar and carbohydrate contents in the anthropogenic foods (e.g., rice, bread, and biscuits) present as an energy bonus (Strum 2010), as human foods were higher in calories and lower in fiber than natural foods (Forthman-Quick and Demment 1988). However, it is difficult to determine whether the exploitation of anthropogenic foods by M. fascicularis in our study site is due to the natural food resource scarcity or a high dependence on anthropogenic foods, as we did not survey the availability of anthropogenic foods in our study site. Further efforts should be made into elucidating the effect of anthropogenic foods on the diet of M. fascicularis.
In this study, we showed that both M. fascicularis and T. o. obscurus consumed relatively high amounts of fruits/seeds; notably, foregut-fermenting T. o. obscurus, which is considered a folivorous animal (Curtin 1980), tended to spend more time eating fruit compared to the hindgut-fermenting M. fascicularis, which is considered to be omnivorous/frugivorous (Lucas and Corlett 1991). Contrary to earlier assumptions that foregut-fermenting primates (i.e., colobines) mainly exploit ubiquitous food sources such as leaves, recent colobine studies have revealed high levels of fruit and/or seed consumption in response to local conditions, for example, T. o. obscurus: 40.1% (this study); P. potenziani: 55.4% for fruit eating (Hadi et al. 2012); Colobus guereza: 33–44% (Fashing 2001); P. rubicunda: 30–84% (Davies 1991; Hanya and Bernard 2012; Ehlers Smith et al. 2013); T. vetulus: 53.7–60.1% (Dela 2007); and Semnopithecus entellus: 23–29.1% (Vandercone et al. 2012). Notably, in our study, over 65% of fruit eating involved the consumption of ripe fruits, and in the majority of cases, the fruit flesh containing more sugar was likely eaten (Table 2). It is believed that colobines avoid sugary food sources (i.e., ripe fruits), which can result in a decrease in fermentation efficiency or cause acidosis, and in extreme cases can even result in death (Collins and Roberts 1978; Kay and Davies 1994). However, not only our study species but also other colobines, for example, T. auratus (Kool 1993), Presbytis entellus (Koenig et al. 1998), Semnopithecus vetulus (Dela 2012), and Piliocolobus tephrosceles (Danish et al. 2006), reportedly include ripe fruits as an important food source. Nonetheless, the detailed digestive mechanism by which these colobines can deal with such sugary sources in their forestomaches and avoid harmful fermentation is poorly known (Danish et al. 2006). In future studies, feeding trials and/or digestibility measured by an in vitro gas production method using ripe fruits, as performed using leaves by Matsuda et al. (2017), together with the investigation of forestomach microbiome analyses (e.g., Amato et al. 2016; Hayakawa et al. 2018) would be helpful in explaining colobines’ digestive mechanism for such sugary items.
Although leaves were always more abundant than fruits in our study site, the amount of monthly fruit eating by M. fascicularis and T. o. obscurus only showed a significant correlation with fruit availability, indicating that both species prefer feeding on fruits when they are available. In this study, the percentage of time spent on leaf eating did not show a clear pattern in relation to leaf availability, supporting the strong fruit preference described in other colobine studies, for example, T. auratus by Kool (1993), Nasalis larvatus by Matsuda et al. (2009), and P. rubicunda by Hanya and Bernard (2012, 2015). Thus, although the digestive systems of M. fascicularis and T. o. obscurus are different, fruits would be their key food source.
Monthly dietary overlap between M. fascicularis and T. o. obscurus in our study showed a positive effect of monthly fruit availability. In addition, contrary to previous studies showing that colobines prefer unripe fruits while macaques prefer ripe fruits, T. o. obscurus consumed a relatively high amount of ripe fruits in this study. In theory, this suggests that their fruit preference mainly leads to contest (or direct) competition for resources rather than scramble (indirect) competition, especially when fruits are abundant and cultivated fruits are available. The dietary overlap with the resource competition between the two species in this study might be more the result of cultivated plants than of natural forest species, suggesting that resource competition becomes higher and niche separation becomes ambiguous between foregut- and hindgut-fermenting primates living in forest edge near adjacent urban areas.
On the other hand, there would also be a possibility that their resource competition is low, as we did not observe the two species to feed on fruits on the same tree, nor did we observe clear conflict or aggressive behaviors between the two species (F.R., pers. obs.), although we did not do alternative evaluation, the degree of food competition between the two sympatric species in our study site. Although the fruits were preferred by the two species in our study site, to avoid food competition, T. o. obscurus would flexibly shift to leaves due to their special digestive system while M. fascicularis could shift to anthropogenic foods, which T. o. obscurus would not be able to consume.
We succeeded in clarifying the detailed feeding ecologies of two sympatric primates, showing their similar dietary preference despite their different physiological systems, although they appeared to avoid or reduce interspecific competition for essential resources. However, to understand the mechanism of coexistence of the two sympatric primates in our study site, further research focusing on the detailed resource competition level to quantify their interspecific competition and niche separation including their ranging patterns is needed.
References
Aldrich-Blake FPG (1980) Long-tailed macaques. In: Chivers DJ (ed) Malayan forest primates. Springer, New York, pp 147–166
Amato KR, Metcalf JL, Song SJ, Hale VL, Clayton J, Ackermann G, Humphrey G, Niu K, Cui D, Zhao H, Schrenzel MD, Tan CL, Knight R, Braun J (2016) Using the gut microbiota as a novel tool for examining colobine primate GI health. Global Ecology and Conservation 7:225–237
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–266
Bartoń K (2013) MuMIn: Multi-model inference. R package version 1.9.0. http://CRAN.R-project.org/package
Brotcorne F (2014) Behavioral ecology of commensal long-tailed macaque (Macaca fascicularis) populations in Bali, Indonesia. PhD Dissertation, University of Liege, Liege
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Chivers DJ (1994) Functional anatomy of the gastrointestinal tract. In: Davies A, Oates J (eds) Colobine monkeys: their ecology, behaviour and evolution. Cambridge University Press, Cambridge, pp 205–227
Collins L, Roberts M (1978) Arboreal folivores in captivity-maintenance of a delicate minority. In: Montgomery GG (ed) The ecology of arboreal folivores. Smithsonian Institution Press, Washington, pp 5–12
Curtin SH (1980) Dusky and banded leaf monkeys. In: Chivers DJ (ed) Malayan forest primates. Springer, Boston, pp 107–146
Danish L, Chapman CA, Hall MB, Rode KD, O’Driscoll Worman C (2006) The role of sugar in diet selection in redtail and red colobus monkeys. In: Hohmann G, Robbins MM, Boesch C (eds) Feeding ecology in apes and other primates. Cambridge University Press, Cambridge, pp 473–487
Davies G (1991) Seed-eating by red leaf monkeys (Presbytis rubicunda) in dipterocarp forest of northern Borneo. Int J Primatol 12:119–144
Davies G, Oates J (1994) Colobine monkeys: their ecology, Behaviour and Evolution. Cambridge University Press, Cambridge
Dela JDS (2007) Seasonal food use strategies of Semnopithecus vetulus nestor, at Panadura and Piliyandala, Sri Lanka. Int J Primatol 28:607–626
Dela JDS (2012) Western purple-faced langurs (Semnopithecus vetulus nestor) feed on ripe and ripening fruits in human-modified environments in Sri Lanka. Int J Primatol 33:40–72
Ehlers Smith DA, Husson SJ, Ehlers Smith YC, Harrison ME (2013) Feeding ecology of red langurs in Sabangau tropical peat-swamp forest, Indonesian Borneo: extreme granivory in a non-masting forest. Am J Primatol 75:848–859
Fashing PJ (2001) Feeding ecology of guerezas in the Kakamega Forest, Kenya: the importance of Moraceae fruit in their diet. Int J Primatol 22:579–609
Fleagle JG, Janson CH, Reed KE (1999) Primate communities. Cambridge University Press, Cambridge
Forthman-Quick DL, Demment M (1988) Dynamics of exploitation: differential energetic adaptations of two troops of baboons to recent human contact. In: Fa JE, Southwick CH (eds) Ecology and behaviour of food enhanced primate groups. AR Liss, New York, pp 25–51
Gumert M, Jones-Engel L, Fuentes A (2011) The common monkey of Southeast Asia: long-tailed macaque populations, ethnophoresy, and their occurrence in human environments. In: Fuentes A, Gumert M, Jones-Engel L (eds) Monkeys on the edge: ecology and management of long-tailed Macaques and their interface with humans. Cambridge University Press, New York, pp 3–44
Hadi S, Ziegler T, Waltert M, Syamsuri F, Mühlenberg M, Hodges JK (2012) Habitat use and trophic niche overlap of two sympatric colobines, Presbytis potenziani and Simias concolor, on Siberut Island, Indonesia. Int J Primatol 33:218–232
Hanya G, Bernard H (2012) Fallback foods of Red Leaf Monkeys (Presbytis rubicunda) in Danum Valley, Borneo. Int J Primatol 33:322–337
Hanya G, Bernard H (2015) Different roles of seeds and young leaves in the diet of Red Leaf Monkeys (Presbytis rubicunda): comparisons of availability, nutritional properties, and associated feeding behavior. Int J Primatol 36:177–193
Hayakawa T, Nathan S, Stark DJ, Saldivar DAR, Sipangkui R, Goossens B, Tuuga A, Clauss M, Sawada A, Fukuda S, Imai H, Matsuda I (2018) First report of foregut microbial community in proboscis monkeys: are diverse forests a reservoir for diverse microbiomes? Environ Microbiol Rep. https://doi.org/10.1111/1758-2229.12677
Holmes RT, Pitelka FA (1968) Food overlap among coexisting sandpipers on northern Alaskan tundra. Syst Zool 17:305–318
Idilfitri S, Mohamad NHN (2012) Role of ornamental vegetation for birds’ habitats in urban parks: case study FRIM, Malaysia. Procedia Soc Behav Sci 68:894–909
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108
Kay RNB, Davies AG (1994) Digestive physiology. In: Davies G, Oates J (eds) Colobine monkeys: their ecology, behaviour and evolution. Cambridge University Press, Cambridge, pp 229–249
Kirkpatrick RC (1999) Colobine diet and social organization. In: Dolhinow P, Fuentes A (eds) The non-human primates. Mayfield, Palo Alto, pp 93–105
Koenig A, Borries C (2001) Socioecology of Hanuman langurs: the story of their success. Evol Anth 10:122–137
Koenig A, Beise J, Chalise MK, Ganzhorn JU (1998) When females should contest for food-testing hypotheses about resource density, distribution, size, and quality with Hanuman langurs (Presbytis entellus). Behav Ecol Sociobiol 42:225–237
Kool KM (1993) The diet and feeding behavior of the silver leaf monkey (Trachypithecus auratus sondaicus) in Indonesia. Int J Primatol 14:667–700
Lucas PW, Corlett RT (1991) Relationship between the diet of Macaca fascicularis and forest phenology. Folia Primatol 57:201–215
MacKinnon JR, MacKinnon KS (1980) Niche differentiation in a primate community. In: Chivers DJ (ed) Malayan forest primates. Springer, New York, pp 167–190
Mat Salleh K (1999) The role and function of Universiti Kebangsaan Malaysia permanent forest reserve in research and education. Pertanika J Trop Agric Sci 22:185–198
Matsuda I, Tuuga A, Higashi S (2009) The feeding ecology and activity budget of proboscis monkeys. Am J Primatol 492:478–492
Matsuda I, Otani Y, Bernard H, Wong A, Tuuga A (2016) Primate survey in a Bornean flooded forest: evaluation of best approach and best timing. Mammal Study 41:101–106
Matsuda I, Clauss M, Tuuga A, Sugau J, Hanya G, Yumoto T, Bernard H, Hummel J (2017) Factors affecting leaf selection by foregut-fermenting proboscis monkeys: new insight from in vitro digestibility and toughness of leaves. Sci Rep 7:42774
Md-Zain BM, Abdullah M (2008) Primat Bukit Belata. In: Muda A, Koh HL, Mustafa NM, Mustafa SN, Nawi SA, Latiff A (eds) Bukit Belata, Selangor: Pengurusan Persekitaran Fizikal, Kepelbagaian Biologi dan Sosio-ekonomi. Jabatan Perhutanan Semenanjung Malaysia, Kuala Lumpur, pp 270–273
Md-Zain BM, Ch’ng CE (2011) The activity patterns of a group of Cantor’s dusky leaf monkeys (Trachypithecus obscurus halonifer). Int J Zool Res 7:59–67
Md-Zain BM, Shaari NA, Mohd-Zaki M, Ruslin F, Idris NI, Kadderi MD, Idris WMR (2010) A comprehensive population survey and daily activity budget on long tailed macaques of Universiti Kebangsaan Malaysia. J Biol Sci 10(7):608–615
Md-Zain BM, Tarmizi MR, Zaki MM (2011) Campus monkeys of Universiti Kebangsaan Malaysia: nuisance problems and students’ perception. In: Fuentes A, Gumert M, Jones-Engel L (eds) Monkeys on the edge: ecology and management of long-tailed macaques and their interface with humans. Cambridge University Press, New York, pp 101–119
Reed KE, Bidner LR (2004) Primate communities: past, present, and possible future. Yearb Phys Anthropol 47:2–39
Sha JCM, Hanya G (2013a) Temporal food resource correlates to the behavior and ecology of food-enhanced long-tailed macaques (Macaca fascicularis). Mamm Study 38:163–175
Sha JCM, Hanya G (2013b) Diet, activity, habitat use, and ranging of two neighboring groups of food-enhanced long-tailed macaques (Macaca fascicularis). Am J Primatol 75:581–592
Singh M, Roy K, Singh M (2011) Resource partitioning in sympatric langurs and macaques in tropical rainforests of the central Western Ghats, south India. Am J Primatol 73:335–346
Strum SC (2010) The development of primate raiding: implications for management and conservation. Int J Primatol 31:133–156
Ungar PS (1995) Fruit preferences of four sympatric primate species at Ketambe, northern Sumatra, Indonesia. Int J Primatol 16:221–245
Vandercone RP, Dinadh C, Wijethunga G, Ranawana K, Rasmussen DT (2012) Dietary diversity and food selection in Hanuman langurs (Semnopithecus entellus) and purple-faced langurs (Trachypithecus vetulus) in the Kaludiyapokuna forest reserve in the dry zone of Sri Lanka. Int J Primatol 33:1382–1405
Acknowledgements
The authors acknowledge Universiti Kebangsaan Malaysia and Department of Wildlife and National Parks, Malaysia Peninsula, for providing necessary funding, facilities, and assistance. The research was conducted under research permit approved by Department of Wildlife and National Parks, Malaysia Peninsula (JPHL&TN(IP):100-34/1.24 Jld 9 (40). We thank Ahmad Fitri Zohari for plant species identification. This research was supported by Grants AP-2015-004, FRGS/1/2012/STWN10/UKM/02/3, TD-2014-022, and DLP-2013-006. This study was also partly financed by Grant-in-Aid for Young Scientists (A) (26711027 to IM), JSPS Core-to-Core Program, Advanced Research Networks (to S. Koshima), and the Cooperative Research Program by KUPRI (2016-B-98 to FR). Finally, we thank Goro Hanya and John C.M. Sha for their helpful suggestions and comments on an earlier draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10329_2018_705_MOESM1_ESM.docx
Appendix I: Food items and parts of each item consumed by M. fascicularis from April 2014 to March 2015. Appendix II: Food items and parts of each item consumed by T. o. obscurus from April 2014 to March 2015
About this article
Cite this article
Ruslin, F., Matsuda, I. & Md-Zain, B.M. The feeding ecology and dietary overlap in two sympatric primate species, the long-tailed macaque (Macaca fascicularis) and dusky langur (Trachypithecus obscurus obscurus), in Malaysia. Primates 60, 41–50 (2019). https://doi.org/10.1007/s10329-018-00705-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-018-00705-w