Abstract
The acoustic structure of primate loud calls can be used as a powerful, inexpensive, and noninvasive tool for intra- and interspecific comparative analyses, reconstruction of phylogeny, and primate surveys. Despite the range of possibilities offered by acoustic analysis, only few studies so far have focused on quantitative descriptions of the acoustic structure of primate loud call repertoires. Here we aimed to assess the vocal repertoire of the solitary Sahamalaza sportive lemur, Lepilemur sahamalazensis, and to investigate potential communication functions. We recorded every sportive lemur vocalization we heard during 1000 h of nocturnal observations of eight collared individuals, as well as opportunistic searches in the Ankarafa Forest, Sahamalaza Peninsula in northwest Madagascar. In addition, we used playback experiments with four call types to clarify call function. We measured both temporal and spectral properties to describe calls quantitatively and used cross-validated discriminant function analysis to validate call types that we identified from a preliminary qualitative inspection of the spectrograms of 107 calls. We identified six distinct loud call types with the possibility of a seventh call type, with six loud call types similar to those of Lepilemur edwardsi and two loud call types similar to those of four other sportive lemur species. The described call types most likely function in mate advertisement, offspring care, and territorial defense. Future studies of loud calling of the Sahamalaza sportive lemur are needed to clarify if certain call types are sex specific and if loud calls could be used for recognition of individuals to enable noninvasive density measurements and species monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The quantitative description of acoustic structure of loud calls, the established term for prominent and explosive primate vocalizations, can be a powerful, inexpensive, and noninvasive tool for intra- and interspecific comparative analyses, e.g., mouse lemurs (Braune et al. 2008; Zimmermann et al. 2000), sportive lemurs (Méndez-Cárdenas et al. 2008), galagos (Ambrose 2003; Anderson et al. 2000; Masters 1991; Zimmermann et al. 1988), and tarsiers (Merker and Groves 2006; Nietsch and Kopp 1998), as well as for the reconstruction of phylogeny in cervids (Cap et al. 2008), anurans (Lehtinen et al. 2011), swifts (Thomassen and Povel 2006), wood-warblers (Farnsworth and Lovette 2008), and different felids (Peters and Tonkin-Leyhausen 1999). In primates, reconstruction of phylogeny based on vocalization has been used for Galagonidae (Zimmermann 1990), Lemuridae (Gamba and Giacoma 2006; Macedonia and Stanger 1994), cheirogaleids (Stanger 1995), as well as for Saimiri (Ploog 1974), Alouatta (Whitehead 1995), Colobus (Oates et al. 2000; Oates and Trocco 1983), Cercopithecus (Gautier 1988), and Pongo (Davila-Ross and Geissmann 2007). Analysis of call structure provides a useful tool for cryptic species, which are morphologically similar, but differ genetically (Mayr 1978; Templeton 1998), for both noninvasive species differentiation (Méndez-Cárdenas et al. 2008 for Lepilemur) and acoustic monitoring (Chiroptera: Brigham et al. 1997; Kalko 1995; O’Farrell 1997; Saunders and Barclay 1992; Orthoptera: Riede 1998; Hylobatidae: Geissmann and Nijman 2006; anurans: Bridges and Dorcas 2000).
Loud calls are frequently used for species differentiation and acoustic monitoring (Méndez-Cárdenas et al. 2008; Zimmermann et al. 2000). The species-specificity of loud calls in strepsirrhine primates (Ambrose 2003; Anderson et al. 2000; Bearder et al. 1995; Zimmermann 1990; Zimmermann et al. 2000), and their species-specific recognition (Braune et al. 2005), imply their importance for sexual selection and speciation (Rasoloharijaona et al. 2006). Acoustic studies of captive nocturnal and solitary foraging strepsirrhine primates suggested that loud calls are used by both males and females for sexual advertisement in the mating context in accordance with the mate attraction/mate defence hypothesis (Büsching et al. 1998; Hafen et al. 1998; Zimmermann and Lerch 1993).
Quantitative descriptions of the acoustic structure of loud call types are a prerequisite for analyses of geographical variation between species of the same genus; individual differences; behavioral, morphophysiological, and ecological correlates; and evolutionary mechanisms (Gamba and Giacoma 2007). However, such descriptions are not available for many species. This is also true for the cryptic and highly endangered sportive lemurs (Lepilemur), a genus that has undergone a substantial increase in species number from just 7 (Harcourt and Thornback 1990; Tattersall 1982) to 26 (Andriaholinirina et al. 2006; Craul et al. 2007; Lei et al. 2008; Louis et al. 2006; Rabarivola et al. 2006; Ramaromilanto et al. 2009) recently. All sportive lemur species are now confirmed to be at risk of extinction, with 4 species being Critically Endangered, 18 Endangered, and 4 Vulnerable (Davies and Schwitzer 2013; IUCN 2014). Many range boundaries of sportive lemurs remain unknown and acoustic analysis could prove extremely useful for species identification and monitoring. Sportive lemurs are generally cryptic and difficult to locate, making species identification and density measurements problematic. Sportive lemur species have been described as highly vocal (Rabesandratana 2006; Rasoloharijaona et al. 2006). So far, detailed comprehensive descriptions of loud call repertoire are available only for 1 of 26 sportive lemur species (Rasoloharijaona et al. 2006), whereas differences of two call types (High-pitched call and Ouah) have been described in 4 different sportive lemur species: Milne-Edwards’ sportive lemur (Lepilemur edwardsi), gray-backed sportive lemur (Lepilemur dorsalis), Ankarana sportive lemur (Lepilemur ankaranensis), and Lepilemur sp. (Méndez-Cárdenas et al. 2008).
Here we aimed to investigate the vocal repertoire and calling behavior of the Sahamalaza sportive lemur (Lepilemur sahamalazensis) from northwestern Madagascar. The Sahamalaza sportive lemur was recently described based on genetic and morphometric data (Andriaholinirina et al. 2006). Since it received species status, it has been included on the list of the World’s Top 25 Most Endangered Primates 2006–2008 (Mittermeier et al. 2007) and has been listed as Critically Endangered by the IUCN (Davies and Schwitzer 2013; IUCN 2014). The species is probably limited to the Sahamalaza Peninsula in northwestern Madagascar (Olivieri et al. 2007), but exact range boundaries and possible range overlaps with the neighboring Mittermeier`s sportive lemur (Lepilemur mittermeieri) and gray-backed sportive lemur remain unclear.
We hypothesized that the Sahamalaza sportive lemur has several types of loud calls, as described for other sportive lemur species, and predicted that loud calls that sound different represent statistically different call types. We hypothesized that different loud call types have different functions and predicted that they are associated mainly with territorial defense, mating, or offspring care, as described for other solitary primates as well as other vertebrate and invertebrate species (Ryan and Kime 2003). To assess the possible function of different call types, we in addition played back four different sportive lemur loud call types to the Sahamalaza sportive lemurs at night and observed their responses.
Methods
Study Site
The Ankarafa Forest is situated in the UNESCO Biosphere Reserve and Sahamalaza – Iles Radama National Park on the Sahamalaza Peninsula, which is located in the Sofia Region, northwest Madagascar (Fig. 1). The Park, officially inaugurated in July 2007 and managed by Madagascar National Parks (MNP), includes both marine and terrestrial ecosystems and is the first park that was created under the Programme Environnemental III of the Malagasy government and the World Bank. The climate is strongly seasonal, with a cool, dry season from May to October and a hot, rainy season from November to April. The Ankarafa Forest lies within a transition zone between the Sambirano domain in the north and the western dry deciduous forest domain in the south, harboring semihumid forests with tree heights of up to 25 m (Schwitzer et al. 2006).
There are no large connected areas of intact primary forest left on the Sahamalaza Peninsula, and the remaining fragments all show some degree of anthropogenic disturbance and/or edge effects (Schwitzer et al. 2007a, b). The forests and forest fragments are separated by grassland with shrubs. All forest fragments were in the process of regeneration after significant anthropogenic disturbance to the original forest vegetation over an extended period during the study period (2009–2011). We considered them to be ≥35 yr old, based on aerial and satellite images and GIS data (Harper et al. 2007), and to exhibit the key characteristics of post-abandonment secondary forest (Chokkalingam and de Jong 2001).
Other lemur species in Sahamalaza include the blue-eyed black lemur (Eulemur flavifrons), the aye-aye (Daubentonia madagascariensis), the western bamboo lemur (Hapalemur occidentalis), the northern giant mouse lemur (Mirza zaza), and the fat-tailed dwarf lemur (Cheirogaleus medius). The lemurs of Sahamalaza are highly threatened by increasing and presumably unsustainable levels of hunting and by forest destruction and degradation, mainly through land conversion for subsistence agriculture (Schwitzer et al. 2006; Seiler et al. 2010, 2012).
Data Collection
In two field seasons (July–October 2009; May–August 2010), we fitted eight individual Sahamalaza sportive lemurs with radio-collars. We used TW3 brass-collar tags and TW3 button cell collars (Biotrack). We captured the sportive lemurs during the day at their sleeping sites (tree hole or tree tangle) with a blowpipe using 1-ml cold air-pressure narcotic syringe projectiles from Telinject. As anesthetic we used Ketasel 50 (50 mg Ketasel ml−1) in the dose recommended by the manufacturer (0.01 ml per 100 g body mass). We anesthetized the lemurs were for a short period of time to take body measurements (weight; length of head and body, tail, femur, tibia, foot, forearm, forearm and hand; distance between ears; collection of fecal samples) and to equip them with radio-collars. We released the lemurs after recovery at their capture site at the onset of their activity period.
During night observations (18:00–06:00 h) we followed the radio-collared lemurs using a portable TR-4 receiver (first field season; Telonics, Inc., Impala, AZ) or a Biotrack receiver (second field season; Biotrack, Dorset, UK) with a three-element yagi-antenna (Biotrack, Dorset, UK) as well as a GPS device (GPS 60, Garmin Ltd., Schaffhausen, Switzerland). We recorded vocalizations of radio-collared individuals using a directional microphone (K6 power module and ME67 recording head, Sennheiser electronic GmbH & Co. KG, Wennebostel, Germany) and a PMD-670 digital recorder (Marantz Japan Inc., Sagamihara, Japan). Followed collared individuals never vocalized while they were in sight, so we were unable to relate calls to specific behaviors. Nonetheless we were able to record vocalizations and immediate behavior of other, uncollared individuals that came into sight during our night tracking. In total, the eight individuals (one male, seven females) were followed 666 h at night: Lepilemur (L)1 (male) and L4: 132 h in 11 nights; L3, L5, L6, and L8: 72 h in 6 nights; L2: 60 h in 5 nights; and L7: 54 h in 4.5 nights. We recorded every sportive lemur vocalization heard during the night observations.
To avoid misclassifying the vocalizations of other abundant species, e.g., the giant mouse lemurs (Mirza zaza), as sportive lemur vocalizations, we analyzed only those loud call types that we had previously recorded from a Sahamalaza sportive lemur. As night tracking did not prove to be efficient to record sportive lemur vocalizations, we also conducted opportunistic sportive lemur searches during 68 additional nights (4–5 h each) and recorded every vocalization, resulting in a total of ca. 1000 h of nocturnal observations. Background noise by various insects and frogs was high, especially in the early rainy season (starting October), making it difficult to achieve sufficient recording quality.
Extraction of Vocalization Properties
All vocalizations were stored as wav–files (Sampling frequency: 48 Khz, bit depth 16 bit). Acoustic features of loud call types (see Table III) were extracted using SASLab Pro (Avisoft Bioacoustics, Berlin, Germany; FFT size 512; Hamming window; overlap 75%, filter bandwidth: 56 Hz, temporal resolution: 1.45 ms). After a preliminary visual qualitative analysis of the entire recordings, all calls were inspected spectrographically and only calls that showed in the power spectrum a minimum difference of 10 dB from the peak of the fundamental frequency to the background noise, were selected for analyses. Of a total of 214 recordings, 107 calls fulfilled this recording quality. Although all precautions had been taken by selecting only the highest quality calls, we cannot completely exclude a certain unquantified influence of background noise on our characterization of Sahamalaza sportive lemur call properties.
We grouped the recorded signals into distinct categories, first by an acoustic assessment of the first author and at least two assistants, then by visually comparing spectrograms on the basis of their temporal and structural properties (Table I). The call types were determined and named according to the vocal repertoire for Milne-Edwards’ sportive lemurs (Rasoloharijaona et al. 2006). A call was defined as a monosyllabic or a multisyllabic vocalization separated from others by a gap of silence of at least twice its call duration. We used the automatic function (automatic parameter measurements; parameters as mentioned in Table III, threshold: -20) in SASLab Pro (Avisoft Bioacoustics, Berlin Germany), which transferred frequency and temporal parameters (Table I) as a dynamic data exchange (DDE parameter file) to an Excel spreadsheet. All automatic measurements were reviewed and manually corrected where needed.
Selection of Study Subjects for Playback Experiments
Between September and November 2011, we conducted a total of 245 playback experiments in four forest fragments (A–D; see Fig. 1). We carried out opportunistic sportive lemur searches starting at 18:00 h and tried to run playback experiments with every sportive lemur we found. During each night we distinguished the individuals by size, special markings, and location; as individual Sahamalaza sportive lemurs have a range of ca. 0.5 ha and are solitary foragers (Seiler et al. 2014) we avoided conducting playback experiments more than once in a location where we already conducted a playback experiment during that night. As nightly ranges of individuals might differ in following nights, we cannot exclude the possibility that we tested the same individuals during successive nights and are thus unable to give an exact number of individuals used for playback experiments. As we found a maximum of 6 sportive lemurs in 3 of the fragments (A–C), and 7 in another fragment (D) every night, we concluded that we tested at least 25 individuals. We did not capture the individuals used for playback experiments and thus are unable to provide information about sex, body mass, or size. The sportive lemurs were resting at a height of 5.8 ± 2.9 m at the start of playbacks.
Playback Stimuli
We used four types of Sahamalaza sportive lemur loud calls (2-parts, Chuckle, Bark 1, and Ouah) as playback stimuli. We recorded these and confirmed that they were Sahamalaza sportive lemur calls in 2009 and 2010. We recorded a further three call types during the 2011 field season, so did not use those for playback experiments.We used four different versions of each loud call type. We equipped all recordings used for playback with a 5-s fade in and fade out using SASLAB Pro (Avisoft Bioacoustics, Berlin, Germany). Table II summarizes acoustic parameters of the calls used, and Fig. 2 shows example spectrograms of each call type (generated in SASLAB Pro; 1024-point Hamming window, 48 kHz sampling rate with 50% window overlap resulting in 47 Hz frequency resolution, and 10.7 ms temporal resolution). We played back the stimuli using an iPod Nano, model A1320 (Apple Inc., Cupertino, CA) and wireless loudspeaker (JBL On Stage Micro II; Harman International Industries, Inc., Stamford, CT; frequency range 80 Hz–20 kHz). We measured the sound pressure level of call playbacks in a semi-anechoic chamber in Bristol using 40BF microphone, 26AB preamplifier, and 12AA power module (all G.R.A.S. Sound & Vibration, Holte, Denmark) calibrated by D1411E acoustic calibrator (Dawe Instruments, Brentford, U.K.). Mean sound pressure levels were between 69.8 and 72.6 dB peak-equivalent SPL re 1 m (Table II).
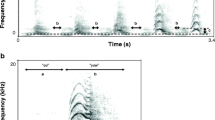
Spectograms (lower panel) and oscillograms (upper panel) generated of typical examples for the seven types of recorded loud calls in the Sahamalaza sportive lemur. (a) Chuckle, High-pitched. (b) Bark 1, Bark 2, Ouah, Tchen-tchen, 2-parts. Generated in SASLAB Pro; FFT length: 512 points; 1024-point Hamming window, 48 kHz sampling rate with 50% window overlap resulting in 47 Hz frequency resolution, and 10.7 ms temporal resolution.
Playback Procedure
For playback experiments, we hid the equipment behind a bush or in a tree at a horizontal distance of ca. 5 m from the tree the sportive lemur was found in. The observer stood ≥5 m away from the playback equipment. Occurrence, frequency, and duration of behavior (Table III) were documented using focal animal sampling for 5 min each before and after the playback. Before starting the 5 min pre-playback observation, we waited for the tested individual to settle to the observers’ presence. Sportive lemurs that are not habituated to human presence are vigilant and constantly stare at the observer, but return to their usual behavior (Seiler et al. 2014) after some minutes if the observer remains calm and does not further approach the lemur. We did not use individuals that did not settle to our presence and/or fled for playback experiments. During the 5-min observation intervals, we noted the exact time (mm: ss) of the onset and offset of each behavior. After 5 min, we played a pre-selected call back using a remote control, and started the 5-min post-playback observation. In addition, we noted immediate behavioral responses (within 5 s) to playback (see Table III, categories II) and recorded all calls given in response to the playbacks.
We presented the four different versions of 2-parts a total of 63 times in 4 forest fragments (A: 15 times; B: 16; C: 16; D: 16) and the 4 versions of Bark 1 65 times (A: 16; B: 17; C: 17; D: 15). We played back the 4 versions of Chuckle 67 times (A: 17; B: 17; C: 16; D: 17 and the 4 different versions of Ouah 50 times in total (A: 13; B: 12; C: 14; D: 11). We presented all calls in a randomized order to avoid repeats of the same call recordings and presented only one call per individual per night. We played back each version of 2-parts, Bark 1, Chuckle, and Ouah between three and five times per forest fragment (A–D).
Data Analyses
Vocalization Properties
To avoid pseudoreplication, we carried out statistical analyses on the individual mean values of each acoustic property per loud call type. To test whether the measured acoustic parameters vary significantly between the loud call types of the Sahamalaza sportive lemur, we performed one-way ANOVAs. We used the Honestly Significantly Different (HSD) test proposed by J. Tukey as a post hoc procedure to test all pairwise comparisons among the mean values of the seven call types (using harmonic means sample size = 10.765). We used discriminant function analysis (DFA) to test whether the seven loud call types identified on a qualitative basis identified distinct vocal groups and to identify linear combinations of predictor variables that maximize the differences among call types (Lehner 1998). We ran the DFA using a stepwise procedure with cross-validation and set F-value thresholds for acceptance or rejection of independent variables at F = 3.84 and F = 2.71 in all analyses.
Playback Experiments
To test for differences in the duration of individual lemurs’ vigilance (measured as seconds of vigilant behavior) before and after the playback of conspecific calls, we performed a Wilcoxon signed rank test (α = 0.025) with each individual’s mean vigilance duration in the 5-min periods before and after the playback of each stimulus type. To test for immediate responses, we recorded immediate scanning and movement reactions as soon as the playback call was presented. As call functions were unknown, we kept categories for immediate responses broad, rating scanning toward the sound, freezing and/or fleeing, or approaching as “response” while no reactions were rated as “no response.” As we were not able to identify individual sportive lemurs, we rated each experiment as independent data set, but lowered the α-level to 0.025 to avoid influences of pseudoreplication. We used binomial tests to test for differences between immediate responses after the playback of call types. Based on control call playbacks that we conducted during earlier playback experiments (Seiler et al. 2013), resulting in immediate scan responses in 15 and immediate locomotion in 1 out of 153 playbacks, we set the probability that immediate responses are just by chance to 1:99 for immediate locomotion and 10:90 for immediate scanning responses (α = 0.025). All statistical tests were carried out using SPSS 19.0 (SPSS Inc., Chicago, IL).
Ethical Note
This study was conducted with permission from the Madagascan Ministere de l’Environnement et des Forets (Autorisation de Recherche #231/11/ MEF/SG/DGF/DCB.SAP/SCB) and adhered to the legal requirements of Madagascar. It was approved by the Welfare & Research Advisory Board of the Bristol, Clifton and West of England Zoological Society.
Results
Call Repertoire
Based on spectral and temporal properties, we manually identified seven distinct loud call types, six of which had a structure similar to those described for Milne-Edwards’ sportive lemurs and were thus named accordingly (Shrill-chuckle related, High-pitched call, Bark 1, Bark 2, Tchen-tchen, and Ouah; Fig. 2; Table IV). As we did not find different types of Shrill-chuckle (as present in Lepilemur edwardsi), we refer to this loud call type only as Chuckle. The ANOVA revealed highly significant differences between call types for all tested properties except Start peak frequency and End maximum frequency (Table IV).
Classical stepwise DFA with cross-validation correctly classified 77.4% of the preclassified calls by vocal type (Table V). Although all cases of High-pitched call and Ouah were correctly classified, lower classification rates (73–82%; see Table V) were produced for Bark 1, Bark 2, and Chuckle. 2-part calls were correctly classified in 60% of the cases, with 35% being classified as Bark 2. Only 40% of Tchen-tchen calls were classified correctly, whilst 60% were classified as Ouah. Five call parameters contributed to the discrimination of call types (Table VI).
Sahamalaza Sportive Lemur Call Types
The Chuckle (N = 15) was a tonal call that consisted of multiple, related, and parabolically frequency-modulated syllables. It was, together with 2-parts, one of the longest call types and also had the longest duration to maximum frequency. This call type had one of the highest mean minimum frequencies and one of the lowest mean maximum frequencies and consequently one of the smallest mean bandwidths (Table IV; Fig. 2a).
Chuckle was uttered during the rare agonistic interactions when individuals chased other individuals away, either from a feeding tree or from sleeping sites. During these interactions both individuals used this call type. Comparing the 5 min before to 5 min after the playback of Chuckle (N = 67), subjects did not show significant differences in stationary vigilance or locomotion (Table VII). Nonetheless, in immediate response to the playback, subjects changed scanning direction and looked toward the sound (binomial test; P < 0.001), and showed significantly more locomotion away from the sound (binomial test; P < 0.001). We received two Bark 2 responses and were able to observe a young individual (about 2 mo old) fleeing to its mother in a nearby tree in response to Chuckle playbacks.
The High-pitched call (N = 6) was a tonal call that consisted of multiple, nonrelated (not connected) syllables with an inverse v-shaped frequency modulation. It was very rare and only recorded late in the year (October–November). It was the longest call type with a short duration to maximum (Table IV; Fig. 2a). The few individuals uttering High-pitched call were moving through the forest faster than usual, stopped abruptly, and uttered the call while stationary and intensively scanning their surroundings, before locomoting again. We never heard an answer after these call displays in the natural context.
The Bark 1 (N = 28) was a monosyllabic and tonal call with only little modulation in frequency. The mean peak frequency was one of the lowest of all call types, as was the case for the mean minimum frequency as well as the mean maximum frequency (Table IV; Fig. 2b). Bark 1 was heard mainly after the start of September when infants were born and was observed only in relation to rearing, e.g., in mother–infant interactions. After playback of Bark 1 locomotion increased significantly, but stationary vigilance remained the same (Table VII). In immediate reaction to the playback, subjects changed scanning direction significantly and looked toward the sound (binomial test; P < 0.001); they showed immediate locomotion after Bark 1 (binomial test; P < 0.001).
Our 65 Bark 1 playbacks elicited two Bark 1 responses, one Chuckle and one Ouah response.
The Bark 2 (N = 13) was a monosyllabic and tonal call. In contrast to the similar Bark 1, the syllable started at a very high frequency, which dropped steeply through the call duration. It was used less frequently as Bark 1. The duration to maximum was significantly shorter than in Chuckle and 2-parts. Together with Tchen-tchen, this call type had one of the highest mean peak frequencies. It also had the highest mean minimum frequency, the highest mean maximum frequency, and a high mean bandwidth (Table IV; Fig. 2b). Like Bark 1, Bark 2 was heard mainly during the lactation period. It was less common than the similar Bark 1.
The 2-parts (N = 17) was a tonal call that consisted of up to nine separated syllables. The first syllable was longer than the following parts and showed little frequency modulation. Similar to Ouah, the shorter syllables were inverse u-shaped or had a downward-modulated frequency contour. It was, like Chuckle, one of the longest call types and had a long duration to maximum. The mean peak frequency was significantly lower than in Chuckle and Bark 2 and the mean maximum frequency was one of the lowest of all call types. This call type was recorded only between April and June (Fig. 2b).
Individuals seen to use 2-parts (N = 17) were stationary and no other individual was observed in the proximity, but distant individuals in response produced the same call type.
After playback of 2-parts the amount of locomotion increased significantly, but stationary vigilance remained the same (Table VII). Individuals changed scanning direction significantly and looked toward the sound after playbacks of 2-parts (binomial test; P < 0.001) and showed significantly more direct locomotion away from the sound (binomial test; P < 0.001). With 63 playbacks of 2-parts, we received five vocal reactions: three 2-parts and two Chuckle. Once a male approached the speaker after playback of 2-parts, continuously uttering the same loud call in all directions for about 10 min after the playback.
The Ouah (N = 22) was one of the most abundant loud call types. It was a monosyllabic and tonal call with an inverse u-shaped or downward-modulated frequency modulation. It was one of the shortest calls with a very short duration to maximum. The mean peak frequency was significantly lower than in Chuckle and Bark 2. Together with Tchen-tchen this call type had the lowest mean minimum frequency (Table IV; Fig. 2b).
Individuals uttering Ouah were stationary both during and after calling. No individuals were observed in the proximity when these calls were uttered and no answers of other individuals were heard. In response to playbacks of Ouah, individuals increased neither locomotion significantly, nor stationary vigilance (Table VII), but showed immediate locomotion (binomial test; P < 0.001) and looked toward the sound (binomial test; P < 0.001). No vocal reaction was ever noted after Ouah playbacks.
The Tchen-tchen (N = 5) was a short tonal call consisting of two hook-like and related syllables. It was heard only rarely. With Bark 2, this call type had the highest mean peak frequency. Mean minimum frequency was significantly lower than in Chuckle and Bark 2 (Table IV; Fig. 2b). Similar to Ouah, individuals uttering this call type were stationary both during and after calling, no further individuals were observed in the proximity, and no answers of other individuals were heard.
Discussion
In this study we were able to identify six distinct classes of loud calls with the possibility of a seventh call type. All loud call types were similar in structure to other sportive lemur species’ loud calls, suggesting that sportive lemurs share a similar call repertoire (Méndez-Cárdenas et al. 2008; Rasoloharijaona et al. 2006). Whereas High-pitched call, Ouah, Bark 1, Bark 2, and Chuckle were correctly classified in nearly all cases by the classical stepwise DFA, about a third of 2-part calls were classified as Bark 2, indicating that these call types are similar.
Based on our behavioral observations we consider 2-parts to be a distinct call type. Like Bark 1, Bark 2 was recorded only during the lactation period and seemed to be associated with parental care and mother–infant communication.Bark 2 was the loud call with the highest mean maximum frequency in our study. The acoustic structure of calls is thought to be related to the caller’s affective state (Fischer et al. 1995; Owings and Morton 1998; Schrader and Todt 1993) with an increase in pitch with perceived stress level of the individual (Fichtel and Hammerschmidt 2002). We thus suggest that Bark 2 is used to warn the offspring of potential danger. Whereas Bark 1 was usually answered by other individuals, Bark 2 never was, suggesting that Bark 1 functions as a contact call and Bark 2 as an alarm call from mother to infant.
The overall structure of 2-parts was similar to a duetting sequence described in the Milne-Edwards’ sportive lemur in which loud calls were most frequent at feeding and sleeping sites (Rasoloharijaona et al. 2006), with regular pair duets that are often followed by a synchronization of movements (Méndez-Cárdenas and Zimmermann 2009). The behavior of Sahamalaza sportive lemurs in response to 2-parts (male approaching the sound and answering with the same call) and our observation that 2-parts was usually used by two individuals suggests that 2-parts could be used in duets. As it was recorded only between April and June and thus during the mating period of the Sahamalaza sportive lemur, this call type might serve for mate attraction. In pair-living Milne-Edwards’ sportive lemurs duetting is most abundant during the offspring care period (Méndez-Cárdenas and Zimmermann 2009). It is possible that females in particular would aim to avoid unknown males during the offspring care period, which was the time when we conducted playback experiments. At least one case of infanticide at the onset of the offspring care period is described for Milne-Edwards’ sportive lemurs (Rasoloharijaona et al. 2000, 2006). This would explain why, during our playback experiments with this call type, all individuals except one male moved away from the sound source.
As 60% of Tchen-tchen calls were classified as Ouah, it is possible that this call type is merely a variation of the Ouah call, which is also supported by our behavioral observations. The Ouah loud call did result in direct flight responses during our playback experiments, as well as in an increased vigilance and locomotion. As we never received answers to this call, we suggest that this call type might function as a territorial call, transmitting the location of the calling individual. We did not conduct playback experiments with Tchen-tchen, but observed that, as with Ouah, individuals uttering Tchen-tchen were stationary and solitary and we heard no answers from other individuals, suggesting a similar function of the two call types. We were able to record the Tchen-tchen only five times, and further records are needed to conclude whether Ouah and Tchen-tchen are distinct call types
If the high frequency of High-pitched call is not due to individual differences or the affective state of the caller, it may function as an alarm call, like Bark 2, as alarm calls are usually higher in frequency and shorter and noisier than other call types (Fischer et al. 2001). Our observations and the reactions of lemurs to playbacks suggest that Chuckle is used in agonistic encounters. The high mean minimum frequency of Chuckle suggests a high level of arousal of individuals uttering this call type, as found in other alarm calls. The long duration of this call type in combination with our observations suggest that this call type is used to intimidate and chase away other individuals, which also might be considered as a type of alarm call.
We found fewer distinct call types than described for pair-living species of the same genus (Méndez-Cárdenas et al. 2008; Rasoloharijaona et al. 2006). In the Milne-Edwards’ sportive lemur, some call types are sex specific (Rasoloharijaona et al. 2006). Males used five different call types including Ouah, Chuckle, and Tchen-tchen, which were also found in the Sahamalaza sportive lemur. Female Milne-Edwards’ sportive lemurs exclusively used Bark 1, Bark 2, and Oaii. Only High-pitched call was found in both sexes (Rasoloharijaona et al. 2006). Our few observations on calling individuals suggest a similar sex-specific use of loud calls as found in the Milne-Edwards’ sportive lemur, but our data set is not sufficient to test this hypothesis. Future studies should test for sex differences in the calling behavior of the Sahamalaza sportive lemur. The same is true for individual recognition of sportive lemurs.
In summary, we have described six distinct classes of loud calls with the possibility of a seventh call type in the Sahamalaza sportive lemur, which are likely to function in mate advertisement, offspring care, and territorial defense. Future studies of loud calling of the species are needed to clarify if some call types are sex specific and if loud calls could be used for recognition of individuals. Once the vocal repertoires of neighboring Mittermeier’s sportive lemur and gray-backed sportive lemur are known, the vocal parameters of Sahamalaza sportive lemurs loud calls could be used for rapid identification of this species to establishing range boundaries in relation to the ranges of the different sportive lemur species as well as for density measurements and acoustic species monitoring.
References
Ambrose, L. (2003). Three acoustic forms of Allen’s galagos (primates; Galagonidae) in the Central African region. Primates, 44, 25–39.
Anderson, M. J., Ambrose, L., Dixson, A. F., & Pullen, S. (2000). Intraspecific variation in the vocalizations and hand pad morphology of southern lesser bush babies (Galago moholi): A comparison with G. senegalensis. International Journal of Primatology, 21, 537–555.
Andriaholinirina, N., Fausser, J. L., Roos, C., Zinner, D., Thalmann, U., Rabarivola, C., Ravoarimanana, I., Ganzhorn, J. U., Meier, B., Hilgartner, R., Walter, L., Zaramody, A., Langer, C., Hahn, T., Zimmermann, E., Radespiel, U., Craul, M., Tomiuk, J., Tattersall, I., & Rumpler, Y. (2006). Molecular phylogeny and taxonomic revision of the sportive lemurs (Lepilemur, Primates). BMC Evolutionary Biology, 6, 17.
Bearder, S. K., Honess, P. E., & Ambrose, L. (1995). Species diversity among galagos with special reference to mate recognition. In L. Alterman, G. A. Doyle, & M. K. Izard (Eds.), Creatures of the dark: The nocturnal prosimians (pp. 331–352). New York: Plenum Press.
Braune, P., Schmidt, S., & Zimmermann, E. (2005). Spacing and group coordination in a nocturnal primate, the golden brown mouse lemur (Microcebus ravelobensis): The role of olfactory and acoustic signals. Behavioural Ecology and Sociobiology, 58, 587–596.
Braune, P., Schmidt, S., & Zimmermann, E. (2008). Acoustic divergence in the communication of cryptic species of nocturnal primates (Microcebus ssp.). BMC Biology, 6, 19.
Bridges, A. S., & Dorcas, M. E. (2000). Temporal variation in anuran calling behavior: Implications for surveys and monitoring programs. Copeia, 2, 587–592.
Brigham, R. M., Grindal, S. D., Firman, M. C., & Morissette, J. L. (1997). The influence of structural clutter on activity patterns of insectivorous bats. Journal of Zoology, 75, 131–136.
Büsching, C. D., Heistermann, M., Hodges, J. K., & Zimmermann, E. (1998). Multimodal oestrus advertisement in a small nocturnal prosimian, Microcebus murinus. Folia Primatologica, 69, 295–308.
Cap, H., Deleporte, P., Joachim, J., & Reby, D. (2008). Male vocal behavior and phylogeny in deer. Cladistics, 24, 917–931.
Chokkalingam, U., de Jong, W. (2001). Secondary forest: a working definition and typology. International Forestry Review 3, 19–26.
Craul, M., Zimmermann, E., Rasoloharijaona, S., Randrianambinina, B., & Radespiel, U. (2007). Unexpected species diversity of Malagasy primates (Lepilemur spp.) in the same biogeographical zone: A morphological and molecular approach with the description of two new species. BMC Evolutionary Biology, 7, 83.
Davies, N., & Schwitzer, C. (2013). Lemur conservation status review: An overview of the lemur Red-Listing results 2012. In C. Schwitzer, R. A. Mittermeier, N. Davies, S. Johnson, J. Ratsimbazafy, J. Razafindramanana, E. E. Louis, Jr., & Rajaobelina, S. (Eds.), Lemurs of Madagascar: A strategy for their conservation 2013–2016 (pp. 13–28). IUCN SSC Primate Specialist Group; Bristol Conservation and Science Foundation; Conservation International.
Davila-Ross, M., & Geissmann, T. (2007). Call diversity of wild male orangutans: A phylogenetic approach. American Journal of Primatology, 69, 305–324.
Farnsworth, A., & Lovette, I. J. (2008). Phylogenetic and ecological effects on interspecific variation in structurally simple avian vocalizations. Biological Journal of the Linnean Society, 94, 155–173.
Fichtel, C., & Hammerschmidt, K. (2002). Responses of redfronted lemurs to experimentally modified alarm calls: Evidence for urgency-based changes in call structure. Ethology, 108, 763–777.
Fischer, J., Hammerschmidt, K., & Todt, D. (1995). Factors affecting acoustic variation in Barbary macaque (Macaca sylvanus) disturbance calls. Ethology, 101, 51–66.
Fischer, J., Metz, M., Cheney, D. L., & Seyfarth, R. M. (2001). Categorical responses of Chacma baboons to graded bark variants? Animal Behaviour, 61, 925–931.
Gamba, M., & Giacoma, C. (2006). Vocal tract modeling in a prosimian primate: The black and white ruffed lemur. Acta Acustica united with Acustica, 92, 749–755.
Gamba, M., & Giacoma, C. (2007). Quantitative acoustic analysis of the vocal repertoire of the crowned lemur. Ethology Ecology and Evolution, 19, 323–343.
Gautier, J. P. (1988). Interspecific affinities among guenons as deduced from vocalizations. In A. Gautier-Hion, F. Bouliére, & J. P. Gautier (Eds.), A primate radiation: Evolutionary biology of the African guenons (pp. 195–226). New York: Cambridge University Press.
Geissmann, T., & Nijman, V. (2006). Calling behavior of wild silvery gibbons (Hylobates moloch) in Java (Indonesia). American Journal Primatology, 68, 1–19.
Hafen, T., Neveu, H., Rumpler, Y., Wilden, I., & Zimmermann, E. (1998). Acoustically dimorphic advertisement calls separate morphologically and genetically homogenous populations of the grey mouse lemur (Microcebus murinus). Folia Primatologica, 69, 342–356.
Harcourt, C., & Thornback, J. (1990). Lemurs of Madagascar and the Comoros. The IUCN Red Data Book. Gland, Switzerland, and Cambridge, U.K: IUCN.
Harper, G., Steininger, M., Tucker, C., Juhn, D., Hawkins, F. (2007). Fifty years of deforestation and forest fragmentation in Madagascar. Environmental Conservation 34, 325–333.
IUCN Red List of Threatened Species. Version 2014.2. www.iucnredlist.org/ (Accessed July 25, 2014).
Kalko, E. K. V. (1995). Echolocation signal design, foraging habitats and guild structure in six Neotropical sheath-tailed bats (Emballonuridae). In P. A. Racey & S. M. Swift (Eds.), Ecology, evolution and behaviour of bats. Oxford: The Zoological Society of London and Clarendon Press.
Lehner, P. N. (1998). Handbook of Ethological Methods. Cambridge: Cambridge University Press, 694 p.
Lehtinen, R. M., Wojtowicz, E. A., & Hailey, A. (2011). Male vocalizations, female discrimination and molecular phylogeny: Multiple perspectives on the taxonomic status of a critically endangered Caribbean frog. Journal of Zoology, 283, 117–125.
Lei, R. H., Engberg, S. E., Andriantompohavana, R., McGuire, S. M., Mittermeier, R. A., Zaonarivelo, J. R., Brenneman, R. A., Louis, E. E. J. (2008). Nocturnal lemur diversity at Masoala National Park. Texas Tech University Museum Special Publications, 53,1-41.
Louis, E. E. J., Engberg, S. E., Lei, R., Geng, H., Sommer, J. A., Randriamampionona, R., Randriamanana, J. C., Zaonarivelo, J. R., Andriantompohavana, R., Randria, G., Prosper, P., Ramaromilanto, B., Rakotoarisoa, G., Rooney, A., Brenneman, R. A. (2006). Molecular and morphological analyses of the sportive lemurs (family Megaladapidae: genus Lepilemur) reveals 11 previously unrecognized species. Texas Tech University Museum Special Publications, 49, 1–49.
Macedonia, J. M., & Stanger, K. F. (1994). Phylogeny of the lemuridae revisited: Evidence from communication signals. Folia Primatologica, 63, 1–43.
Masters, J. C. (1991). Loud calls of Galago crassicaudatus and G. garnettii and their relation to habitat structure. Primates, 32, 153–167.
Mayr, E. (1978). Darwin and natural selection. American Scientific, 65, 312–327.
Méndez-Cárdenas, M., Randrianambinina, B., Rabesandratana, A., Rasoloharijaona, S., & Zimmermann, E. (2008). Geographic variation in loud calls of sportive lemurs (Lepilemur ssp.) and their implications for conservation. American Journal of Primatology, 70, 828–838.
Méndez-Cárdenas, M., & Zimmermann, E. (2009). Duetting: A mechanism to strengthen pair bonds in a dispersed pair-living primate (Lepilemur edwardsi)? American Journal of Physical Anthropology, 139, 523–532.
Merker, S., & Groves, C. P. (2006). Tarsius lariang: A new primate species from Western Central Sulawesi. International Journal of Primatology, 27, 465–485.
Mittermeier, R. A., Ratsimbazafy, J., & Rylands, A. B. (2007). Primates in peril: The world’s 25 Most Endangered Primates 2006–2008. Primate Conservation, 22, 1–40.
Nietsch, A., & Kopp, M. L. (1998). Role of vocalization in species differentiation of Sulawesi Tarsiers. Folia Primatologica, 69, 371–378.
Oates, J. F., Bocian, C. M., & Terranova, C. J. (2000). The loud calls of black-and-white colobus monkeys: Their adaptive and taxonomic significance in light of new data. In P. F. Whitehead & C. J. Jolly (Eds.), Old World monkeys (pp. 431–452). Cambridge, U.K.: Cambridge University Press.
Oates, J. F., & Trocco, T. F. (1983). Taxonomy and phylogeny of black- and-white colobus monkeys: Inferences from an analysis of loud call variation. Folia Primatologica, 40, 83–113.
O’Farrell, M. J. (1997). Use of echolocation calls for the identification of free-flying bats. Transactions of the Western Section of the Wildlife Society, 33, 1–8.
Olivieri, G., Zimmermann, E., Randrianambinina, B., Rasoloharijaona, S., Rakotondravony, D., Guschanski, K., Radespiel, U. (2007). The ever-increasing diversity in mouse lemurs: three new species in north and northwestern Madagascar. Molecular Phylogenetics and Evolution, 43, 309–327.
Owings, D. H., & Morton, E. S. (1998). Animal vocal communication: A new approach. Cambridge, U.K.: Cambridge University Press.
Peters, G., & Tonkin-Leyhausen, B. A. (1999). Evolution of acoustic communication signals of mammals: Friendly close-range vocalizations in Felidae (Carnivora). Journal of Mammalian Evolution, 2, 129–159.
Ploog, D. (1974). Phylogenetic and ontogenetic aspects of vocal behavior in squirrel monkeys. Neuroscience Research Program Bulletin, 12, 611–618.
Rabarivola, C., Zaramody, A., Fausser, J. L., Andriaholinirina, N., Roos, C., Zinner, D., Rumpler, Y. (2006). Cytogenetic and molecular characteristics of a new species of Lepilemur from Northern Madagascar. Lemur News, 11, 45–49.
Rabesandratana, A. Z. (2006). Variation microgeographiques et bioacoustiques de Lepilemur edwardsi (Geoffroy, 1850) dans le Parc National Ankarafantsika (region nord-ouest de Madagascar). Doctoral dissertation, Universite´ d’Antananarivo, Madagascar.
Ramaromilanto, B., Lei, R. H., Engberg, S. E., Johnson, S. E., Sitzmann, B. D., & Jouis, E. E. J. (2009). Sportive lemur diversity at Mananara-Nord Biosphere Reserve, Madagascar. Occasional Papers of the Museum of Texas Tech University, 286, 1–22.
Rasoloharijaona, S., Rakotosamimanana, B., Zimmermann, E. (2000). Infanticide by a male Milne-Edwards' sportive lemur (Lepilemur edwardsi) in Ampijoroa, NW-Madagascar. International Journal of Primatology, 21, 41–45.
Rasoloharijaona, S., Randrianambinina, B., Braune, P., & Zimmermann, E. (2006). Loud calling, spacing, and cohesiveness in a nocturnal primate, the Milne Edwards’ sportive lemur (Lepilemur edwardsi). American Journal of Physical Anthropology, 129, 591–600.
Riede, K. (1998). Acoustic monitoring of Orthoptera and its potential for conservation. Journal of Insect Conservation, 2, 217–223.
Ryan, M. J., & Kime, N. M. (2003). Selection on long-distance acoustic signals. In A. M. Simmons, A. N. Popper, & R. R. Fay (Eds.), Acoustic communication (pp. 225–274). New York: Springer-Verlag.
Saunders, M. B., & Barclay, R. M. R. (1992). Ecomorphology of insectivorous bats: A test of predictions using two morphologically similar species. Ecology, 73, 1335–1345.
Schrader, L., & Todt, D. (1993). Contact call parameters covary with social context in common marmosets, Callithrix j. jacchus. Animal Behaviour, 46, 1026–1028.
Schwitzer, C., Schwitzer, N., Randriatahina, G. H., Rabarivola, C., & Kaumanns, W. (2006). “Programme Sahamalaza”: New perspectives for the in situ and ex situ study and conservation of the blue-eyed black lemur (Eulemur macaco flavifrons) in a fragmented habitat. In C. Schwitzer, S. Brandt, O. Ramilijaona, M. Rakotomalala Razanahoera, D. Ackermand, T. Razakamanana, J. U. Ganzhorn (Eds.), Proceedings of the German-Malagasy research cooperation in life and earth sciences (pp. 135–149). Berlin: Concept Verlag.
Schwitzer, N., Kaumanns, W., Seitz, P. C., & Schwitzer, C. (2007a). Cathemeral activity patterns of the blue-eyed black lemur Eulemur macaco flavifrons in intact and degraded forest fragments. Endangered Species Research, 3, 293–247.
Schwitzer, N., Randriatahina, G. H., Kaumanns, W., Hoffmeister, D., & Schwitzer, C. (2007b). Habitat utilization of blue-eyed black lemurs, Eulemur macaco flavifrons (Gray, 1867), in primary and altered forest fragments. Primate Conservation, 22, 79–87.
Seiler, M., Holderied, M., & Schwitzer, C. (2013). Anti-predator behaviour of Sahamalaza sportive lemurs, Lepilemur sahamalazensis, at diurnal sleeping sites. Contributions to Zoology, 82, 131–143.
Seiler, M., Holderied, M., & Schwitzer, C. (2014). Habitat selection and use in the Critically Endangered Sahamalaza sportive lemur, Lepilemur sahamalazensis, in altered habitat. Endangered Species Research, 24, 273–286.
Seiler, M., Randriatahina, G. H., & Schwitzer, C. (2010). Ongoing threats to lemurs and their habitat inside the Sahamalaza – Iles Radama National Park. Lemur News, 15, 7–9.
Seiler, M., Randriatahina, G. H., & Schwitzer, C. (2012). The rapid boost of forest destruction and poaching of lemurs inside the Sahamalaza – Iles Radama National Park. Lemur News, 16, 28–30.
Stanger, K. (1995). Vocalizations of some cheirogaleid prosimians evaluated in a phylogenetic context. In L. Alterman, G. Doyle, & M. Izard (Eds.), Creatures of the dark (pp. 353–376). New York: Plenum Press.
Tattersall, I. (1982). The primates of Madagascar. New York: Columbia University Press.
Templeton, A. R. (1998). The role of molecular genetics in speciation studies. In R. DeSalle & B. Schierwater (Eds.), Molecular approaches to ecology and evolution (pp. 131–156). Basel: Birkhäuser.
Thomassen, H. A., & Povel, G. D. E. (2006). Comparative and phylogenetic analysis of the echo clicks and social vocalizations of swiftlets (Aves: Apodidae). Biological Journal of the Linnean Society, 88, 631–643.
Whitehead, J. M. (1995). Vox Alouattinae: A preliminary survey of acoustic characteristics of long-distance calls of howling monkeys. International Journal of Primatology, 16, 121–144.
Zimmermann, E. (1990). Differentiation of vocalizations on bushbabies (Galaginae, Prosimiae, Primates) and significance for assessing phylogenetic relationships. Journal of Zoological Systematics and Evolutionary Research, 28, 217–239.
Zimmermann, E., Bearder, S. K., Doyle, G. A., & Andersson, A. B. (1988). Variations in vocal patterns of Senegal and South African lesser bushbabies and their implications for taxonomic relationships. Folia Primatologica, 51, 87–105.
Zimmermann, E., & Lerch, C. (1993). The complex acoustic design of an advertisement call in male mouse lemurs (Microcebus murinus, Prosimii, Primates) and sources of its variation. Ethology, 93, 211–224.
Zimmermann, E., Vorobieva, E., Wrogemann, D., & Hafen, T. (2000). Use of vocal fingerprinting for specific discrimination of gray (Microcebus murinus) and rufous mouse lemurs (Microcebus rufus). International Journal of Primatology, 21, 837–852.
Acknowledgments
We thank Madagascar National Parks (MNP), especially the director of Sahamalaza – Iles Radama National Park, M. Isaia Raymond, for their continuing collaboration. Thank you also to the DGEF and CAFF/CORE for granting us research permits for our work in Sahamalaza, and to Prof. Rabarivola Clément for his ongoing help. Tantely Nirina Ralantoharijaona, Bronwen Daniel, Chris Ingold, Sam Gatley, Jeremy Cusack, Lucy Todd, Lisa Knudsen, and Gabby Bell, along with all Ankarafa field guides, contributed substantially to the data collection. We further thank the editor and two anonymous reviewers for valuable comments on a former version of the manuscript. M. Seiler was funded by Bristol Conservation and Science Foundation, Association Européenne pour l’Etude et la Conservation des Lémuriens (AEECL), Conservation International Primate Action Fund, Margot Marsh Biodiversity Foundation, Mohamed bin Zayed Species Conservation Fund, International Primatological Society, and Christian-Vogel-Fonds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seiler, M., Schwitzer, C. & Holderied, M. Call Repertoire of the Sahamalaza Sportive Lemur, Lepilemur sahamalazensis . Int J Primatol 36, 647–665 (2015). https://doi.org/10.1007/s10764-015-9846-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-015-9846-0