Abstract
Macaques are characterized by their wide distribution and ability to adapt to a variety of habitats. Activity budgets are affected by habitat type, season, and food availability in relation to differing age–sex class and individual requirements. We conducted a comparative study on two commensal rhesus groups, one living in a rural village and the other in the center of urban Dhaka, Bangladesh. The study was conducted in three different seasons between 2007 and 2009 in order to evaluate how habitat type and season affects their behavioral activities. Differences in food type and its availability between these two habitats were mainly responsible for the variations in activity budgets between groups. Feeding time in the rural group was significantly longer than that in the urban group. In contrast, grooming and object manipulation/play were significantly greater in the urban than the rural group. Seasonal variations in all major behaviors were significantly affected by group, with more time spent feeding in summer than in winter/dry season, and more time spent grooming and moving in winter/dry season than summer in the rural group. In contrast, time spent resting was greater in the monsoon and summer seasons than the winter/dry season in the urban group. Grooming time was greater in the winter/dry season than the monsoon and summer seasons. In both groups, immature of both sexes spent significantly more time on feeding and object manipulation/playing and less time resting than adults. Adult females spent more time grooming than males and immatures, of both sexes, in both groups. Moreover, the rural group spent most of their time feeding on garden/crop produce and wild plant food resources, while the urban group spent more time feeding on provisioned foods. These results showed that differences in the activity budgets of rural and urban dwelling macaques were due largely to the differences in available food resources. Commensal rhesus macaques show a high degree of behavioral flexibility in response to habitat and resource variability, and knowledge of these differences is important for the conservation and management of highly commensal primates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behavioral activity is influenced by environmental constraints such as food resources and their seasonal availability, plant species diversity, and physiological constraints such as individual food requirements and thermoregulation (Agetsuma and Nakagawa 1998; Bean 1999; El Alami et al. 2012; Hanya 2004; Jaman and Huffman 2008). These constraints are all determined by broad climatic and geographical variables (Lindburg 1971; Goldstein and Richard 1989) that lead species to adapt to a specific habitat or geographical location, maximizing their opportunity for survival over evolutionary time. Due to the species’ high behavioral flexibility (particularly for feeding and resting behavior), which is related to metabolism and energy conservation, rhesus macaques are able to inhabit a wide range of geographical locations with differing climatic and ecological environments (Goldstein and Richard 1989; Richard et al. 1989; Dunbar 1992; Seth et al. 2001).
Activity budgets can be affected by season, as seasonal differences are prominent in terms of temperature, rainfall, plant growth, and fruiting seasons according to habitat type (Dunbar 1992; Agetsuma and Nakagawa 1998). Different age–sex classes respond differently to their environment in ways such as acquiring access to limited food resources and exploiting new food resources (Marriott, 1988; Jaman and Huffman 2008). Thus, age–sex differences in behavioral patterns should also be habitat specific (Mitchell 1979). The degree of difference in body mass between adults and immatures strongly influences resting and foraging (Bean 1999; Jaman and Huffman 2010). These differences are functions of energetic (Bean 1999) and social constraints. While activity budgets of rhesus macaques have been studied in different ecological settings (Altmann 1962; Bernstein and Mason 1963; Southwick et al. 1982; Shukla 1979; Marriott 1988; Goldstein and Richard 1989; Seth et al. 1989; Akter 2002) and some studies have compared activity budgets in primates across season and age–sex class within groups (Maruhashi 1981; Ciani and Chiarelli 1988; Nakayama et al. 1999; Saj et al. 1999; Agetsuma 2001; Hanya 2003, 2004; Jaman and Huffman 2008; Kamilar and Pokempner 2008), no studies have examined the effect of multiple factors, such as group, season, and age–sex class together on activity patterns in the rhesus macaque.
The main objective of the study described in this paper was to better understand the adaptability of commensal rhesus macaques in Bangladesh, for which there is currently little information. Rhesus macaques, like many other macaques and baboons, are highly commensal, and this close relationship with humans is gaining increasing attention (e.g., Kamal et al. 1997; Paterson and Wallis 2005; Hill and Webber 2010; Radhakrishna et al. 2012). However, to the best of our knowledge, comparative studies of rhesus macaques living under extreme commensal habitats are few (e.g., Lata 1980; Oppenheimer et al. 1983; Pirta 1984; Agetsuma and Nakagawa 1998; Wolf 2002). To improve our understanding of how commensalism affects the activity budgets of rhesus macaques in Bangladesh, we selected two groups living under contrasting forms of commensalism (urban versus rural) for our study, taking into account differences in habitat type, season, individual age–sex class behavioral budgets, and feeding behaviors. The urban group lives in the middle of Dhaka city and receives provisioned foods, which they supplement with a few wild and garden plants obtained from a public park nearby. They seek shelter inside factory buildings, cause little damage to human property, and do not compete for local human resources. Their presence is viewed favorably (Jaman and Huffman, in prep.). In contrast, the rural group, located nearby Borme village, does not receive provisioned foods, and subsists on natural vegetation as well as crops and food items stolen from houses and traditional outdoor shops. These monkeys damage human resources to acquire their foods and conflict with humans is high, so most people consider them to be pests (Jaman and Huffman, in prep.).
Behavioral activity budget studies of commensal macaque groups are important to understand how a species copes under human-induced environmental pressures. Such studies should be beneficial for conservation and management efforts, as they facilitate our understanding of primate adaptations within human-modified environments (Teas et al. 1975; Oppenheimer et al. 1983; Saj et al. 1999).
Here we assess rhesus macaque behavioral flexibility in response to commensalism. We test whether activity budgets are influenced by various food resources, habitat type, season, and age–sex class differences. Specifically, we make three predictions. (1) We predict that the rural group will spend more time feeding on a diverse variety of indigenous plants and risky-to-obtain planted garden food resources, while the urban group will spend less time feeding, consuming large quantities of an energy-rich, risk-free provisioned food resource. (2) We predict that the rural group will spend more time feeding and less time resting and grooming during seasons when the diversity of natural food is higher. In contrast, we predict that he urban group will spend more time resting, but no seasonal variation in the feeding time budget is expected, due to their strong dependence on provisioned foods year-round. (3) We predict that age–sex class will affect both feeding and social activity patterns. We suggest that feeding activities will be influenced by differences in metabolic requirements, and predict that immature will spend more time engaged in feeding and object manipulation/play than adults, while adults will spend more time resting and grooming than immatures, particularly in the rural group, since they are not provisioned.
We then compare our results with studies on rhesus and other macaque species performed in other geographical regions to better understand the behavioral flexibility of this species and the possible impact of commensalism on their future survival.
Methods
Study subjects and locations
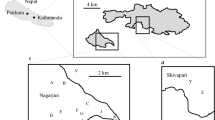
This research was conducted in accordance with all national and institutional guidelines for the care and management of primate species under the Wildlife Protection Law 1973, Government of Bangladesh and the Primate Research Institute’s guidelines for field research of non-human primates (http://www.pri.kyoto-u.ac.jp/research/guide-e2008.html). We collected data from two free-ranging rhesus groups in Bangladesh, one living in association with a rural village and the other living in the urban center of the capital city Dhaka.
The rural Borme group
The rural group lives in a habitat surrounding Borme village in the Gazipur District, about 70 km north of Dhaka, the capital city. This group consisted of 47 individuals at the start of the study and had increased to 57 by the end of the study (Table 1). This group subsists largely on natural vegetation (grasses, bushes, and more than 64 species of trees) and food items obtained by raiding crops of agricultural vegetables (pea Pisum sativum, string bean Vigna sesquipedalis, tomato Lycopersicon esculentum, drumstick Moringa oleifera, brinjal Solanum melongena, radish Raphanus sativus), home gardens (berry Zizyphus mauritiana, betelnut palm Areca catechu, black berry Syzygium cumini, guava Psidium guajava, date Phoenix sylvestris, jackfruit Artocarpus heterophyllus, banana Musa, etc.), and prepared food items (cooked rice, cooked vegetables, etc.) removed from houses and shops in the traditional market. A rough estimation of each group’s home range size was made using GPS points recorded during daily observations of the group. The approximate home range area was estimated each study period using the furthest points that the group traveled to in all directions. During this study, the home range of the rural group ranged between 31.2 and 42.9 ha.
The urban Dhaka group
This group lives in an urban habitat in the Gendaria–Shutrapur area in and around the property of Sadhona Traditional Pharmaceutical Co. in Dhaka. This urban group consisted of 81 individuals at the start of the study, and had increased to 94 individuals by the end of the study (Table 1). Monkeys in this group subsist mainly on provisioned foods (mainly chick peas, bread, biscuits, crackers) supplemented by some wild and garden plants, mainly grasses, and 13 species of trees (berry Zizyphus mauritiana, betel nut palm Areca catechu, blackberry Syzygium cumini, guava Psidium guajava, jackfruit Artocarpus heterophyllus, mango Mangifera indica, green coconut Cocos nucifera, etc.) from a small park next to the grounds of the property. A total of 400 medium-sized bananas were provided daily by the government authorities. The Sadhona authorities provided 10 kg in total of chick peas daily: 5 kg in the morning (0800–0900 hours) and 5 kg in the afternoon (1500–1600 hours). Bread, biscuits, crackers, and other items were also given to the monkeys by visitors. During the study, the urban group’s estimated home range ranged from 18.1 to 29.2 ha.
Climate at the study sites
Bangladesh has three seasons a year: July to October—monsoon; November to February—winter/dry season; and March to June—summer season (Ahmed and Elias 1988; Mourshed 2011). The main characteristic seasonal difference is heavy rain in the monsoon and little rain in summer except for June. Temperature and rainfall data were obtained from the Meteorology Department, Agargaon, Dhaka, Bangladesh. They automatically recorded the temperature every 3 h throughout the year at locations near our study sites. From these data, the temperatures recorded from 0600 to 1800 hours were retrieved and used. The daily mean temperature at the rural study site was 27.6 °C ± 2.8 in the monsoon season, 21.0 °C ± 4.7 in the winter/dry season, and 29.7 °C ± 4.2 in the summer season. At the urban study site, the mean temperature was 27.8 °C ± 2.7 in the monsoon season, 22.9 °C ± 4.3 in the winter/dry season, and 30.1 °C ± 3.6 in the summer season. The monthly total rainfall at the rural site was 1405.5 mm in the monsoon season and 707.5 mm in the summer season. At the urban site, the monthly total rainfall was 1678 mm in the monsoon season and 952.5 mm in the summer season. No measurable rainfall was recorded in the winter/dry season at either site.
Behavioral observation
Data was collected in three different periods between September 2007 and January 2009. Period I was in September–October 2007 (monsoon), period II in May–June 2008 (summer), and period III in December 2008–January 2009 (winter/dry season). The core behavioral data were collected by M.F. Jaman, using focal animal sampling by continuous recording methods (Altmann 1974; Martin and Bateson 1993), and each focal session lasted 10 min. After careful training in coding methods and an initial monitoring period, an assistant observer also collected focal data (5.7 %, 384 focal sessions) during study periods I and III. To justify whether combining these data was appropriate in the overall analysis, we compared all statistical tests before and after the addition of this supplemental data. There were no differences in the level of significance of any of the results, so we combined the data for both observers to maintain a balanced number of focal samples, observation hours per individual/season and time of day.
A total of 552 h of observations (3312 focal samples) were performed for each group (two groups), distributed equally across each focal subject (12 focal subjects from each group); four age–sex classes over three seasons and across times of day (four time blocks). At the end of our observations, a total of 1104 h of behavioral data were obtained from a total of 6624 focal sessions. Prior to this, one and a half months (February–March 2007) were spent habituating the monkeys of both groups to our presence. All group members (except for juveniles and infants) were identified using individually distinct morphological characteristics recognized during the habituation period.
We classified all focal subjects into one of two age classes: immature (male age: three years; female age: three years) and adult (male: >5 years and female: >5 years). All observers estimated individual age based on body size compared to individuals studied in the enclosures at Dhaka Zoo, the relative ages of which were known. Three focal subjects were selected from each of the four age–sex classes for both groups, making a combined total of 24 individuals for the two study groups. Adult focal subjects were chosen from both groups based on similarity of age. All focal subjects were given a name to identify them during focal sampling. Before starting data collection in each period, we spent two days observing each group from dawn to dusk to confirm the identities of all focal subjects by their previously given names.
Observation periods were divided into four different time blocks; early morning (EM 0700–0930 hours), late morning (LM 0930–1200 hours), early afternoon (EA 1200–1430 hours), and late afternoon (LA 1430–1700 hours). An equal number of samples were collected in each time block of the day for each focal subject. We attempted to sample some focal subjects the next day when we could not collect equal amounts of data from them that day. We also compiled equal amounts of observation hours for each focal animal in each group and in each study period to facilitate unbiased group-wise, seasonal, and age–sex class comparisons of behavioral data.
We used the same ethogram as that employed in our previous research on this topic in Japanese macaques (see Jaman and Huffman 2008). The behavioral categories are resting, feeding, grooming, moving, object manipulation/play, vigilance, and dominance interactions. Feeding includes foraging for and the actual ingestion of food. Feeding records were broken down into time spent on each food item and the locations of these food resources (i.e., gardens/crop lands, wild plants, provisioned foods, houses/shops). Animal matter and soil ingestion were also recorded. Two closely related behaviors—aggression and submission—are combined into “dominance interactions.” “Aggression” includes any aggressive physical contact, gesture, or vocalization (supplanting, grabbing, hitting, chasing, biting, stare threat, head bobbing, threat bark, etc.) that is typically directed toward a subordinate individual. “Submission” includes any submissive behavior, gesture, or vocalization (retreat, avoidance of eye contact, crouching, grimace, cry, etc.) in response to aggressive behaviors received from a more dominant individual.
Data analysis
For statistical analysis, we used data on time spent for each behavioral category by group (two groups), season (three seasons), age–sex class (adult male, adult female, immature male, and immature female), and focal subject (12 focal subjects in each group). The data failed tests of normality and equal variance (i.e., Kolmogorov–Smirnov, p < 0.05), and thus all tests were done using square-root n + 0.5 transformed data (Sokal and Rohlf 1969). We analyzed the duration (time in seconds) of all behavioral activities with multivariate analysis of variance (MANOVA) using group, season, and age–sex class (see above for the number in each category), and their interactions as explanatory variables. We employed univariate analysis of variance using a general linear model (GLM) to compare time spent for each of the five most frequent behavioral categories (>1 % of time spent)—resting, feeding, grooming, moving, and object manipulation/play—by group, season, and age–sex class and their interactions. Post hoc pair-wise contrasts were also employed using the univariate analysis of variance test to compare time spent for each behavioral activity across season and between age–sex class in each group. Finally, we employed the paired-sample t test to compare the average time spent feeding on each food resource between groups (n = 12 focal subjects). The significance of all statistical tests was set at P ≤ 0.05, and all tests were done using SPSS (version 13.0).
Results
Overall behavioral differences between groups
The general linear model multivariate analysis of variance test revealed that the overall time spent on seven behavioral activities significantly varied, regardless of season and age–sex class (F 6,336 = 4215.8, P < 0.0001). There was a significant interaction between time spent on behavioral activities overall and group (F 6,336 = 91.35, P < 0.0001; Table 2; Fig. 1). The univariate analysis of variance test for each behavioral activity revealed that only the time spent feeding was significantly higher in the rural group than in the urban group (F 1,48 = 283.3, P < 0.0001; Table 3). In contrast, time spent grooming and on object manipulation/play was significantly higher in the urban group than in the rural group (F 1,48 = 48.7, P < 0.0001 and F 1,48 = 7.2, P = 0.01, respectively; Table 3; Fig. 1). Although time spent in dominance interactions was negligible, the urban group spent fivefold more time on such interactions than the rural group. These results indicate that the time spent in different behavioral activities varied between groups in relation to habitat type, and thus support prediction 1.
Next we focused on a comparison of the five most frequent behavioral activities: resting, feeding, grooming, moving, and object manipulation/play (total >1 % time allocated for each behavior) to assess whether habitat differences affect the overall behavioral activities of rhesus macaques living in these two different sites. The matrix showing the effects of group, season, and age–sex class on time spent in behavioral activities is presented in Table 2.
Effect of seasonality
Regardless of group, season significantly affected the time spent on all activities (F 12,336 = 37.2, P < 0.0001; Table 2). Season and group also significantly affected activities (F 12,336 = 23.3, P < 0.0001). Univariate analysis of variance revealed that the time spent on each behavioral activity varied significantly across season, regardless of group (Table 3). Post hoc pair-wise comparisons showed that monkeys in the rural group spent significantly more time feeding in summer than in winter/dry season (P < 0.05), more time grooming in winter/dry season than in summer, and more time moving in winter/dry season than during the monsoon (P < 0.05; Fig. 2). In the urban group, monkeys spent significantly more time resting in the monsoon and summer seasons than in winter/dry season (P < 0.05 and P < 0.0001, respectively). The time spent grooming was greater in the monsoon and winter/dry seasons than in summer (P < 0.01 and P < 0.05, respectively). Moving time was greater in the winter/dry season than in the monsoon and summer seasons (P < 0.01 and P < 0.01, respectively). Time spent on object manipulation/play was greater in the summer than in winter/dry season (P < 0.01; Fig. 2). These results show that behavioral activities varied seasonally between the groups, thus supporting prediction 2.
Effect of age–sex class
Time spent on all activities significantly varied across age–sex class, regardless of group and season (F 18,336 = 152.3, P < 0.0001). Age–sex class, group, and season significantly affected behavioral activities (F 36,336 = 2.7, P < 0.0001; Table 2). Regardless of the group, a univariate analysis of variance test revealed that time spent on each behavioral activity significantly varied across age–sex class (see Table 3). A post hoc pair wise comparison showed that in the rural group, adult males spent more time resting and less time feeding than adult females (P = 0.001 and P < 0.05) and immatures of both sexes (P < 0.0001, same value for all comparisons, respectively; Fig. 3). Immatures spent more time feeding than adult females (P < 0.0001, same value), thus supporting prediction 3. Adult females spent more time grooming and less time moving than adult males (P = 0.001 and P < 0.05, respectively), immature males (P < 0.0001, same value), and immature females (P < 0.0001 and P < 0.05, respectively). Immature of both sexes spent more time on object manipulation/playing than adults of both sexes (P < 0.0001, same value for all comparisons; Fig. 3). In contrast, a post hoc pair wise comparison for the urban group showed that immature of both sexes spent more time feeding and on object manipulation/playing than adults of both sexes (P < 0.0001, same value for all comparisons; Fig. 3). Adult males spent more time resting than immature males and immature females (P < 0.05 and P < 0.0001, respectively). Adult males also spent more time feeding and moving and less time grooming than adult females (P < 0.05, P < 0.0001, and P = 0.001, respectively). Adult females spent more time resting than immature females (P = 0.001) and more time grooming than immature males (P < 0.0001). Adult females also spent less time moving than immature males and immature females (P < 0.0001 and P = 0.001, respectively). Immature females spent more time grooming than immature males (P < 0.05). These results show that activity budgets varied across age–sex class, and that this is affected by the group’s habitat, thus supporting prediction 3.
Effect of food sources on time spent feeding
Monkeys in the rural group spent significantly more time feeding on foods derived from gardens/crop lands (39.7 %) followed by foods in houses/shops (17.1 %) and wild plants (17.1 %) (Table 4). Monkeys in the urban group, on the other hand, mostly consumed provisioned foods (69.1 %), followed by wild plants (21.7 %) and foods from gardens/crop lands (5.1 %) (Table 4).
Discussion
We analyzed and compared possible factors affecting the activity budgets of two rhesus macaque groups living in habitats (rural and urban groups) in Bangladesh with differing levels of commensalism in order to better understand the behavioral flexibility of this species. Time spent on behavioral activities (feeding, resting, grooming, moving, and object manipulation/playing) varied between groups, and we suggest that individuals modified their activity budgets in response to changing ecological factors in the habitat based on seasonal variation of food resources and a combination of age–sex class food requirements.
We found that the rural group spent a greater amount of time feeding (36.2 %) than the urban group (22.4 %), and also more time doing so than any other previously studied group (Teas et al. 1975; Marriott 1988; Malik and Southwick 1988; Goldstein and Richards 1989; Seth et al. 2001). This is supported by another study showing that a Nepalese forest rhesus group spent more time feeding than did the provisioned Cayo Santiago group (Marriott 1988). El Alami et al. (2012) also reported that Barbary macaques decreased their foraging time at the tourist site and spent significantly less time foraging and moving than the rural group, which was dependent on wild plant food resources. During our observations, we found that the rural group had greater access to gardens/crop lands and also utilized more naturally occurring vegetated areas, resulting in them spending more time feeding than did the urban group.
In this study, we found that the urban rhesus group spent a greater amount of time resting (46.1 %) than previously studied groups (Teas et al. 1975; Malik and Southwick 1988; Marriott 1988; Seth et al. 2001). We suggest that time spent resting and feeding in the urban group was influenced by the regular supply of provisioned foods, which provide more energy to meet metabolic demands in a smaller amount of food and in a shorter amount of time than do wild plant foods (Baulu and Redmond 1980; Brennan et al. 1985; Fa 1986; Saj et al. 1999). Furthermore, the urban group spent more time resting and grooming, which might be due to their limited access to natural foraging sites and/or due to becoming satiated much more quickly. After eating provisioned food, they frequently rested and engaged in social grooming. It has been suggested that social grooming functions to maintain cohesion and strengthen social bonds between individual kin groups (Lindburg 1973). The overall grooming patterns in this study are similar to groups studied in India, Nepal, and Cayo Santiago (Teas et al. 1975; Malik and Southwick 1988; Marriott 1988; Seth et al. 2001); however, the urban group of this study and the captive Cayo Santiago group spent more time grooming (Marriott 1988). Since both groups were provisioned, their energetic requirements may be met more quickly than wild or less food-dependent commensal groups, leaving more time available for grooming, which would in turn help to maintain interindividual cohesiveness in a group that is likely to be more spatially drawn together to fairly clumped resources. No differences in moving time between the rural and the urban group were found in this study, but previously reported groups of rhesus macaque in forest (Southwick et al. 1982; Marriott 1988; Seth et al. 2001), mountain (Goldstein and Richards 1989), temple (Teas et al. 1975), and urban areas (Malik and Southwick 1988) spent more time moving than both of our study groups or the provisioned Cayo Santiago group (Marriott 1988). Interestingly, object manipulation/playing time was actually shorter in our study groups than in groups reported elsewhere (Teas et al. 1975; Malik and Southwick 1988; Seth et al. 2001).
Kurup and Kumar (1993) reported that the time devoted to feeding was inversely related to resting. However, we found that monkeys of the rural group fed more on garden sources and wild plant foods (see Table 4), as these items were more abundant in their habitat. This is also supported by the study of Saj et al. (1999), who found that monkeys in Entebbe, Uganda, preferred garden plant items because they are nutrient rich (particularly fruit items) and are easier to find, though raiding garden foods is presumably a riskier feeding strategy. Feeding time was extended in our rural group. These results support Siddiqi and Southwick (1980), who showed that a group found near to a forest patch spent more than 50 % of their time feeding on natural vegetation. In contrast, the urban group spent most of their feeding time on provisioned foods provided by local visitors and government authorities (see Table 4). This is also consistent with the results of an urban rhesus group in India that spent more than 80 % of their time feeding on foods offered by local people (Siddiqi and Southwick 1980), in spite of the fact that feeding on higher quality foods, such as fruits and nuts, can reduce the time spent feeding (Nakagawa 1989). The semi-provisioned Barbary macaques showed a high level of competition and aggression (El Alami et al. (2012). Intertroop agonistic behavior was also much higher in urban rhesus macaques than in their forest-dwelling conspecifics (Ciani 1986). Our results too showed that the provisioned urban rhesus group exhibited more dominance interactions than the rural group. In all of the above instances, dominance interactions are attributable to competition over highly clumped food resources.
Overall, differences in the activity budgets of the two groups were related mainly to the food resources available, reflecting different behavioral modifications overall to these two different habitats. In less human-impacted habitats, food availability is expected to fluctuate more seasonally (Halle and Stenseth 2000). Thus, time allocated to feeding and resting should also fluctuate seasonally, depending mainly on food availability, as was reported for red ruffed lemurs in the lowland coastal rain forest of Madagascar (Vasey 2005) and also for Japanese macaques in the temperate area of Takagoyama, northern Japan (Yotsumoto 1976). A study of Geoffroy’s marmosets showed that time spent resting and foraging significantly varied between the dry and wet seasons and was related to insect-food availability (Passamani 1998). Seasonal trends in resting and foraging were also noted for Japanese macaques, and were related to the availability of natural plant and insect foods (Hill 1997; Hanya 2004; Jaman and Huffman 2008). Time spent feeding and resting is constant across the months for Japanese macaques living in the subtropical lowland areas of Yakushima, whereas time spent grooming increases from October to December and moving decreases during this period (Maruhashi 1981). Thus, we expected (prediction 2) that the time spent feeding and resting as well as on other activities in the rhesus groups should vary seasonally according to the sources of food available to meet an individual’s nutritional requirements. Indeed, we found that regardless of group, overall time spent on behavioral activities was significantly affected by season. More specifically, in the rural group, time spent feeding, grooming, and moving varied significantly across seasons, while time spent on all activities other than feeding varied across seasons in the urban group. This suggests that season did not affect time spent feeding by the urban group, most likely because roughly the same amount of provisioned foods was provided to them each season by humans. They spent more time resting in summer than in winter/dry season, probably to avoid the high temperature and also because of the lack of available trees for shade. This study found that seasonal variations in behavioral activities, particularly feeding time, in the rural group might be related to the seasonal variation in food resources, as monkeys had access to a variety of wild and garden fruits and agricultural crops in the summer and during the monsoon season. The rural group rested more in the winter/dry season, in part due to the early morning fog, although it was also the season of relative food scarcity (only 5–7 species of fruit plants were available then).
We found that time spent on all activities, regardless of group and season, varied across age–sex class in relation to food access and quality. Our results showed that age–sex class variations in activity patterns were similar in both groups; adults spent more time resting and less time feeding, while immatures spent more time feeding and less time resting. This result is consistent with the different activity budgets of adult versus immature Japanese macaques (Jaman and Huffman 2008). This suggests that, regardless of habitat differences, immatures spend more time feeding, perhaps to satisfy the energetic requirements of physical development and maturation (National Research Council 2003). We also found that immatures in both groups spent more time on object manipulation/play than adults of both sexes, and we suggest that the energy required for this activity was another reason for their extended feeding time. Moreover, in the rural group, while adults initiated raids on gardens, houses, and shops for food, immatures stayed and continued to feed even after the adults stopped—until the group was driven away. This is one reason perhaps why immatures in the rural group could spent significantly more time feeding than their counterparts in the urban group. A similar tendency was observed in baboons, in that juveniles were noted to be greater agricultural pests than adults (Forthman-Quick 1986). Interestingly, it seemed that once immature rhesus macaques of the rural group had visited newly available garden sites with adult group members, they continued to raid houses and gardens more frequently than the adults did. Adult females in the rural group spent more time feeding than adult males, but the reverse was true in the urban group. One interpretation of this difference may be that urban females do not have the same access to food as rural females because males monopolize provisioned foods in the urban group.
Adult females groom more than adult males (Smith 1977; Waser 1977; Maruhashi 1981; Watanuki and Nakayama 1993). Possible reasons for this may be that (1) males are dominant over females as well as subordinate males (interestingly, adult males allowed adult females who groomed them to also forage with them, which would allow some females greater access to high-quality food and maximum access to available food; Soumah and Yokota 1991) and (2) males tend to feed faster than females and juveniles (Clutton-Brock 1977).
Adult females, in this study, groomed more than both adult and immature males and immature females. Immature females also groomed more than immature males. Due to the observation protocol of our study, we did not record detailed aspects of each groups’ social-sexual behavior. Even so, the levels we observed were too small to be included in our activity budget analyses. In the future, it would be interesting to investigate possible differences in reproductive behavior as an effect of differences in the activity budgets of groups under different levels of commensalism. Such information could have implications for population management.
In conclusion, behavioral patterns in the rural group were different from those in the urban rhesus macaque group, and these differences were related to habitat type, seasonality/availability of food resources (natural food sources and the level and stability of access to human foods), and differences in age–sex class nutritional requirements. In particular, daily access to a wider variety of plant food resources by the rural group and daily provisioning in the urban group seems to be largely responsible for the differences in the daily activity budgets of these two commensal rhesus groups. An important issue for rural macaque populations warranting further investigation is how food shortages caused by human-induced degradation of their natural habitat can increase crop raiding and in turn human–macaque conflict.
In an earlier study performed in the same two areas, Oppenheimer et al. (1983) mentioned that the group size of rhesus monkeys was higher in the forest than in the city. However, some 30 years later, we found the opposite trend in our two study groups. The urban group was twice the size of the rural group. The diet of the urban group comes mainly from food given by local people and visitors. The improved nutritional value of a provisioned diet is known to enhance fecundity, resulting in population growth (Mori 1979; Lyles and Dobson 1988). Increased group size and a growing reliance on humans for food may bring them into increased conflict with urban residents in the future. However, unlike the rural group site, where human–macaque conflict is high, in urban Dhaka problems associated with the rhesus macaques are manageable and there is no human–macaque conflict (Jaman and Huffman, in preparation). On the contrary, these monkeys are perceived to be an important part of the environment; feeding them brings intrinsic joy and religious benefits, so the people want to protect them through appropriate management practices (Jaman and Huffman, in preparation). This study adds to our growing understanding of commensalism in macaques (e.g., Paterson and Wallis, 2005; Radhakrishna et al. 2012).
References
Agetsuma N (2001) Relation between age–sex classes and dietary selection of wild Japanese monkeys. Ecol Res 16:759–763
Agetsuma N, Nakagawa N (1998) Effects of habitat differences on feeding behaviors of Japanese monkeys: comparison between Yakushima and Kinkazan. Primates 39:275–289
Ahmed J, Elias SM (1988) Socio-economic study of winter maize in some selected areas of Bangladesh. J Rural Dev (Bangladesh) 18:60–68
Akter T (2002) Ecology and behavior of rhesus macaques (Macaca mulatta) at Charmuguria (Masters thesis). Jahangir Nagar University, Savar
Altmann SA (1962) A field study of the sociobiology of rhesus monkeys. Ann N Y Acad Sci 102:338–435
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267
Baulu J, Redmond DE (1980) Social and nonsocial behaviors of sex- and age matched enclosed and free ranging rhesus monkeys (Macaca mulatta). Folia Primatol (Basel) 34:239–258
Bean A (1999) Ecology of sex differences in great ape foraging. In: Lee PC (ed) Comparative primate socioecology. Cambridge University Press, Cambridge, pp 339–362
Bernstein IS, Mason WA (1963) Activity patterns of rhesus monkeys in a social group. Anim Behav 11:455–460
Brennan EJ, Else JG, Altmann J (1985) Ecology and behaviour of a pest primate: vervet monkeys in a tourist lodge habitat. Afr J Ecol 23:35–44
Ciani AC (1986) Intertroop agonistic behavior of a feral rhesus macaque troop ranging in town and forested areas in India. Aggress Behav 12:433–439
Ciani AC, Chiarelli B (1988) Age and sex differences in the feeding strtategies of a free ranging population of Macaca mulatta Zimmerman, 1788 (Primates: Cercopithecidae), in Simla (India). Monit Zool Italy 22:171–182
Clutton-Brock TH (1977) Some aspect of intraspecific variation in feeding and ranging behaviour in primates. In: Clutton-Brock TH (ed) Primate ecology. Academic, London, pp 539–556
Dunbar RIM (1992) A model of the gelada socio-ecological system. Primates 33(1):69–83
El Alami A, ELS VAN Lavieren EV, Rachida A, Chait A (2012) Differences in activity budgets and diet between semiprovisioned and wild-feeding groups of the endangered Barbary macaque (Macaca sylvanus) in the Central High Atlas Mountains, Morocco. Am J Primatol 74(3):210–216
Fa JE (1986) Use of time and resources by provisioned troops of monkeys: social behavior, time and energy in the Barbary macaque (Mcaca sylvanus L.) at Gibraltar (Contrib Primatol 23). Karger, Basle
Forthman-Quick DL (1986) Controlling primate pests: the feasibility of conditioned taste aversion. In: Taub DM, King FA (eds) Current perspectives in primate social dynamics. Van Nostrand Reinhold, New York, pp 252–273
Goldstein SJ, Richard AF (1989) Ecology of rhesus macaques (Macaca mulatta) in northwest Pakistan. Int J Primatol 10:531–567
Halle S, Stenseth NC (2000) Introduction. In: Halle S, Stenseth NC (eds) Activity patterns in small mammals: an ecological approach. Springer, Berlin, pp 3–17
Hanya G (2003) Age differences in food intake and dietary selection of wild male Japanese macaques. Primates 44:333–339
Hanya G (2004) Seasonal variation in the activity budget of Japanese macaques in the coniferous forest of Yakushima: effects of food and temperature. Am J Primatol 63:165–177
Hill DA (1997) Seasonal variation in the feeding behavior and diet of Japanese macaques (Macaca fuscata yakui) in lowland forest of Yakushima. Am J Primatol 43:305–322
Hill CM, Webber AD (2010) Perceptions of non-human primates in human–wildlife conflict scenarios. Am J Primatol 72:909–924
Jaman MF, Huffman MA (2008) Enclosure environment affects the activity budgets of captive Japanese macaques (Macaca fuscata). Am J Primatol 70:1133–1144
Jaman MF, Huffman MA (2010) Age class differences in the feeding strategies of captive Japanese macaques (Macaca fuscata) in a forested enclosure. Zoo Biol 30(3):260–274
Kamal K, Boug A, Brain P (1997) Effects of food provisioning on the behavior of commensal Hamadryas baboons, Papio hamadryas, at Al Hada Mountains in Western Saudi Arabia. Mammalia 14:11–22
Kamilar JM, Pokempner AA (2008) Does body mass dimorphism increase male–female dietary niche separation? A comparative study of primates. Behaviour 145:1211–1234
Kurup GU, Kumar A (1993) Time budget and activity patterns of the lion-tailed macaque (Macaca silenus). Int J Primatol 14:27–39
Lata K (1980) Impact of urban environment on the development of cognitive behaviour in rhesus monkeys (Ph.D. thesis). Meerut University, Meerut
Lindburg DG (1971) The rhesus monkey in north India: an ecology and behaviour study. In: Rosenblum LA (ed) Primate behaviour: developments in the field and laboratory research, vol 1. Academic, New York, pp 83–104
Lindburg DG (1973) Grooming behavior as a regulator of social interactions in rhesus monkeys. In: Carpenter CR (ed) Behavioral regulators of behavior in primates. Bucknell University Press, Lewisburg, pp 124–148
Lyles AM, Dobson AP (1988) Dynamics of provisioned and unprovisioned primate populations. In: Fa JE, Southwick CH (eds) Ecology and behavior of food-enhanced primate groups. Alan R. Liss Inc., New York, pp 167–198
Malik I, Southwick CH (1988) Feeding behavior and activity patterns of rhesus monkeys (Macaca mulatta) at Tughlaqabad, India. In: Southwick CH, Fa JE (eds) Ecology and behavior of food-enhanced primate groups. Alan R. Liss Inc., New York, pp 95–112
Marriott BM (1988) Time budgets of rhesus monkeys (Macaca mulatta) in a forest habitat in Nepal and on Cayo Santiago. In: Ecology and behavior of food-enhanced primate groups, vol 11. Alan R. Liss, Inc, New York, pp 125–149
Martin P, Bateson P (1993) Measuring behavior: an introductory guide. Cambridge University Press, Cambridge, p 222
Maruhashi T (1981) Activity patterns of a troop of Japanese monkeys (Macaca fuscata yakui) on Yakushima Island, Japan. Primates 22:1–14
Mitchell G (1979) Behavioral sex differences in nonhuman primates. Van Nostrad and Reinhold, New York
Mori A (1979) Analysis of population changes by measurement of body weight in the Koshima troop of Japanese macaques. Primates 20:371–397
Mourshed M (2011) The impact of the projected changes in temperature on heating and cooling requirements in buildings in Dhaka, Bangladesh. Appl Energy 88:3737–3746
Nakagawa N (1989) Activity budget and diet of Patas monkeys in Kala Maloue National Park, Cameroon: a preliminary report. Primates 30:27–34
Nakayama Y, Matsuoka S, Watanuki Y (1999) Feeding rates and energy deficits of Juvenile and adult Japanese monkeys in a cool temperate area with snow coverage. Ecol Res 14:291–301
National Research Council (2003) Nutrient requirements of nonhuman primates, 2nd edn. The National Academies Press, Washington, DC
Oppenheimer JR, Akonda AW, Husain KZ (1983) Rhesus monkeys: effect of habitat structure, human contact and religious beliefs on population size. In: Seth PK (ed) Perspectives on primate biology. Today’s & Tomorrow’s Printers and Publishers, New Delhi, pp 193–199
Passamani M (1998) Activity budget of Geoffroy’s marmoset (Callithrix geoffroyi) in an Atlantic forest in southeastern Brazil. Am J Primatol 46:333–340
Paterson JD, Wallis J (2005) Commensalism and conflict: the primate–human interface. American Society of Primatologists, Norman
Pirta RS (1984) Cooperative behaviour in rhesus monkeys (Macaca mulatta) living in urban and forest areas. I. Genesis. In: Roonwal ML, Mohnot SM, Rathore NS (eds) Current primate research. University of Jodhpur Press, Jodhpur, pp 271–283
Radhakrishna S, Huffman MA, Sinha A (2012) The macaque connection: cooperation and conflict between humans and macaques (Developments in Primatology: Progress and Prospects, vol 43). Springer, New York
Richard AF, Goldstein SJ, Dewar RE (1989) Weed macaques: the evolutionary implications of macaque feeding ecology. Int J Primatol 10:569–594
Saj T, Sicotte P, Paterson JD (1999) Influence of human food consumption on the time budget of vervets. Int J Primatol 20:977–994
Seth PK, Seth S, Chopra PK, Reddy (1989) Behavioural phylogeny of rhesus monkeys in India. In: Seth PK, Seth S (eds) Perspectives on primate biology, vol 3. Today’s & Tomorrow’s Printers, New Delhi, pp 219–243
Seth PK, Chopra PK, Seth S (2001) Indian rhesus macaque: habitat, ecology and activity patterns of naturally occurring populations. ENVIS Bull Wildl Prot Areas 1(1):68–80
Shukla AK (1979) Activity patterns in the rhesus monkeys (Macaca mulatta) (research proceedings). Department of Anthropology, University of New Delhi, New Delhi
Siddiqi MF, Southwick CH (1980) Food habits of rhesus monkeys (Macaca mulatta) in the north India plains. Proc Zool Soc Calcutta 31:53–61
Smith CC (1977) Feeding behavior and social organization in howling monkeys. In: Clutton-Brock TH (ed) Primate ecology. Academic, New York, pp 97–126
Sokal RR, Rohlf FJ (1969) Biometry, 2nd edn. WH Freeman and Company, New York
Soumah G, Yokota N (1991) Female rank and feeding strategies in a free-ranging provisioned troop of Japanese macaques. Folia Primatol 57:191–200
Southwick CH, Teas J, Richie T, Taylor H (1982) Ecology and behavior of rhesus monkeys (Macaca mulatta) in Nepal. Nat Geogr Soc Res Rep 14:619–630
Teas J, Taylor H, Richie T, Southwick CH (1975) Nepal rhesus studies: ecological influences on the behavior of rhesus monkeys in Kathmandhu Valley (Progress Reports of the National Geographic Society). National Geographic Society, Washington, DC (unpublished)
Vasey N (2005) Activity budgets and activity rhythms in red ruffed lemurs (Varecia rubra) on the Masoala Peninsula, Madagascar: seasonality and reproductive energetics. Am J Primatol 66:23–44
Waser P (1977) Feeding, ranging and group size in the mangabey, Cercocebus albigena. In: Clutton-Brock TH (ed) Primate ecology. Academic, London, pp 183–222
Watanuki Y, Nakayama Y (1993) Age difference in activity pattern of Japanese monkeys: effects of temperature, snow and diet. Primates 34(4):419–430
Wolf LD (2002) Rhesus macaques: a compressive study of two sites, Jaipur in India and Silver Spring, Florida, vol 8. Cambridge University Press, Cambridge, pp 87–93
Yotsumoto N (1976) The daily activity rhythm in a troop of wild Japanese monkey. Primates 17(2):183–204
Acknowledgments
We would like to thank the Wildlife Circle of the Forest Department, Ministry of Environment of Bangladesh Government, for giving us permission to conduct this study. We are grateful to Sadhona Pharmaceutical Co. for permission to conduct observations on their property. We are most grateful to Dr. Jean-Baptist Leca for his suggestion regarding statistical analysis and Drs. A. Mori, K. Watanabe, T. Furuichi, C. Hashimoto, Y. Tsuji, and other faculty of the Department of Ecology and Social Behavior, PRI, for their critical comments and suggestions during the research period. This study was supported in part by a Graduate Studies Scholarship “Monbukagakusho” (Ministry of Education, Culture, Sports, Science and Technology, Japan) to M.F.J.. We thank the HOPE Project, a core-to-core program sponsored by the Japan Society for the Promotion of Science (JSPS), for providing travel funds to Bangladesh to carry out research between the 2007 and 2010 field visits. Our sincere thanks to Dr. M. Zashim Uddin and the Herbarium of the Botany Department, University of Dhaka, for identifying all of the plant species. Special thanks are also due to the Meteorological Department in Agargaon, Dhaka, for providing us with meteorological data for the study period. We are grateful to Mrs. D. Roy, D. Shah, M. Islam, and M. Hossain for assistance in the field. Finally, we express our sincere gratitude to Professor S. U. Sarker for his guidance and many helpful recommendations during the fieldwork.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Jaman, M.F., Huffman, M.A. The effect of urban and rural habitats and resource type on activity budgets of commensal rhesus macaques (Macaca mulatta) in Bangladesh. Primates 54, 49–59 (2013). https://doi.org/10.1007/s10329-012-0330-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-012-0330-6